Benefits of Cardio-Pulmonary Rehabilitation in Moderate to Severe Forms of COVID-19 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Pulmonary Function Testing
2.3. CPET
2.4. Cardiopulmonary Rehabilitation
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 3 October 2022).
- Pascarella, G.; Strumia, A. COVID-19 diagnosis and management: A comprehensive review. J. Intern. Med. 2020, 288, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.J.; Gattinoni, L. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Madid, M.; Safavi-Naeini, P. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farshidfar, F.; Koleini, N.; Ardehali, H. Cardiovascular complications of COVID-19. JCI Insight 2021, 6, e148980. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Cares-Marambio, K.; Montenegro-Jiménez, Y. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Chronic Respir. Dis. 2021, 18, 14799731211002240. [Google Scholar] [CrossRef]
- Spruit, M.A.; Holland, A.E. COVID-19: Interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society and American Thoracic Society-coordinated International Task Force. Eur. Respir. J. 2020, 56, 2002197. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanger, J.; Clausen, J.L. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, N.; Crapo, R.O. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef] [Green Version]
- Bjarnason-Wehrens, B.; Schwaab, B. Resistance Training in Patients With Coronary Artery Disease, Heart Failure, and Valvular Heart Disease: A review with special emphasis on old age, frailty, and physical limitations. J. Cardiopulm. Rehabil. Prev. 2022, 42, 304–315. [Google Scholar] [CrossRef]
- Bai, C.; Chotirmall, S.H. Updated guidance on the management of COVID-19: From an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020). Eur. Respir. Rev. 2020, 29, 200287. [Google Scholar] [CrossRef]
- Belli, S.; Balbi, B. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur. Respir. J. 2020, 56, 2002096. [Google Scholar] [CrossRef]
- Motiejunaite, J.; Balagny, P. Hyperventilation as one of the mechanisms of persistent dyspnea in SARS-CoV-2 survivors. Eur. Respir. J. 2021, 58, 2101578. [Google Scholar] [CrossRef]
- Rinaldo, R.R.; Mondoni, M. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur. Respir. J. 2021, 58, 2100870. [Google Scholar] [CrossRef]
- Raman, B.; Cassar, M.P. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021, 31, 100683. [Google Scholar] [CrossRef]
- Medrinal, C.; Prieur, G. Muscle weakness, functional capacities and recovery for COVID-19 ICU survivors. BMC Anesthesiol. 2021, 21, 64. [Google Scholar] [CrossRef]
- Soares, M.N.; Eggelbusch, M.; Naddaf, E.; Gerrits, K.H.; van der Schaaf, M.; van den Borst, B.; Wiersinga, W.J.; van Vugt, M.; Weijs, P.J.; Murray, A.J.; et al. Skeletal muscle alterations in patients with acute COVID-19 and post-acute sequelae of COVID-19. J. Cachexia Sarcopenia Muscle 2022, 13, 11–22. [Google Scholar] [CrossRef]
- Simon, M.; Simmons, J.E. A Review of Respiratory Post-Acute Sequelae of COVID-19 (PASC) and the Potential Benefits of Pulmonary Rehabilitation. Rhode Isl. Med. J. 2022, 105, 11–15. [Google Scholar]
- Wirth, K.J.; Scheibenbogen, C. Dyspnea in Post-COVID Syndrome following Mild Acute COVID-19 Infections: Potential Causes and Consequences for a Therapeutic Approach. Medicina 2022, 58, 419. [Google Scholar] [CrossRef]
- Mezzani, A.; Hamm, L.F. Association for Cardiovascular Prevention and Rehabilitation; American Association of Cardiovascular and Pulmonary Rehabilitation; Canadian Association of Cardiac Rehabilitation. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: A joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur. J. Prev. Cardiol. 2013, 20, 442–467. [Google Scholar] [PubMed]
- Soril, L.J.; Damant, R.W.; Lam, G.Y.; Smith, M.P.; Weatherald, J.; Bourbeau, J.; Hernandez, P.; Stickland, M.K. The effectiveness of pulmonary rehabilitation for post-COVID symptoms: A rapid review of the literature. Respir. Med. 2022, 195, 106782. [Google Scholar] [CrossRef] [PubMed]
- Winnige, P.; Vysoky, R.; Dosbaba, F.; Batalik, L. Cardiac rehabilitation and its essential role in the secondary prevention of cardiovascular diseases. World J. Clin. Cases 2021, 9, 1761–1784. [Google Scholar] [CrossRef]
- Salzwedel, A.; Jensen, K. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence-based medicine: Update of the Cardiac Rehabilitation Outcome Study (CROS-II). Eur. J. Prev. Cardiol. 2020, 27, 1756–1774. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Oldridge, N. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2016, 67, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, E.C.; Franke, W.D. Comparative effectiveness of aerobic, resistance, and combined training on cardiovascular disease risk factors: A randomized controlled trial. PLoS ONE 2019, 14, e0210292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balady, G.J.; Williams, M.A. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update. A scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2007, 115, 2675–2682. [Google Scholar]
- Guiraud, T.; Nigam, A. High-intensity interval training in cardiac rehabilitation. Sports Med. 2012, 42, 587–605. [Google Scholar] [CrossRef]
- Wisløff, U.; Støylen, A. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef] [Green Version]
- Leanna, M.; RossRyan, R. High-intensity interval training (HIIT) for patients with chronic diseases. J. Sport Health Sci. 2016, 5, 139–144. [Google Scholar]
- Vogiatzis, G.; Terzis, S. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest 2005, 128, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
| COVID-19 Patients | CONTROLS | |||
|---|---|---|---|---|
| Before | Post | Before | Post | |
| Male/Female (n = 25) | 16/9 (n = 25) | 16/9 (n = 25) | ||
| Age (years) | 61 ± 8 | 62 ± 10 | ||
| Height (cm) | 173 ± 11 | 170 ± 10 | ||
| Weight (kg) | 86 ± 16 | 88 ± 15 | 81 ± 13 | 81 ± 13 |
| BMI (kg/m2) | 28.7 ± 4.2 | 29.2 ± 3.8 | 28.1 ± 3.8 | 28 ± 3.7 |
| History of smoking | 10 | 5 | ||
| Hypertension | 12 | 18 | ||
| Diabetes | 3 | 8 | ||
| Dyslipidemia | 6 | 22 $$$ | ||
| MEDICAL HISTORY | ||||
| Intensive Care Unit | 13 | Stenting | 16 | |
| Mechanical ventilation | 12 | CABG | 9 | |
| Noninvasive ventilation | 16 | |||
| Oxygen supplementation | 25 | |||
| Betablockers | 5 | 20 | ||
| Before (n = 17) | After (n = 17) | |
|---|---|---|
| LVC (L) | 3.1 ± 0.7 | 3.5 ± 0.7 *** |
| LVC (% predicted value) | 79 ± 22 | 91 ± 21 *** |
| FEV1 (L) | 2.7 ± 0.8 | 2.9 ± 0.7 |
| FEV1 (% predicted value) | 84 ± 20 | 94 ± 19 * |
| DLCO (mL/min/mmHg) | 14.2 ± 3.8 | 17.1 ± 4.4 *** |
| DLCO (% predicted value) | 52 ± 11 | 63 ± 12 *** |
| KCO (mL/min/mmHg/L) | 3.4 ± 0.8 | 3.6 ± 0.7 |
| KCO (% predicted value) | 82 ± 18 | 87 ± 18 |
| COVID-19 Patients | CONTROLS | |||
|---|---|---|---|---|
| Before | Post | Before | Post | |
| REST | ||||
| HR (bpm) | 93 ± 15 | 89 ± 15 | 78 ± 13 $$ | 75 ± 10 $$ |
| MBP (mmHg) | 90 ± 10 | 93 ± 10 | 88 ± 12 | 89 ± 10 |
| SpO2 (%) | 98 ± 2 | 98 ± 1 | 98 ± 1 | 98 ± 1 |
| VENTILATORY TRESHOLD 1 | ||||
| VO2 (L/min) | 1.0 ± 0.3 | 1.2 ± 0.6 *** | 0.9 ± 0.2 | 1.2 ± 0.3 *** |
| VO2 (% predicted value) | 51 ± 17 | 60 ± 19 *** | 47 ± 12 | 61 ± 13 *** |
| Workload (W) | 63 ± 23 | 86 ± 30 *** | 65 ± 19 | 88 ± 30 *** |
| EqCO2 | 39 ± 6 | 36 ± 5 * | 37 ± 4 | 35 ± 4 ** |
| PetCO2 (mmHg) | 36 ± 4 | 38 ± 4 * | 37 ± 4 | 38 ± 4 |
| HR (bpm) | 117 ± 13 | 119 ± 13 | 98 ± 15 $$$ | 103 ± 13 $$$ |
| MAXIMAL EFFORT | ||||
| Workload (W) | 107 ± 28 | 137 ± 38 *** | 111 ± 31 | 138 ± 45 *** |
| Workload (% predicted value) | 70 ± 25 | 88 ± 27 *** | 75 ± 20 | 93 ± 29 *** |
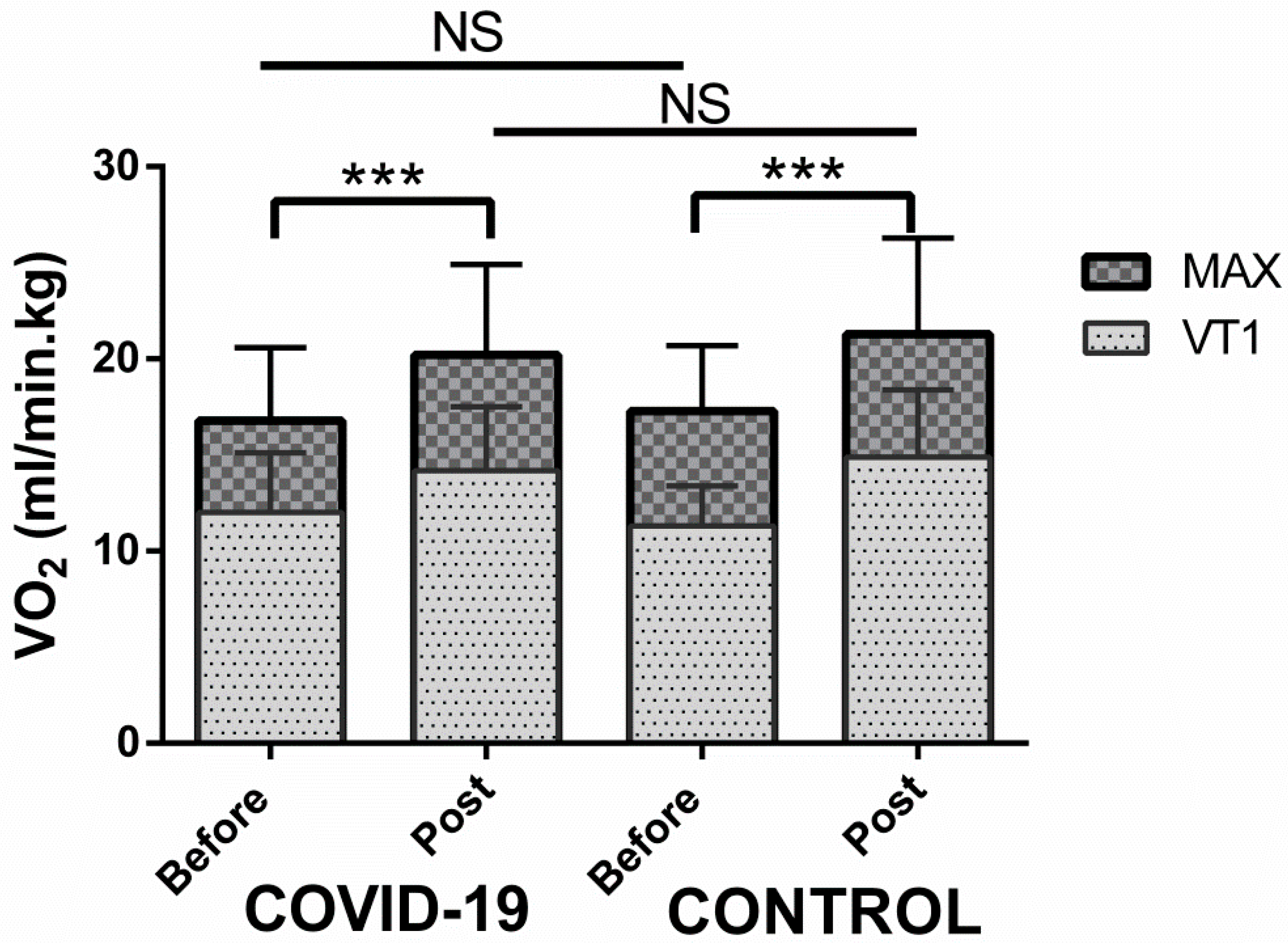
| VO2 (L/min) | 1.4 ± 0.3 | 1.8 ± 0.5 *** | 1.4 ± 0.3 | 1.7 ± 0.4 *** |
| VO2 (mL/min/kg) | 16.8 ± 3.8 | 20.2 ± 4.7 *** | 17.3 ± 3.4 | 21.3 ± 5 *** |
| VO2 (% predicted value) | 71 ± 21 | 84 ± 22 *** | 72 ± 15 | 87 ± 16 *** |
| RER | 1.18 ± 0.10 | 1.16 ± 0.06 | 1.23 ± 0.10 | 1.20 ± 0.09 |
| VE (L/min) | 68 ± 14 | 78 ± 18 *** | 71 ± 20 | 81 ± 24 *** |
| VE/MVV (%) | 63 ± 13 | 83 ± 19 * | 63 ± 9 | 76 ± 16 ** |
| HR (bpm) | 140 ± 17 | 144 ± 24 | 126 ± 20 $$ | 134 ± 21 * |
| HR (% theoretical max HR) | 88 ± 9 | 90 ± 13 | 80 ± 11 $$$ | 85 ± 10 *$ |
| MBP (mmHg) | 115 ± 13 | 119 ± 13 | 117 ± 17 | 121 ± 19 |
| SpO2 (%) | 95 ± 4 | 95 ± 2 | 98 ± 1 $$ | 98 ± 1 $$ |
| SLOPE | ||||
| VO2/W | 9 ± 2 | 10 ± 1 | 9 ± 2 | 9 ± 2 |
| FC/VO2 | 4.2 ± 1.4 | 3.8 ± 0.7 | 4.0 ± 1.3 | 3.7 ± 1.1 |
| VE/VCO2 | 37 ± 6 | 34 ± 6 | 38 ± 7 | 35 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douin, C.; Forton, K.; Lamotte, M.; Gillet, A.; Van de Borne, P. Benefits of Cardio-Pulmonary Rehabilitation in Moderate to Severe Forms of COVID-19 Infection. Healthcare 2022, 10, 2044. https://doi.org/10.3390/healthcare10102044
Douin C, Forton K, Lamotte M, Gillet A, Van de Borne P. Benefits of Cardio-Pulmonary Rehabilitation in Moderate to Severe Forms of COVID-19 Infection. Healthcare. 2022; 10(10):2044. https://doi.org/10.3390/healthcare10102044
Chicago/Turabian StyleDouin, Clara, Kevin Forton, Michel Lamotte, Alexis Gillet, and Philippe Van de Borne. 2022. "Benefits of Cardio-Pulmonary Rehabilitation in Moderate to Severe Forms of COVID-19 Infection" Healthcare 10, no. 10: 2044. https://doi.org/10.3390/healthcare10102044
APA StyleDouin, C., Forton, K., Lamotte, M., Gillet, A., & Van de Borne, P. (2022). Benefits of Cardio-Pulmonary Rehabilitation in Moderate to Severe Forms of COVID-19 Infection. Healthcare, 10(10), 2044. https://doi.org/10.3390/healthcare10102044





