Development and Implementation of a Professional Practices Evaluation during Radiopharmaceuticals Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Elaboration of the Evaluation Grid
- Criterion met (O) if all aspects of the item were fulfilled (the item scores the totality of the points related to its weighting);
- Criterion partially met (I) when one of the aspects of the item was not satisfactory (the item scores half of the points related to its weighting);
- Criterion not met (N) when none of the aspects of the item were correctly covered (the item scores no points);
- Not applicable criteria (NA) when the aspects related to the item are not relevant to the activity audited; NA criteria are therefore not considered in the calculation of rating scores.
2.2. Audit Process
2.3. Data Analysis
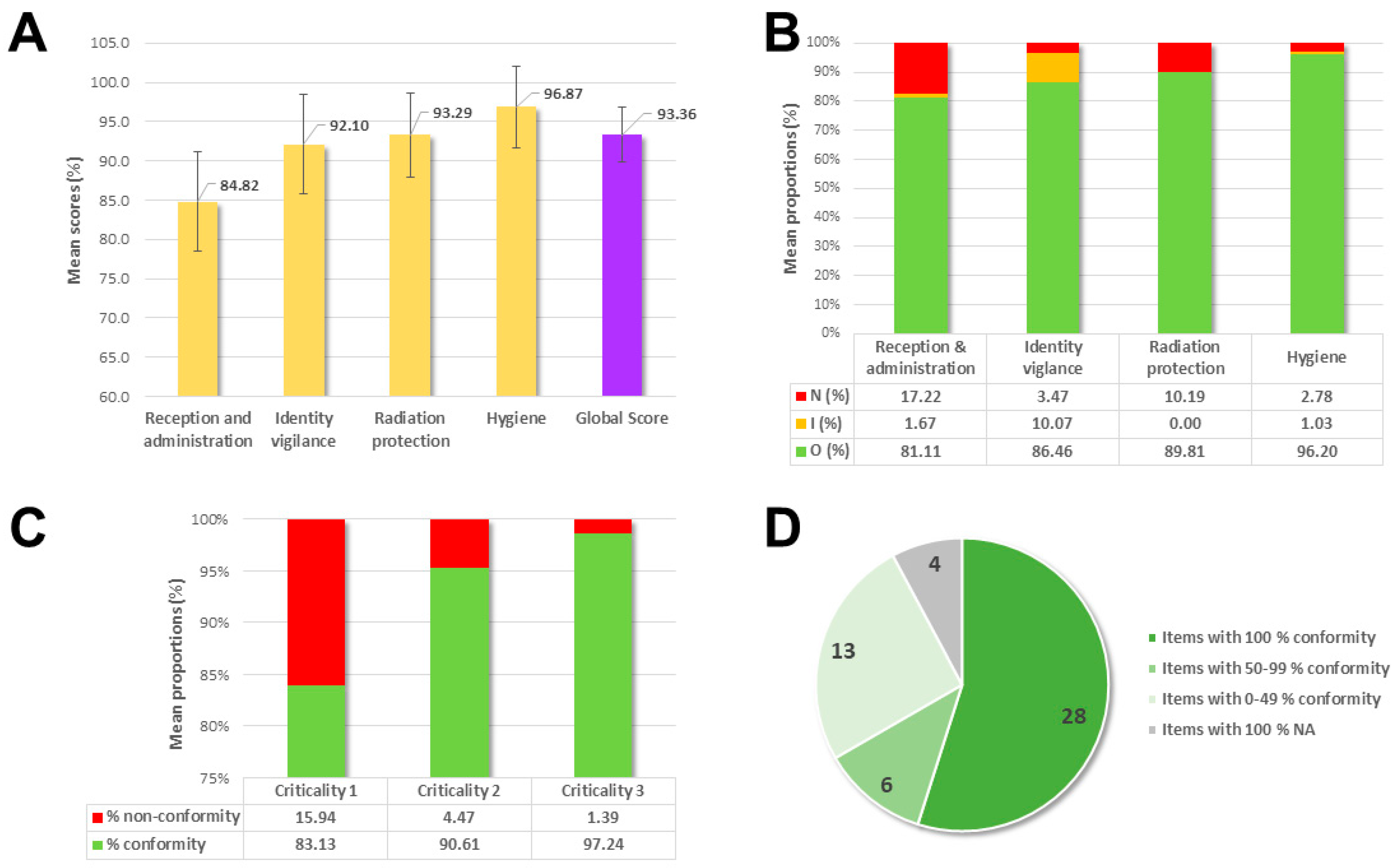
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhonsle, J.; Chianelli, M.; Hartman, N.G.; Jeong, J.M.; Ozker, K.; Savio, E.; Saw, M.M.; Solanki, K.K. Operational Guidance on Hospital Radiopharmacy: A Safe and Effective Approach; International Atomic Energy Agency: Vienna, Austria, 2008. [Google Scholar]
- Shukla, U.; Chowdhury, I.H.; Beckta, J.M.; Witt, J.S.; McFarlane, M.; Miller, C.J.; Huber, K.E.; Katz, M.S.; Royce, T.J.; Chowdhary, M. Unsealed source: Scope of practice for radiopharmaceuticals among United States radiation oncologists. Adv. Radiat. Oncol. 2021, 7, 100827. [Google Scholar] [CrossRef]
- Dinet, J.; Becker, S.; Le Cloirec, J.; Bohn, P. The added value of clinical radiopharmacists in Nuclear Medicine: The example of glomerular filtration rate assessment in kidney donors. J. Clin. Pharm. Ther. 2020, 45, 1114–1119. [Google Scholar] [CrossRef]
- Leclerc, P.; Marie, S.; Fouque, J.; Olivier, M.; Blondeel-Gomes, S. How can we optimise the pharmaceutical analysis of radiopharmaceutical pediatric prescriptions? Eur. J. Hosp. Pharm. 2021; in press. [Google Scholar] [CrossRef]
- Kim, J.; Bates, D.W. Medication administration errors by nurses: Adherence to guidelines. J. Clin. Nurs. 2013, 22, 590–598. [Google Scholar] [CrossRef]
- Williams, E.D.; Harding, L.K. Radiopharmaceutical maladministration: What action is required? Nucl. Med. Commun. 1995, 16, 721–723. [Google Scholar] [CrossRef]
- Joint Working Party of the NSW Branch of ANZSNM and HURSOG. Proposed guidelines for the administration of diagnostic therapeutic radiopharmaceuticals. ANZ Nucl. Med. 1999, 30, 63–65. [Google Scholar]
- Marengo, M.; Martin, C.J.; Rubow, S.; Sera, T.; Amador, Z.; Torres, L. Radiation safety and accidental radiation exposures in nuclear medicine. Semin. Nucl. Med. 2022, 52, 94–113. [Google Scholar] [CrossRef]
- Potdevin-Verdier, J.; Le Meur, C.; Maget, A.; Calas-Chane-Woon-Ming, L.; Carre, A.-L.; Galvez, D.; Hammer-Lefevre, C.; Lao, S.; Debordeaux, F.; Véran, N. Validation of a professional practice assessment tool in radiopharmacy and results of a multisite study. Pharm. Hosp. Clin. 2021, 56, 334–344. [Google Scholar] [CrossRef]
- Ben Reguiga, M.; Rotaru, I.; Ait Abdeslam, S.; Pons-Kerjean, N. EPP en radiopharmacie: Expérimentation d’un outil international d’auto-évaluation des pratiques. Pharm. Hosp. Clin. 2016, 51, 353–354. [Google Scholar] [CrossRef]
- Ebel-Lao, S.; Collomp, R.; Dompe, J.; Ruitort, S.; Carrier, P.; Girma, A.; Koulibaly, P.; Darcourt, J.; Mousnier, A. Formation initiale et continue des préparateurs en radiopharmacie: Mise en place d’une démarche qualité. J. Pharm. Clin. 2008, 27, 235–243. [Google Scholar] [CrossRef]
- World Health Organization. WHO Best Practices for Injections and Related Procedures Toolkit. No. WHO/EHT/10.02; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Gillings, N.; Hjelstuen, O.; Ballinger, J.; Behe, M.; Decristoforo, C.; Elsinga, P.; Ferrari, V.; Peitl, P.K.; Koziorowski, J.; Laverman, P.; et al. Guideline on current good radiopharmacy practice (CGRPP) for the small-scale preparation of radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2021, 6, 8. [Google Scholar] [CrossRef]
- McCambridge, J.; Witton, J.; Elbourne, D.R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J. Clin. Epidemiol. 2014, 67, 267–277. [Google Scholar] [CrossRef]
- Giannoula, E.; Panagiotidis, E.; Katsikavelas, I.; Chatzipavlidou, V.; Sachpekidis, C.; Bamidis, P.; Raftopoulos, V.; Iakovou, I. Quality & safety aspects of nuclear medicine practice: Definitions and review of the current literature. Hell. J. Nucl. Med. 2020, 23, 60–66. [Google Scholar] [CrossRef]
- Kasalak, Ö.; Yakar, D.; Dierckx, R.A.J.O.; Kwee, T.C. Patient safety in nuclear medicine: Identification of key strategic areas for vigilance and improvement. Nucl. Med. Commun. 2020, 41, 1111–1116. [Google Scholar] [CrossRef]
- Dondi, M.; Paez, D.; Torres, L.; Marengo, M.; Delaloye, A.B.; Solanki, K.; Van Zyl Ellmann, A.; Lobato, E.E.; Miller, R.N.; Giammarile, F.; et al. Implementation of quality systems in nuclear medicine: Why it matters. An outcome analysis (quality management audits in nuclear medicine Part III). Semin. Nucl. Med. 2018, 48, 299–306. [Google Scholar] [CrossRef]
- Larcos, G.; Prgomet, M.; Georgiou, A.; Westbrook, J. A work observation study of nuclear medicine technologists: Interruptions, resilience and implications for patient safety. BMJ Qual. Saf. 2017, 26, 466–474. [Google Scholar] [CrossRef]
- De Neef, L.; Peyronnet, D.; Blondeel-Gomes, S. How to validate radiopharmaceuticals management software? Pharm. Technol. 2020, 5, 20200010. [Google Scholar] [CrossRef]
- Hakala, J.L.; Hung, J.C.; Mosman, E.A. Minimizing Human Error in Radiopharmaceutical Preparation and Administration via a Bar Code-Enhanced Nuclear Pharmacy Management System. J. Nucl. Med. Technol. 2012, 40, 183–186. [Google Scholar] [CrossRef]
- Yenson, T.; Larcos, G.; Collins, L.T. Radiopharmaceutical maladministrations in New South Wales. Nucl. Med. Commun. 2005, 26, 1037–1041. [Google Scholar] [CrossRef]
- Kearney, N.; Denham, G. Recommendations for nuclear medicine technologists drawn from an analysis of errors reported in Australian radiation incident registers. J. Nucl. Med. Technol. 2016, 44, 243–247. [Google Scholar] [CrossRef][Green Version]
- Martin, C.J. A survey of incidents in radiology and nuclear medicine in the west of Scotland. Br. J. Radiol. 2005, 78, 913–921. [Google Scholar] [CrossRef]
- Larcos, G.S.; Collins, L.T.; Georgiou, A.; Westbrook, J.I. Maladministrations in nuclear medicine: Revelations from the Australian radiation incident register. Med. J. Aust. 2014, 200, 37–40. [Google Scholar] [CrossRef]
- Larcos, G.; Collins, L.T.; Georgiou, A.; Westbrook, J.I. Nuclear medicine incident reporting in Australia: Control charts and notification rates inform quality improvement. Intern. Med. J. 2015, 45, 609–617. [Google Scholar] [CrossRef]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug. Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Berman, J.; Moadel, R.M.; Goldman-Yassen, A.E.; Abraham, T.; Ye, K.; Volansky, J.; Goldberg-Stein, S. Impact of patient-centered care on the patient experience in nuclear medicine. Curr. Probl. Diagn. Radiol. 2020, 49, 326–332. [Google Scholar] [CrossRef]
- Braithwaite, J.; Wears, R.L.; Hollnagel, E. Resilient health care: Turning patient safety on its head. Int. J. Qual. Health Care 2015, 27, 418–420. [Google Scholar] [CrossRef]
- Dondi, M.; Torres, L.; Marengo, M.; Massardo, T.; Mishani, E.; Van Zyl Ellmann, A.; Solanki, K.; Bischof Delaloye, A.; Lobato, E.E.; Miller, R.N.; et al. Comprehensive auditing in nuclear medicine through the international atomic energy agency quality management audits in nuclear medicine (QUANUM) program. Part 1: The QUANUM program and methodology. Semin. Nucl. Med. 2017, 47, 680–686. [Google Scholar] [CrossRef]
- Gillings, N.; Hjelstuen, O.; Behe, M.; Decristoforo, C.; Elsinga, P.H.; Ferrari, V.; Kiss, O.C.; Kolenc, P.; Koziorowski, J.; Laverman, P.; et al. EANM guideline on quality risk management for radiopharmaceuticals. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3353–3364. [Google Scholar] [CrossRef]
| Topics | Reception and Administration | Identity Vigilance | Hygiene | Radiation Protection | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Criteria and Criticality | |||||||||||
| Total number of criteria in the category | High criticality (3) | 7 | 0 | 9 | 2 | 20 | 5 | 15 | 4 | 51 | 11 |
| Intermediate criticality (2) | 1 | 7 | 10 | 7 | 25 | ||||||
| Low criticality (1) | 6 | 0 | 5 | 4 | 15 | ||||||
| Theme | Item | Partially Compliant Records (n) | Non-Compliant Records (n) | Overall Non-Compliance Proportion (%) |
|---|---|---|---|---|
| A | The nuclear medicine technologist introduces himself | 1/36 | 29/36 | 83.3 |
| In case of injection of blood-derived drugs: verbal information of the patient before injection | 0 | 2/3 | 66.7 | |
| In case of injection of blood-derived drugs: written information provided to the patient | 0 | 2/3 | 66.7 | |
| I | Concordance with the examination checked in the patient file | 0 | 5/36 | 13.9 |
| Once installed, the patient asked to confirm their last name | 0 | 3/36 | 8.3 | |
| Once installed, the patient asked to confirm their first name | 0 | 2/36 | 5.6 | |
| Patient was asked to confirm the purpose of the examination by open question (O)/By closed question (I) | 25/36 | 0 | 69.4 | |
| Concordance between the examination and the radiopharmaceutical checked just before administration | 4/36 | 0 | 11.1 | |
| R | Woman of childbearing age asked about breastfeeding | 0 | 5/6 | 83.3 |
| Secure transport of the RP syringe | 0 | 1/1 | 100 | |
| Shielded case used for RP syringe transport | 0 | 1/1 | 100 | |
| Mobile cart used for RP syringe transport | 0 | 1/1 | 100 | |
| Shielded apron worn for RP injection | 0 | 20/36 | 55.6 | |
| H | Preparation of an injection tray with pads, plasters, needles, gloves, tourniquet, and syringe | 5/36 | 0 | 13.9 |
| Catheter placed after 4-step detersion | 0 | 5/5 | 100 | |
| Check of the catheter line by opening infusion of NaCl 0.9% | 0 | 4/5 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donzé, C.; Rubira, L.; Santoro, L.; Kotzki, P.O.; Deshayes, E.; Fersing, C. Development and Implementation of a Professional Practices Evaluation during Radiopharmaceuticals Administration. Healthcare 2022, 10, 2247. https://doi.org/10.3390/healthcare10112247
Donzé C, Rubira L, Santoro L, Kotzki PO, Deshayes E, Fersing C. Development and Implementation of a Professional Practices Evaluation during Radiopharmaceuticals Administration. Healthcare. 2022; 10(11):2247. https://doi.org/10.3390/healthcare10112247
Chicago/Turabian StyleDonzé, Charlotte, Léa Rubira, Lore Santoro, Pierre Olivier Kotzki, Emmanuel Deshayes, and Cyril Fersing. 2022. "Development and Implementation of a Professional Practices Evaluation during Radiopharmaceuticals Administration" Healthcare 10, no. 11: 2247. https://doi.org/10.3390/healthcare10112247
APA StyleDonzé, C., Rubira, L., Santoro, L., Kotzki, P. O., Deshayes, E., & Fersing, C. (2022). Development and Implementation of a Professional Practices Evaluation during Radiopharmaceuticals Administration. Healthcare, 10(11), 2247. https://doi.org/10.3390/healthcare10112247







