Protocols of Investigation of Neonatal Cholestasis—A Critical Appraisal
Abstract
1. Definition
2. Differential Diagnosis
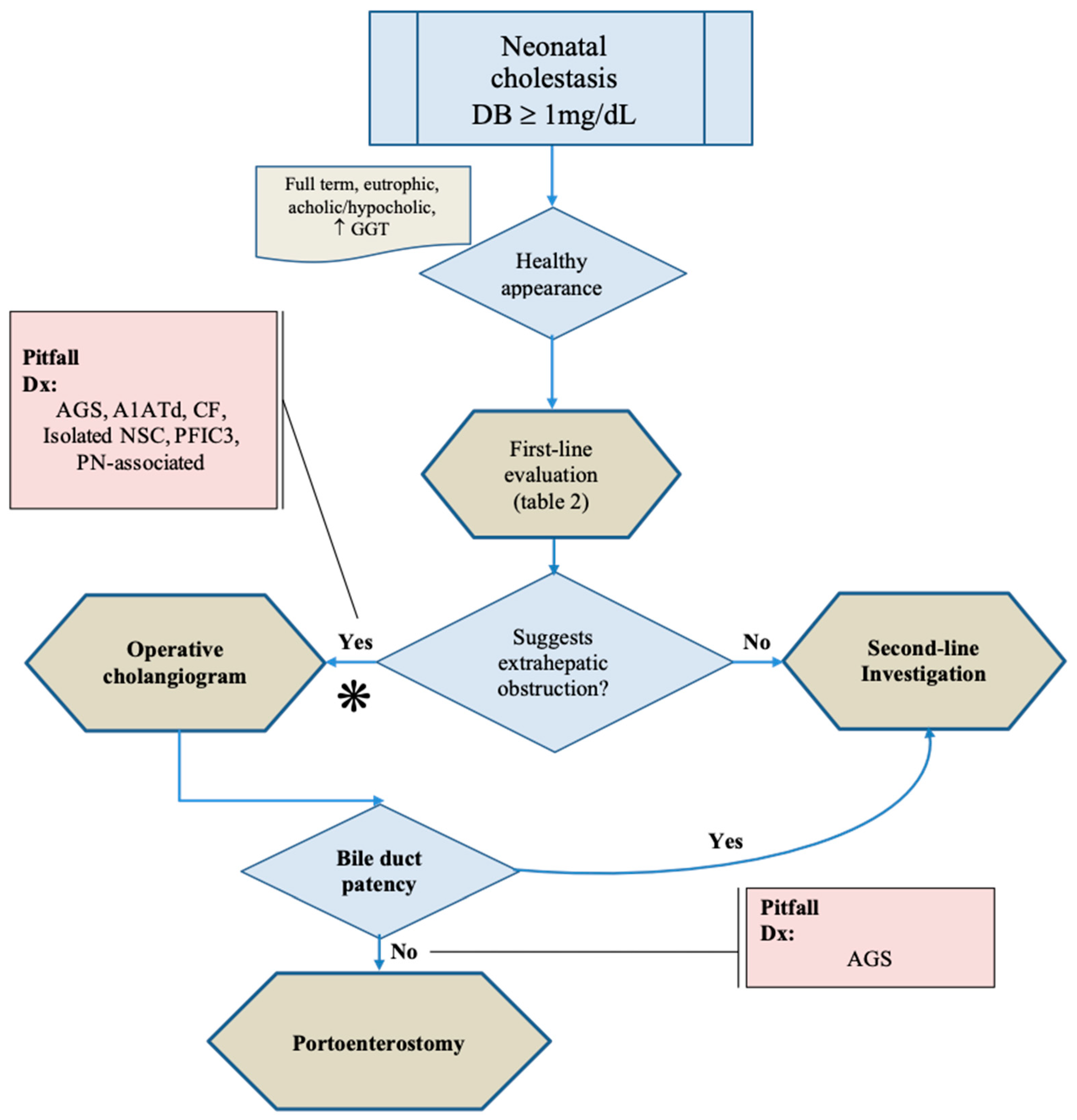
3. The First Challenge: Pitfalls in the Diagnosis of Biliary Atresia
3.1. Novel Approaches for Identification of BA
3.1.1. Serum Markers
3.1.2. Arterial Vascular Abnormalities
3.1.3. Are Genetic Studies Useful to Differentiate BA from Intrahepatic Causes of NC?
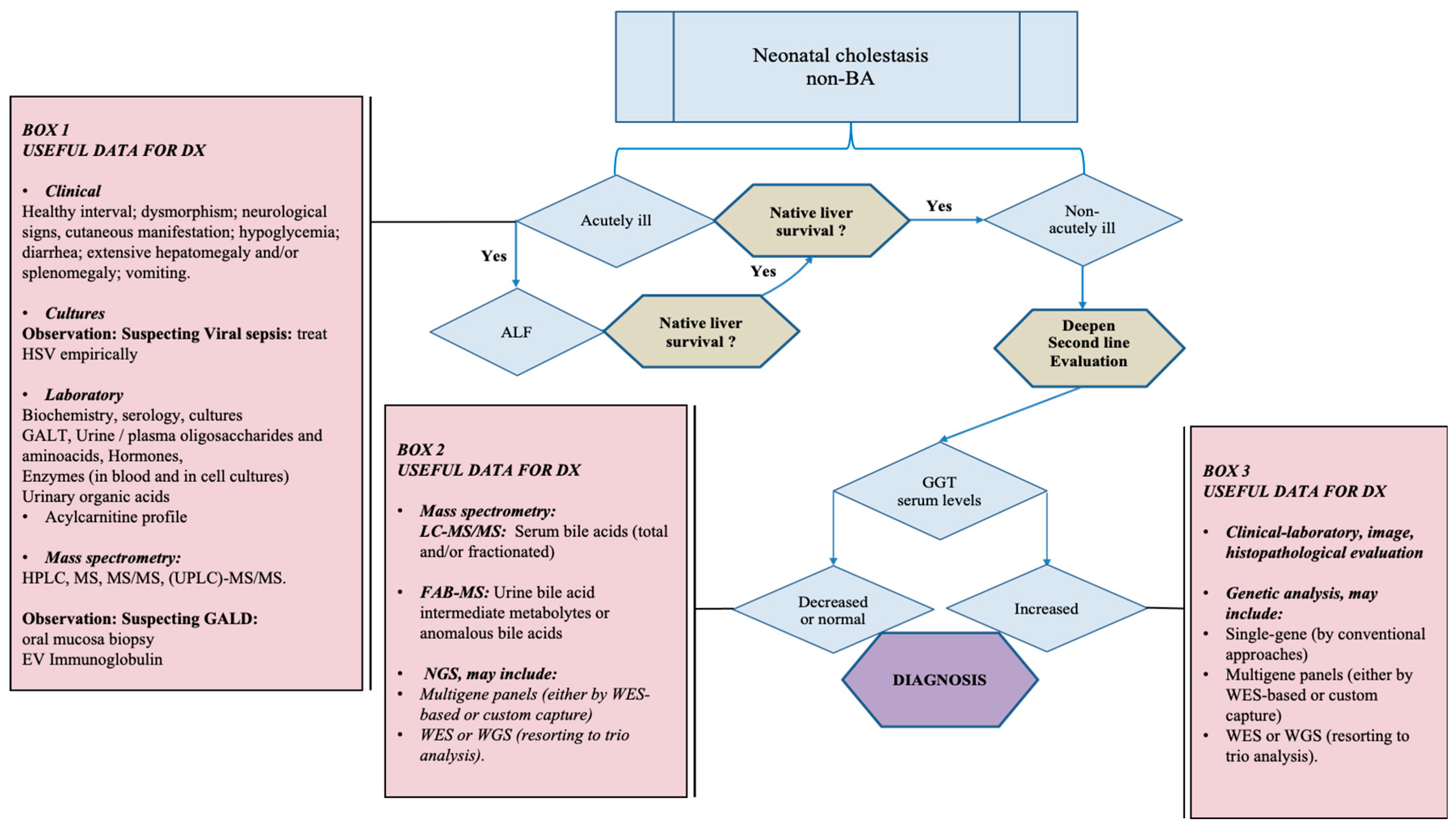
4. The Second Challenge: The Identification of the Neonatal Intrahepatic Disease
4.1. Clinical-Laboratory Investigation
Acutely-Ill Appearing Child
4.2. Integrative Approach of Clinical-Laboratory, Molecular, Histopathological, and Genetic Investigation for the Diagnosis of NC
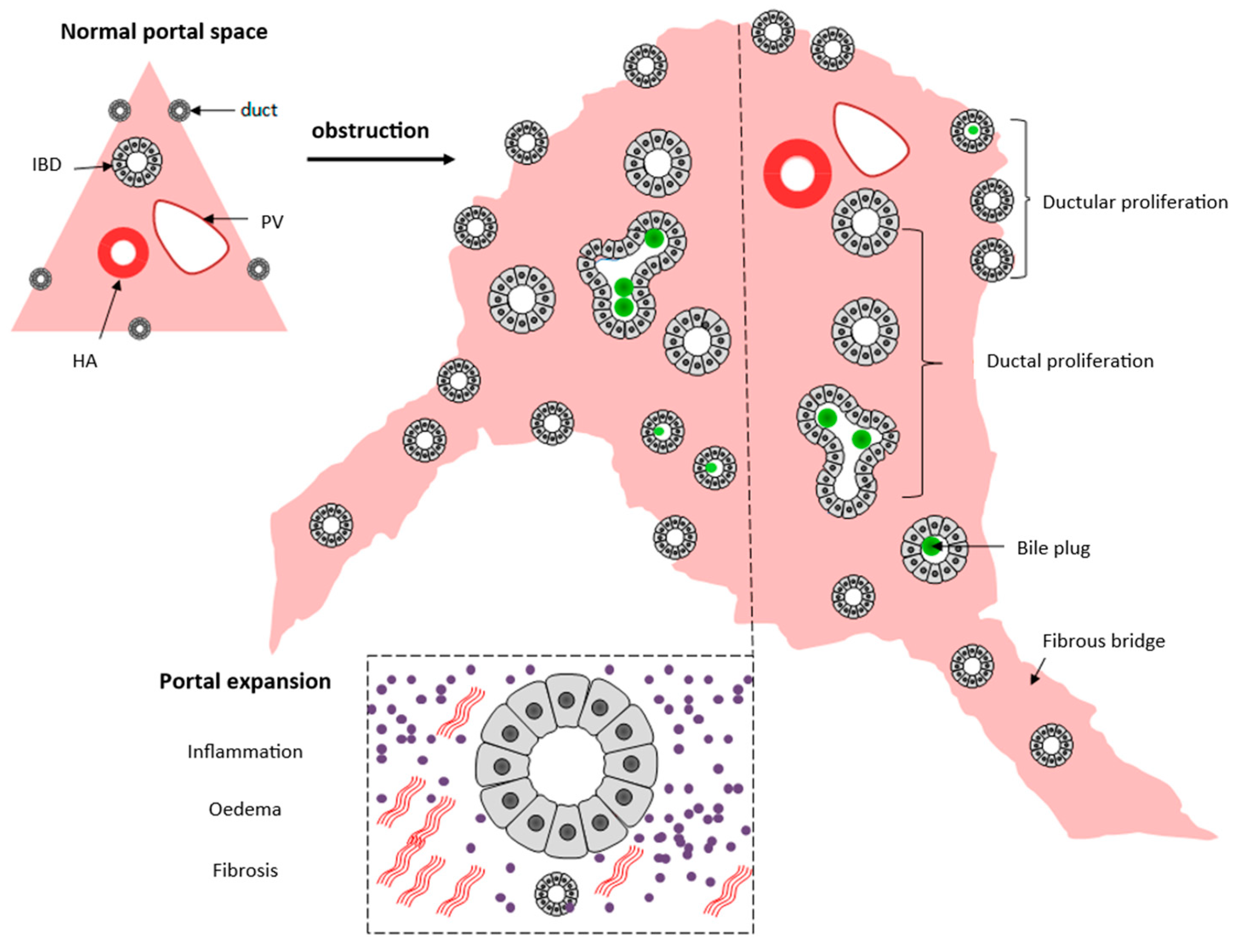
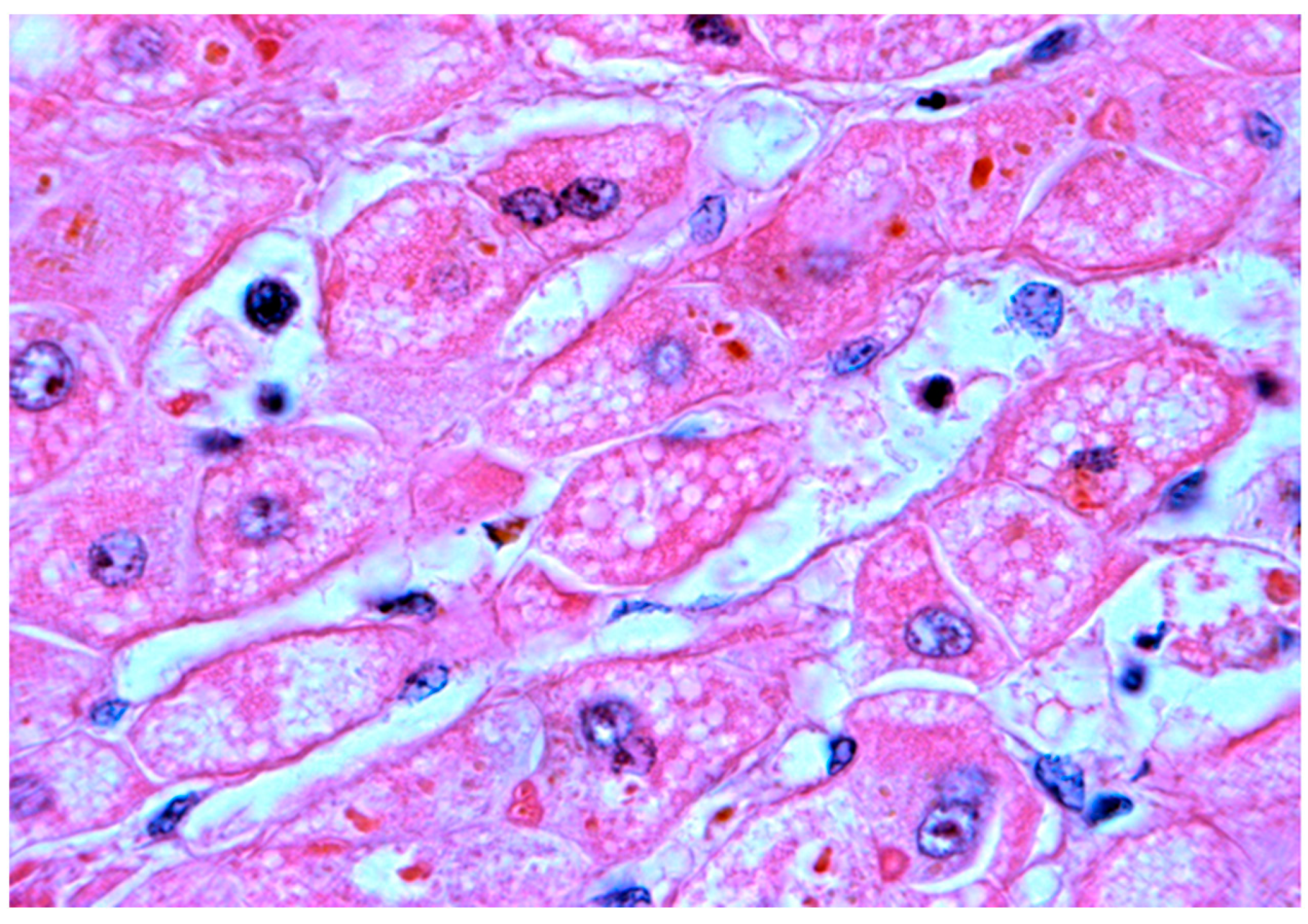
4.3. The Role of Histopathological Investigation in the Differentiation of Intrahepatic Neonatal Cholestasis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, J.L.; Choquette, M.; Bezerra, J.A. Cholestatic Liver Disease in Children. Curr. Gastroenterol. Rep. 2010, 12, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, W.F. Neonatal cholestasis. J. Pediatr. 1985, 106, 171–184. [Google Scholar] [CrossRef]
- McKiernan, P.J. Neonatal cholestasis. Semin. Neonatol. 2002, 7, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Fischler, B.; Lamireau, T. Cholestasis in the newborn and infant. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.; Rajindrajith, S. Neonatal and infantile cholestasis: An overlooked health burden with unmet needs. Indian J. Gastroenterol. 2020, 39, 531–538. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, I.; Lee, H.J.; Oh, H.J.; Ahn, M.K.; Baek, W.I.; Kim, Y.E.; Oh, S.H.; Lee, B.S.; Namgoong, J.M. Clinical characteristics of neonatal cholestasis in a tertiary hospital and the development of a novel prediction model for mortality. EBioMedicine 2022, 77, 103890. [Google Scholar] [CrossRef] [PubMed]
- Fawaz, R.; Baumann, U.; Ekong, U.; Fischler, B.; Hadzic, N.; Mack, C.L.; McLin, V.A.; Molleston, J.P.; Neimark, E.; Ng, V.L. Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 154–168. [Google Scholar] [PubMed]
- Harpavat, S.; Garcia-Prats, J.A.; Anaya, C.; Brandt, M.L.; Lupo, P.J.; Finegold, M.J.; Obuobi, A.; ElHennawy, A.A.; Jarriel, W.S.; Shneider, B.L. Diagnostic yield of newborn screening for biliary atresia using direct or conjugated bilirubin measurements. JAMA 2020, 323, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.G.; Sokol, R.J. Neonatal cholestasis: Emerging molecular diagnostics and potential novel therapeutics. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 346–360. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, E.; dos Santos, J.L.; da Silveira, T.R.; Kieling, C.O.; Silva, L.R.; Porta, G.; Kazue, I.; Tommaso, A.M.A.; Brandão, M.A.B.; Ferreira, A.R.; et al. Atresia biliar: A experiência Brasileira. J. Pediatr. 2010, 86, 473–479. [Google Scholar] [CrossRef]
- Mieli-Vergani, G.; Howard, E.R.; Portman, B.; Mowat, A.P. Late referral for biliary atresia—Missed opportunities for effective surgery. Lancet 1989, 1, 421–423. [Google Scholar] [CrossRef]
- Serinet, M.-O.; Broué, P.; Jacquemin, E.; Lachaux, A.; Sarles, J.; Gottrand, F.; Gauthier, F.; Chardot, C. Management of patients with biliary atresia in France: Results of a decentralized policy 1986–2002. Hepatology 2006, 44, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Chardot, C.; Buet, C.; Serinet, M.O.; Golmard, J.L.; Lachaux, A.; Roquelaure, B.; Gottrand, F.; Broué, P.; Dabadie, A.; Gauthier, F.; et al. Improving outcomes of biliary atresia: French national series 1986–2009. J. Hepatol. 2013, 58, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Fanna, M.; Masson, G.; Capito, C.; Girard, M.; Guerin, F.; Hermeziu, B.; Lachaux, A.; Roquelaure, B.; Gottrand, F.; Broue, P.; et al. Management of Biliary Atresia in France 1986 to 2015: Long-term Results. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.A. Biliary atresia in Brazil: Where we are and where we are going. J. Pediatr. 2010, 86, 445–447. [Google Scholar] [CrossRef][Green Version]
- Andrianov, M.G.; Azzam, R.K. Cholestasis in Infancy. Pediatr. Ann. 2016, 45, e414–e419. [Google Scholar] [CrossRef]
- Chen, H.L.; Wu, S.H.; Hsu, S.H.; Liou, B.Y.; Chen, H.; Chang, M.H. Jaundice revisited: Recent advances in the diagnosis and treatment of inherited cholestatic liver diseases. J. Biomed. Sci. 2018, 25, 75. [Google Scholar] [CrossRef]
- Tanimizu, N. The neonatal liver: Normal development and response to injury and disease. Semin. Fetal. Neonatal. Med. 2022, 27, 101229. [Google Scholar] [CrossRef]
- Potter, C.J. Cholestasis in the Premature Infant. Clin. Perinatol. 2020, 47, 341–354. [Google Scholar] [CrossRef]
- Santos Silva, E.; Almeida, A.; Frutuoso, S.; Martins, E.; Valente, M.J.; Santos-Silva, A.; Lopes, A.I. Neonatal Cholestasis Over Time: Changes in Epidemiology and Outcome in a Cohort of 154 Patients From a Portuguese Tertiary Center. Front. Pediatr. 2020, 30, 351. [Google Scholar] [CrossRef]
- Boyer, J.L.; Soroka, C.J. Bile formation and secretion: An update. J. Hepatol. 2021, 75, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.C.; Pisarello, M.J.; LaRusso, N.F. Pathobiology of biliary epithelia. BBA Mol. Basis Dis. 2018, 1864, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Shneider, B.L.; Moore, J.; Kerkar, N.; Magee, J.C.; Ye, W.; Karpen, S.J.; Kamath, B.M.; Molleston, J.P.; Bezerra, J.A.; Murray, K.F.; et al. Childhood Liver Disease Research Network. Initial assessment of the infant with neonatal cholestasis—Is this biliary atresia? PLoS ONE 2017, 12, e0176275. [Google Scholar] [CrossRef] [PubMed]
- El-Guindi, M.A.; Sira, M.M.; Sira, A.M.; Salem, T.A.H.; El-Abd, O.L.; Konsowa, H.A.S.; El-Azab, D.S.; Allam, A.A.H. Design and validation of a diagnostic score for biliary atresia. J. Hepatol. 2014, 61, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Lertudomphonwanit, C.; Mourya, R.; Fei, L.; Zhang, Y.; Gutta, S.; Yang, L.; Bove, K.E.; Shivakumar, P.; Bezerra, J.A. Large-scale proteomics identifies MMP-7 as a sentinel of epithelial injury and of biliary atresia. Sci. Transl. Med. 2017, 9, eaan8462. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rong, L.; Tang, J.; Niu, H.; Jin, Z.; Zhou, Y.; Cao, G.; Zhang, X.; Chi, S.; Tang, S. Re-evaluation of Laparoscopic Hepatic Subcapsular Spider-Like Telangiectasis Sign: A Highly Accurate Method to Diagnose Biliary Atresia in Infants. Front. Pediatr. 2022, 10, 850449. [Google Scholar] [CrossRef]
- Hoerning, A.; Raub, S.; Dechêne, A.; Brosch, M.N.; Kathemann, S.; Hoyer, P.F.; Gerner, P. Diversity of disorders causing neonatal cholestasis—The experience of a tertiary pediatric center in Germany. Front. Pediatr. 2014, 2, 65. [Google Scholar] [CrossRef]
- Yoon, H.M.; Suh, C.H.; Kim, J.R.; Lee, J.S.; Jung, A.Y.; Cho, Y.A. Diagnostic performance of sonographic features in patients with Biliary Atresia: A systematic review and meta-analysis. J. Ultrasound Med. 2017, 36, 2027–2038. [Google Scholar] [CrossRef]
- Karrer, M.; Roach, J.P. Error Traps and Culture of Safety in Biliary Atresia. Semin. Pediatric Surg. 2019, 18, 135–138. [Google Scholar] [CrossRef]
- Ho, A.; Sacks, M.A.; Sapra, A.; Khan, F.A. The Utility of gallbladder Absence on Ultrasound for Children with Biliary Atresia. Front. Pediatr. 2021, 9, 685268. [Google Scholar] [CrossRef] [PubMed]
- Di Serafino, M.; Gioioso, M.; Severino, R.; Esposito, F.; Vezzali, N.; Ferro, F.; Pelliccia, P.; Caprio, M.G.; Iorio, R.; Vallone, G. Ultrasound findings in paediatric cholestasis: How to image the patient and what to look for. J. Ultrasound 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Han, S.; Jeon, T.Y.; Hwang, S.M.; Yoo, S.Y.; Lee, S.K.; Kim, J.H. Imaging findings of Alagille syndrome in young infants: Differentiation from biliary atresia. Br. J. Radiol. 2017, 90, 20170406. [Google Scholar] [CrossRef]
- Cho, H.-H.; Kim, W.S.; Choi, Y.H.; Cheon, J.-E.; Lee, S.M.; Kim, I.-O.; Shin, S.-M.; Ko, J.S.; Moon, J.S. Ultrasonography evaluation of infants with Alagille syndrome: In comparison with biliary atresia and neonatal hepatitis. Eur. J. Radiol. 2016, 85, 1045–1052. [Google Scholar] [CrossRef]
- Takamizawa, S.; Zaima, A.; Muraji, T.; Kanegawa, K.; Akasaka, Y.; Satoh, S.; Nishijima, E. Can biliary atresia be diagnosed by ultrasonography alone? J. Pediatr. Surg. 2007, 42, 2093–2096. [Google Scholar] [CrossRef]
- Götze, T.; Blessing, H.; Grillhösl, C.; Gerner, P.; Hoerning, A. Neonatal Cholestasis—Differential Diagnoses, Current Diagnostic Procedures, and Treatment. Front. Pediatr. 2015, 3, 43. [Google Scholar] [CrossRef][Green Version]
- Alagille, D. Cholestasis in the first three months of life. Prog. Liver Dis. 1979, 6, 471–485. [Google Scholar]
- Agana, M.; Frueh, J.; Kamboj, M.; Patel, D.R.; Kanungo, S. Common metabolic disorder (inborn errors of metabolism) concerns in primary care practice. Ann. Transl. Med. 2018, 6, 469. [Google Scholar] [CrossRef]
- Robie, D.K.; Overfelt, S.R.; Xie, L. Differentiating biliary atresia from other causes of cholestatic jaundice. Am. Surg. 2014, 80, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Abreu, J.C.; Green, A. Gamma-Glutamyltransferase: Value of its measurement in paediatrics. Ann. Clin. Biochem. 2002, 39, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, M.C.; Gallucci, S.D.; Maezono, R.; Maksoud, J.G.; Gayotto, L.C. Liver biopsy in neonatal cholestasis: A review on statistical grounds. Mod. Pathol. 1997, 10, 793–799. [Google Scholar]
- Lee, J.Y.; Sullivan, K.; El Demellawy, D.; Nasr, A. The value of preoperative liver biopsy in the diagnosis of extrahepatic biliary atresia: A systematic review and meta-analysis. J. Pediatr. Surg. 2016, 51, 753–761. [Google Scholar] [CrossRef]
- Pierre, R.; Magee, J.C.; Anders, R.A.; Bove, K.E.; Chung, C.; Cummings, O.W.; Finegold, M.J.; Finn, L.S.; Kim, G.E.; Mark, A.; et al. Childhood Liver Disease Research Network (ChiLDReN). Key histopathologic features of liver biopsies that distinguish biliary atresia from other causes of infantile cholestasis and their correlation with outcome. Am. J. Surg. Pathol. 2016, 40, 1601–1615. [Google Scholar] [CrossRef]
- Grammatikopoulos, T.; Sambrotta, M.; Strautnieks, S.; Foskett, P.; Knisely, A.S.; Wagner, B.; Deheragoda, M.; Starling, C.; Mieli-Vergani, G.; Smith, J.; et al. Mutations in DCDC2 (doublecortin domain containing protein 2) in neonatal sclerosing cholangitis. J. Hepatol. 2016, 65, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.; Benstz, J.; Feist, D.; Nutzenadel, W.; Otto, H.F.; Hofmann, W.J. Bile duct to portal space ratio and ductal plate remnants in liver disease of infants aged less than 1 year. Pathology 2008, 40, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Talbot, I.C.; Mowat, A.P. Liver disease in infancy: Histological features and relationship to alpha-antitrypsin phenotype. J. Clin. Pathol. 1975, 28, 559–563. [Google Scholar] [CrossRef]
- Mysore, K.R.; Shneider, B.L.; Harpavat, S. Biliary atresia as a disease starting in utero: Implications for treatment, diagnosis, and pathogenesis. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Hope, W.W.; Fanelli, R.; Walsh, D.S.; Narula, V.K.; Price, R.; Stefanidis, D.; Richardson, W.S. SAGES clinical spotlight review: Intraoperative cholangiography. Surg. Endosc. 2017, 31, 2007–2016. [Google Scholar] [CrossRef]
- Kaneshiro, M.; Okamoto, T.; Sonoda, M.; Sonoda, M.; Ogawa, E.; Okajima, H.; Uemoto, S. Outcomes of liver transplantation for Alagille syndrome after Kasai portoenterostomy: Alagille Syndrome with agenesis of extrahepatic bile ducts at porta hepatis. J. Pediatr. Surg. 2019, 54, 2387–2391. [Google Scholar] [CrossRef]
- Lin, H.; Zoll, B.; Russo, P.; Spinner, N.B.; Spinner, N.B.; Loomes, K.M. Challenging case of focal extrahepatic duct obstruction/hypoplasia in Alagille syndrome. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e18–e22. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Sakamoto, S.; Uchida, H.; Shimizu, S.; Yanagi, Y.; Fukuda, A.; Shimizu, S.; Yanagi, Y.; Fukuda, A.; Yoshioka, T.; et al. The morphological and histopathological assessment of Alagille syndrome with extrahepatic bile duct obstruction: The importance of the differential diagnosis with subgroup “o” biliary atresia. Pediatr. Surg. Int. 2021, 37, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Emerick, K.M.; Rand, E.B.; Goldmuntz, E.; Krantz, I.D.; Spinner, N.B.; Piccoli, D.A. Features of Alagille syndrome in 92 patients: Frequency and relation to prognosis. Hepatology 1999, 29, 822–829. [Google Scholar] [CrossRef]
- Lee, H.P.; Kang, B.; Choi, S.Y.; Lee, S.; Lee, S.-K.; Ho, Y. Outcome of Alagille syndrome patients who had previously received kasai operation during infancy: A single center study. Pediatr. Gas-Troenterol. Hepatol. Nutr. 2015, 18, 175–179. [Google Scholar] [CrossRef][Green Version]
- Fujishiro, J.; Suzuki, K.; Watanabe, M.; Uotani, C.; Takezoe, T.; Takamoto, N.; Hayashi, K. Outcomes of Alagille syndrome following the Kasai operation: A systematic review and meta-analysis. Pediatr. Surg. Int. 2018, 34, 1073–1077. [Google Scholar] [CrossRef]
- Kishore, R.; Kisku, S.M.C.; Thomas, R.J.; Jeenipalli, S.K. Laparoscopic cholangiogram in biliary atresia: A refinement in the gallbladder hitch technique. Pediatr. Surg. Int. 2018, 34, 395–398. [Google Scholar] [CrossRef]
- Nose, S.; Hasegawa, T.; Soh, H.; Sasaki, T.; Kimura, T.; Fukuzawa, M. Laparoscopic cholecystocholangiography as an effective alternative exploratory laparotomy for the differentiation of biliary atresia. Surg. Today 2005, 35, 925–928. [Google Scholar] [CrossRef]
- Alkan, M.; Tutus, K.; Fakıoglu, E.; Ozden, O.; Hatipoglu, Z.; Iskit, S.H.; Tuncer, R.; Zorludemir, U. Laparoscopy-Assisted Percutaneous Cholangiography in Biliary Atresia Diagnosis: Comparison with Open Technique. Gastroenterol. Res. Pract. 2016, 2016, 5637072. [Google Scholar] [CrossRef]
- Narayanaswamy, B.; Gonde, C.; Tredger, J.M.; Hussain, M.; Vergani, D.; Davenport, M. Serial circulating markers of inflammation in biliary atresia—Evolution of the postoperative inflammatory process. Hepatology 2007, 46, 180–187. [Google Scholar] [CrossRef]
- Bessho, K.; Mourya, R.; Shivakumar, P.; Walters, S.; Magee, J.C.; Rao, M.; Jegga, A.G.; Bezerra, J.A. Gene expression signature for biliary atresia and a role for interleukin-8 in pathogenesis of experimental disease. Hepatology 2014, 60, 211–223. [Google Scholar] [CrossRef]
- He, L.; Ip, D.K.M.; Tam, G.; Lui, V.C.H.; Tam, P.K.H.; Chung, P.H.Y. Biomarkers for the diagnosis and post- Kasai portoenterostomy prognosis of biliary atresia: A systematic review and meta- analysis. Sci. Rep. 2021, 11, 11692. [Google Scholar] [CrossRef]
- Nomden, M.; Beljaars, L.; Verkade, H.J.; Hulscher, J.B.F.; Peter Olinga, P. Current concepts of biliary atresia and matrix metalloproteinase-7: A review of literature. Front. Med. 2020, 7, 617261. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Y.; Xu, P.P.; Mourya, R.; Lei, H.-Y.; Cao, G.-Q.; Xiong, X.-L.; Xu, H.; Duan, X.-F.; Wang, N.; et al. Diagnostic accuracy of serum matrix metal-loproteinase-7 for biliary atresia. Hepatology 2018, 68, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.L.; da Silveira, T.R.; da Silva, V.D.; Cerski, C.T.; Wagner, M.B. Medial thickening of hepatic artery branches in biliary atresia. A morphometric study. J. Pediatr. Surg. 2005, 40, 637–642. [Google Scholar] [CrossRef]
- Uflacker, R.; Pariente, D.M. Angiographic findings in biliary atresia. Cardiovasc. Interv. Radiol. 2004, 27, 486–490. [Google Scholar] [CrossRef]
- Caruso, S.; Miraglia, R.; Milazzo, M.; Maruzzelli, L.; Pasta, A.; Spada, M.; Riva, S.; Luca, A.; Gridelli, B. Multidetector computed tomography hepatic findings in children with end-stage biliary atresia. Eur. Radiol. 2010, 20, 1468–1475. [Google Scholar] [CrossRef]
- El-Guindi, M.A.; Sira, M.M.; Konsowa, H.A.S.; El-Abd, O.L.; Salem, T.A.-H. Value of hepatic subcapsular flow by color Doppler ultrasonography in the diagnosis of biliary atresia. J. Gastroenterol. Hepatol. 2013, 28, 867–872. [Google Scholar] [CrossRef]
- Humphrey, T.M.; Stringer, M.D. Biliary atresia: US diagnosis. Radiology 2007, 244, 845–851. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, M.J.; Lee, M.J.; Yoon, C.S.; Han, S.J.; Koh, H. Ultrasonographic findings of type IIIa biliary atresia. Ultrasonography 2014, 33, 267–274. [Google Scholar] [CrossRef]
- Mu, S.L.; Kim, M.J.; Lee, M.J.; Yoon, C.S.; Han, S.J.; Oh, J.-T.; Park, Y.N. Biliary Atresia: Color Doppler US findings in neonates and infants. Radiology 2009, 252, 282–289. [Google Scholar] [CrossRef]
- Ramesh, R.L.; Murthy, G.V.; Jadhav, V.R.S. Hepatic subcapsular flow: An early marker in diagnosing biliary atresia. Indian J. Radiol. Imaging 2015, 25, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.K.; Cheon, J.E.; Byung, J.Y.; Yoo, S.-Y.; Kim, W.Y.; Kim, I.-O.; Yeon, K.M.; Seo, J.K.; Park, K.-W. Hepatic arterial diameter measured with US: Adjunct for US diagnosis of biliary atresia. Radiology 2007, 245, 549–555. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, M.; Tang, S.T.; Yang, L.; Zhang, X.; Yang, D.-H.; Xiong, M.; Li, S.; Cao, G.-Q.; Wang, Y. Laparoscopic finding of a hepatic subcapsular spider-like telangiectasis sign in biliary atresia. World J. Gastroenterol. 2017, 23, 7119–7128. [Google Scholar] [CrossRef]
- Perlmutter, D.H. Alpha-1-antitrypsin deficiency: Diagnosis and treatment. Clin. Liver Dis. 2004, 8, 839–859. [Google Scholar] [CrossRef]
- Wang, A.W.; Newton, K.; Kling, K. Successful outcome and biliary drainage in an infant with concurrent alpha-1-antitrypsin deficiency and biliary atresia. Case Rep. Surg. 2017, 2017, 9348461. [Google Scholar] [CrossRef]
- Nord, K.S.; Saad, S.; Joshi, V.V.; McLoughlin, L.C. Concurrence of alpha 1-antitrypsin deficiency and biliary atresia. J. Pediatr. 1987, 111, 416–418. [Google Scholar] [CrossRef]
- Sangkhathat, S.; Laochareonsuk, W.; Maneechay, W.; Kayasut, K.; Chiengkriwate, P. Variants associated with infantile cholestatic syndromes detected in extrahepatic biliary atresia by Whole Exome studies: A 20-case series from Thailand. J. Pediatr. Genet. 2018, 7, 67–73. [Google Scholar] [CrossRef]
- Santos, J.L.; Pinto Pais, I.; Ramos, D. Neonatal Cholestasis: Implementation of a National Program of Genetic Screening in Portugal; International Symposium on Gastroenterology: Festschrift Honoring Professor Ernest Seidman—13–14 April 2018; McGill University Health Centre (MUHC): Montreal, QC, Canada, 2018. [Google Scholar]
- Feldman, A.G.; Sokol, R.J. Recent developments in diagnostics and treatment of neonatal cholestasis. Semin. Pediatr. Surg. 2020, 29, 150945. [Google Scholar] [CrossRef]
- Nicastro, E.; D’Antiga, L. Next generation sequencing in pediatric hepatology and liver transplantation. Liver Transpl. 2018, 24, 282–293. [Google Scholar] [CrossRef]
- Nicastro, E.; Di Giorgio, A.; Marchetti, D.; Barboni, C.; Cereda, A.; Iascone, M.; D’Antiga, L. Diagnostic Yield of an Algorithm for Neonatal and Infantile Cholestasis Integrating Next-Generation Sequencing. J. Pediatr. 2019, 211, 54–62.e4. [Google Scholar] [CrossRef]
- Clark, M.M.; Stark, Z.; Farnaes, L.; Tan, T.Y.; White, S.M.; Dimmock, D.; Kingsmore, S.F. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. NPJ Genom. Med. 2018, 3, 16. [Google Scholar] [CrossRef]
- Sun, H.; Yu, G. New insights into the pathogenicity of non-synonymous variants through multi-level analysis. Sci. Rep. 2019, 9, 1667. [Google Scholar] [CrossRef]
- Henkel, A.S. Genetic Disorders of Bile Acid Transport. Clin. Liver Dis. 2021, 18, 237–242. [Google Scholar] [CrossRef]
- Jeyaraj, R.; Bounford, K.M.; Ruth, N.; Lloyd, C.; MacDonald, F.; Hendriksz, C.J.; Baumann, U.; Gissen, P.; Kelly, D. The Genetics of Inherited Cholestatic Disorders in Neonates and Infants: Evolving Challenges. Genes 2021, 12, 1837. [Google Scholar] [CrossRef]
- Taylor, S.A.; Whitington, P.F. Neonatal acute liver failure. Liver Transpl. 2016, 22, 677–685. [Google Scholar] [CrossRef]
- Hoffmann, G.F.; Engelmann, G. Liver Disease. In Inherited Metabolic Diseases—A Clinical Approach; Hoffmann, G.F., Zschocke, J., Nyhan, W.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Lak, R.; Yazdizadeh, B.; Davari, M.; Nouhi, M.; Kelishadi, R. Newborn screening for galactosaemia. Cochrane Database Syst. Rev. 2017, 12, CD012272. [Google Scholar] [CrossRef]
- Berry, G.T. Galactosemia: When is it a newborn screening emergency? Mol. Genet. Metab. 2012, 106, 7–11. [Google Scholar] [CrossRef]
- Demirbas, D.; Coelho, A.I.; Rubio-Gozalbo, M.E.; Berry, G.T. Hereditary Galactosemia. Metabolism 2018, 83, 188–196. [Google Scholar] [CrossRef]
- De Jesús, V.R.; Adam, B.W.; Mandel, D.; Cuthbert, C.D.; Matern, D. Succinylacetone as primary marker to detect tyrosinemia type I in newborns and its measurement by newborn screening programs. Mol. Genet. Metab. 2014, 113, 67–75. [Google Scholar] [CrossRef]
- Chinsky, J.M.; Singh, R.; Ficicioglu, C.; Karnebeek, C.D.M.V.; Grompe, M.; Mitchell, G.; Waisbren, S.E.; Gucsavas-Calikoglu, M.; Wasserstein, M.P.; Coakley, K.; et al. Diagnosis and treatment of Tyrosinemia type I: A US and Canadian consensus group review and recommendations. Genet. Med. 2017, 19, 1380. [Google Scholar] [CrossRef]
- Äärelä, L.; Hiltunen, P.; Soini, T.; Vuorela, N.; Huhtala, H.; Nevalainen, P.I.; Heikinheimo, M.; Kivelä, L.; Kurppa, K. Type 1 tyrosinemia in Finland: A nationwide study. Orphanet. J. Rare Dis. 2020, 15, 281. [Google Scholar] [CrossRef]
- Dehghani, S.M.; Haghighat, M.; Imanieh, M.H.; Karamnejad, H.; Malekpour, A. Clinical and para-clinical findings in the children with Tyrosinemia referring for liver transplantation. Int. J. Prev. Med. 2013, 4, 1380–1385. [Google Scholar]
- Kliegman, R.M. Defects in Metabolism of Amino Acids. In Nelson Textbook of Pediatrics, 21st ed.; Kliegman, R.M., St Geme, J.W., Blum, N.J., Eds.; Chapter 103; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 695–739. ISBN 978-0-323-52950-1. [Google Scholar]
- Pronicka, E.; Adamowicz, M.; Kowalik, A.; Płoski, R.; Radomyska, B.; Rogaszewska, M.; Rokicki, D.; Sykut-Cegielska, J. Elevated carbohydrate-deficient transferrin (CDT) and its normalization on dietary treatment as a useful biochemical test for hereditary fructose intolerance and galactosemia. Pediatr. Res. 2007, 62, 101–105. [Google Scholar] [CrossRef]
- Gaughan, S.; Ayres, L.; Baker, P.R.I.I. Hereditary Fructose Intolerance. In GeneReviews® [Internet]; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Eds.; University of Washington: Seattle, WA, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/ (accessed on 28 June 2022).
- Whitington, P.F.; Hibbard, J.U. High-dose immunoglobulin during pregnancy for recurrent neonatal haemochromatosis. Lancet. 2004, 364, 1690–1698. [Google Scholar] [CrossRef]
- Deugnier, Y.; Turlin, B. Pathology of hepatic iron overload. World J. Gastroenterol. 2007, 13, 4755–4760. [Google Scholar] [CrossRef] [PubMed]
- Rand, E.B.; Karpen, S.J.; Kelly, S.; Mack, C.L.; Malatack, J.J.; Sokol, R.J.; Whitington, P.F. Treatment of neonatal hemochromatosis with exchange transfusion and intravenous immunoglobulin. J. Pediatr. 2009, 155, 566–571. [Google Scholar] [CrossRef]
- Smith, S.R.; Shneider, B.L.; Magid, M.; Martin, G.; Rothschild, M. Minor salivary gland biopsy in neonatal hemochromatosis. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 760–763. [Google Scholar] [CrossRef]
- Feldman, A.G.; Whitington, P.F. Neonatal hemochromatosis. J. Clin. Exp. Hepatol. 2013, 3, 313–320. [Google Scholar] [CrossRef]
- Whitington, P.F.; Kelly, S. Outcome of pregnancies at risk for neonatal hemochromatosis is improved by treatment with high-dose intravenous immunoglobulin. Pediatrics 2008, 121, e1615–e1621. [Google Scholar] [CrossRef]
- Bilreiro, C.; Noruegas, M.J.; Gonçalves, I.; Moreira, Â. Ultrasound-guided Liver Biopsies in Children: A Single-center Experience and Risk Factors for Minor Bleeding. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 137–140. [Google Scholar] [CrossRef]
- Short, S.S.; Papillon, S.; Hunter, C.J.; Stanley, P.; Kerkar, N.; Wang, L.; Azen, C.; Wang, K. Percutaneous liver biopsy: Pathologic diagnosis and complications in children. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 644–648. [Google Scholar] [CrossRef]
- Fiorotto, R.; Strazzabosco, M. Pathophysiology of Cystic Fibrosis Liver Disease: A Channelopathy Leading to Alterations in Innate Immunity and in Microbiota. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 197–207. [Google Scholar] [CrossRef]
- Servidoni, M.F.; Gomez, C.C.S.; Marson, F.A.L.; Toro, A.; Ribeiro, M.; Ribeiro, J.; Ribeiro, A. Grupo Colaborativo de Estudos em Fibrose Cística. Sweat test and cystic fibrosis: Overview of test performance at public and private centers in the state of São Paulo, Brazil. J. Bras. Pneumol. 2017, 43, 121–128. [Google Scholar] [CrossRef]
- Bosma, P.J.; Wits, M.; Oude-Elferink, R.P.J. Gene Therapy for Progressive Familial Intrahepatic Cholestasis: Current Progress and Future Prospects. Int. J. Mol. Sci. 2021, 22, 273. [Google Scholar] [CrossRef] [PubMed]
- Gopan, A.; Sarma, M.S. Mitochondrial hepatopathy: Respiratory chain disorders—‘breathing in and out of the liver’. World J. Hepatol. 2021, 13, 1707–1726. [Google Scholar] [CrossRef]
- Aldrian, D.; Vogel, G.F.; Frey, T.K.; Civan, H.; Aksu, A.; Avitzur, Y.; Boluda, E.; Çakır, M.; Demir, A.; Deppisch, C.; et al. Congenital Diarrhea and Cholestatic Liver Disease: Phenotypic Spectrum Associated with MYO5B Mutations. J. Clin. Med. 2021, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, C.; Ermarth, A.K.; Putnam, A.R.; Deneau, M. Neonatal cholestasis and hepatosplenomegaly caused by congenital dyserythropoietic anemia type 1: A case report. World J. Hepatol. 2019, 11, 477–482. [Google Scholar] [CrossRef]
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef]
- Michelakakis, H.; Moraitou, M.; Mavridou, I.; Dimitriou, E. Plasma lysosomal enzyme activities in congenital disorders of glycosylation, galactosemia and fructosemia. Clin. Chim. Acta 2009, 401, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Nagatomo, T.; Sugiyama, Y.; Tsuruoka, T.; Osone, Y.; Shimura, M.; Tajika, M.; Matsuhashi, T.; Ichimoto, K.; Matsunaga, A.; et al. Neonatal-onset mitochondrial disease: Clinical features, molecular diagnosis and prognosis. Arch. Dis. Child. Fetal. Neonatal. Ed. 2022, 107, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Reichert, M.C.; Hall, R.A.; Krawczyk, M.; Lammert, F. Genetic determinants of cholangiopathies: Molecular and systems genetics. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt B, 1484–1490. [Google Scholar] [CrossRef]
- Bull, L.N.; Thompson, R.J. Progressive Familial Intrahepatic Cholestasis. Clin. Liver Dis. 2018, 22, 657–669. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Kamath, B.M.; Loomes, K.M.; Karpen, S.J. Cholestatic liver diseases of genetic etiology: Advances and controversies. Hepatology 2022, 75, 1627–1646. [Google Scholar] [CrossRef] [PubMed]
- Li, M.K.; Crawford, J.M. The pathology of cholestasis. Semin Liver Dis. 2004, 24, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Clouston, A.D. Pathologic Features of Hereditary Cholestatic Diseases. Surg Pathol Clin. 2018, 11, 313–327. [Google Scholar] [CrossRef]
- Govender, P.; Jonas, M.M.; Alomari, A.I.; Padua, H.M.; Dillon, B.J.; Landrigan-Ossar, M.F.; Chaudry, G. Sonography-guided percutaneous liver biopsies in children. AJR Am. J. Roentgenol. 2013, 201, 645–650. [Google Scholar] [CrossRef]
- Ovchinsky, N.; Moreira, R.K.; Lefkowitch, J.H.; Lavine, J.E. Liver biopsy in modern clinical practice: A pediatric point-of-view. Adv. Anat. Pathol. 2012, 19, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Kieling, C.O.; Meurer, L.; Vieira, S.; Ferreira, C.T.; Lorentz, A.; Silveira, T.R. The extent of biliary proliferation in liver biopsies from patients with biliary atresia at portoenterostomy is associated with the postoperative prognosis. J. Pediatr. Surg. 2009, 44, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, E.; El-Hennawy, M.; Ehrenkranz, R.A.; Zelterman, D.; Reyes-Múgica, M. Total Parenteral Nutrition induced Liver Pathology: An Autopsy Series of 24 Newborn Cases. Pediatr. Dev. Pathol. 2004, 7, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, F.W.; Regano, N.; Mazzuoli, S.; Fregnan, S.; Leogrande, G.; Guglielmi, A.; Merli, M.; Pironi, L.; Penco, J.M.P.; Francavilla, A. Cholestasis Induced by Total Parenteral Nutrition. Clin. Liver Dis. 2008, 12, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.A. Preventing parenteral nutrition liver disease. Early Hum. Dev. 2010, 86, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; Jaeschke, H. Inflammation and Cell Death During Cholestasis: The Evolving Role of Bile Acids. Gene Expr. 2019, 19, 215–228. [Google Scholar] [CrossRef]
- Jevon, G.P.; Dimmick, J.E. Histopathologic approach to metabolic liver disease: Part 1. Pediatr. Dev. Pathol. 1998, 1, 179–199. [Google Scholar] [CrossRef]
- Zheng, Q.-Q.; Zhang, Z.-H.; Zeng, H.-S.; Lin, W.-X.; Yang, H.-W.; Yin, Z.-N.; Song, Y.-Z. Identification of a Large SLC25A13 Deletion via Sophisticated Molecular Analyses Using Peripheral Blood Lymphocytes in an Infant with Neonatal Intrahepatic Cholestasis Caused by Citrin Deficiency (NICCD): A Clinical and Molecular Study. Biomed. Res. Int. 2016, 2016, 4124263. [Google Scholar] [CrossRef]
- Ilhan, O.; Ozer, E.A.; Ozdemir, S.A.; Akbay, S.; Memur, S.; Kanar, B. 5, Mustafa M Tatli 6. Arthrogryposis-renal tubular dysfunction-cholestasis syndrome: A cause of neonatal cholestasis. Case report. Arch. Argent. Pediatr. 2016, 114, e9–e12. [Google Scholar] [CrossRef] [PubMed]
- Alsaleem, M.; Saadeh, L.; Misra, A.; Madani, S. Neonatal isolated ACTH deficiency (IAD): A potentially life-threatening but treatable cause of neonatal cholestasis. BMJ Case Rep. 2016, 2016, bcr2016215032. [Google Scholar] [CrossRef]
- Alissa, F.T.; Jaffe, R.; Shneider, B.L. Update on Progressive Familial Intrahepatic Cholestasis. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 241–252. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Heubi, J.E. Defects in Bile Acid Biosynthesis-Diagnosis and Treatment. J. Pediatr. Gastroenterol. Nutr. 2006, 43 (Suppl. S1), S17–S22. [Google Scholar] [CrossRef]
- Subramaniam, P.; Clayton, P.T.; Portmann, B.C.; Mieli-Vergani, G.; Hadzić, N. Variable Clinical Spectrum of the Most Common Inborn Error of Bile Acid Metabolism—3β-hydroxy-Δ5-C27-steroid Dehydrogenase Deficiency. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 61–66. [Google Scholar] [CrossRef]
- Gumus, E.; Haliloglu, G.; Karhan, A.N.; Demir, H.; Gurakan, F.; Topcu, M.; Yuce, A. Niemann-Pick disease type C in the newborn period: A single-center experience. Eur. J. Pediatr. 2017, 176, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Klouwer, F.C.C.; Berendse, K.; Ferdinandusse, S.; Wanders, R.; Engelen, M.; Poll, B.T. Zellweger spectrum disorders: Clinical overview and management approach. Orphanet. J. Rare Dis. 2015, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Arvind, A.; Osganian, S.A.; Cohen, D.E.; Corey, K.E. Lipid and Lipoprotein Metabolism in Liver Disease. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2019. [Google Scholar]
- Repa, A.; Lochmann, R.; Unterasinger, L.; Weber, M.; Berger, A.; Haiden, N. Aggressive nutrition in extremely low birth weight infants: Impact on parenteral nutrition associated cholestasis and growth. PeerJ. 2016, 4, e2483. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.; Karpen, S. Intestinal Failure-Associated Liver Disease: Management and Treatment Strategies Past, Present, and Future. Semin. Liver Dis. 2007, 27, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.M. Classical galactosaemia revisited. J. Inherit. Metab. Dis. 2006, 29, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Aldámiz-Echevarría, L.; de Las Heras, J.; Couce, M.L.; Alcalde, C.; Vitoria, I.; Bueno, M.; Blasco-Alonso, J.; García, M.C.; Ruiz, M.; Suárez, R.; et al. Non-alcoholic fatty liver in hereditary fructose intolerance. Clin. Nutr. 2020, 39, 455–459. [Google Scholar] [CrossRef]
- Russo, P.A.; Mitchell, G.A.; Tanguay, R.M. Tyrosinemia: A review. Pediatr. Dev. Pathol. 2001, 4, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.C. Congenital cholestatic syndromes: What happens when children grow up? Can. J. Gastroenterol. 2007, 21, 743–751. [Google Scholar] [CrossRef]
- Libbrecht, L.; Spinner, N.B.; Moore, E.C.; Cassiman, D.; Damme-Lombaerts, R.V.; Roskams, T. Peripheral bile duct paucity and cholestasis in the liver of a patient with Alagille syndrome: Further evidence supporting a lack of postnatal bile duct branching and elongation. Am. J. Surg. Pathol. 2005, 29, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, P.; Knisely, A.; Portmann, B.; Qureshi, S.A.; Aclimandos, W.A.; Karani, J.B.; Baker, A.J. Diagnosis of Alagille Syndrome—25 Years of Experience at King’s College Hospital. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 84–89. [Google Scholar] [CrossRef]
- Roberts, E.A. Neonatal hepatitis syndrome. Semin. Neonatol. 2003, 8, 357–374. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef]





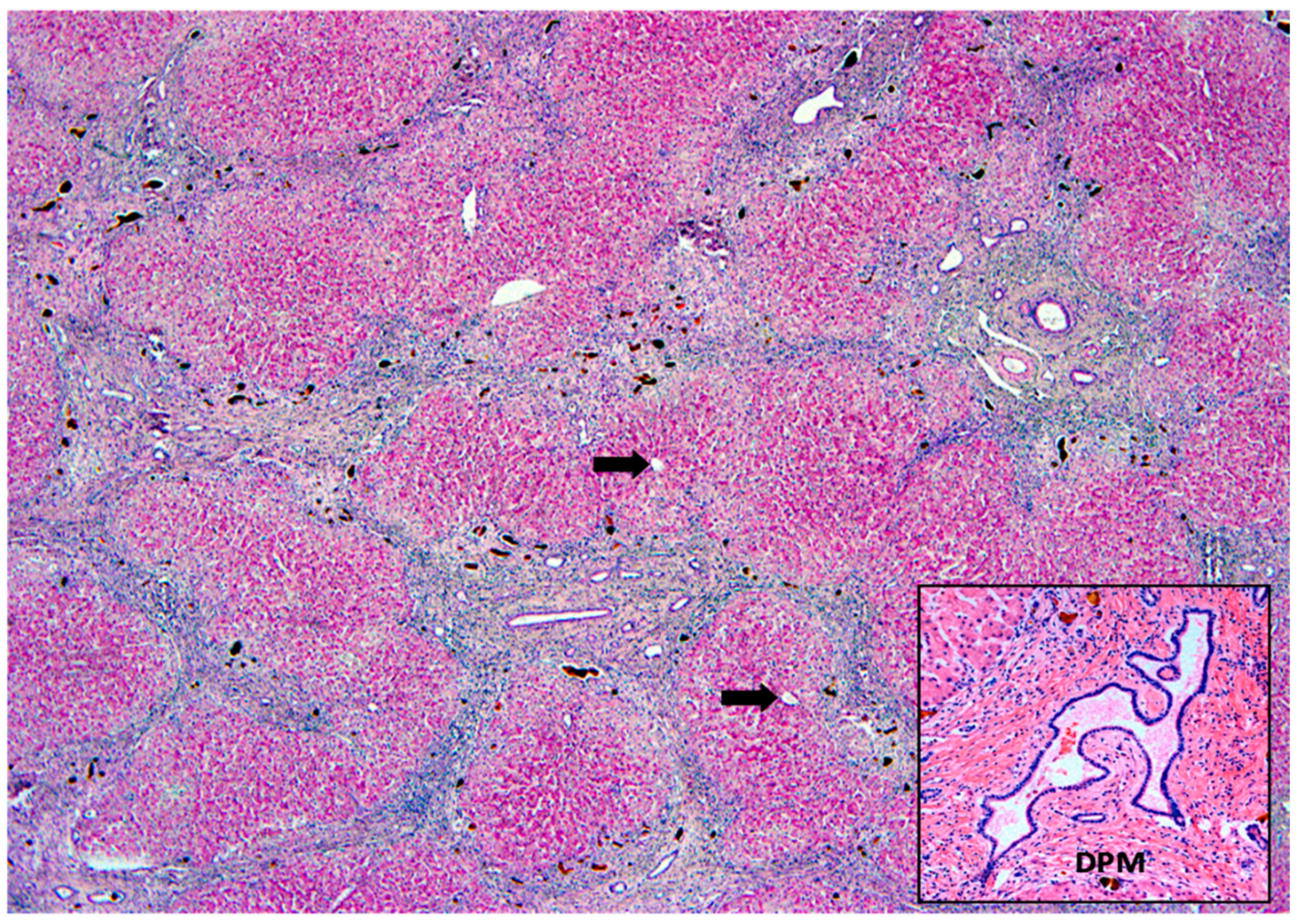
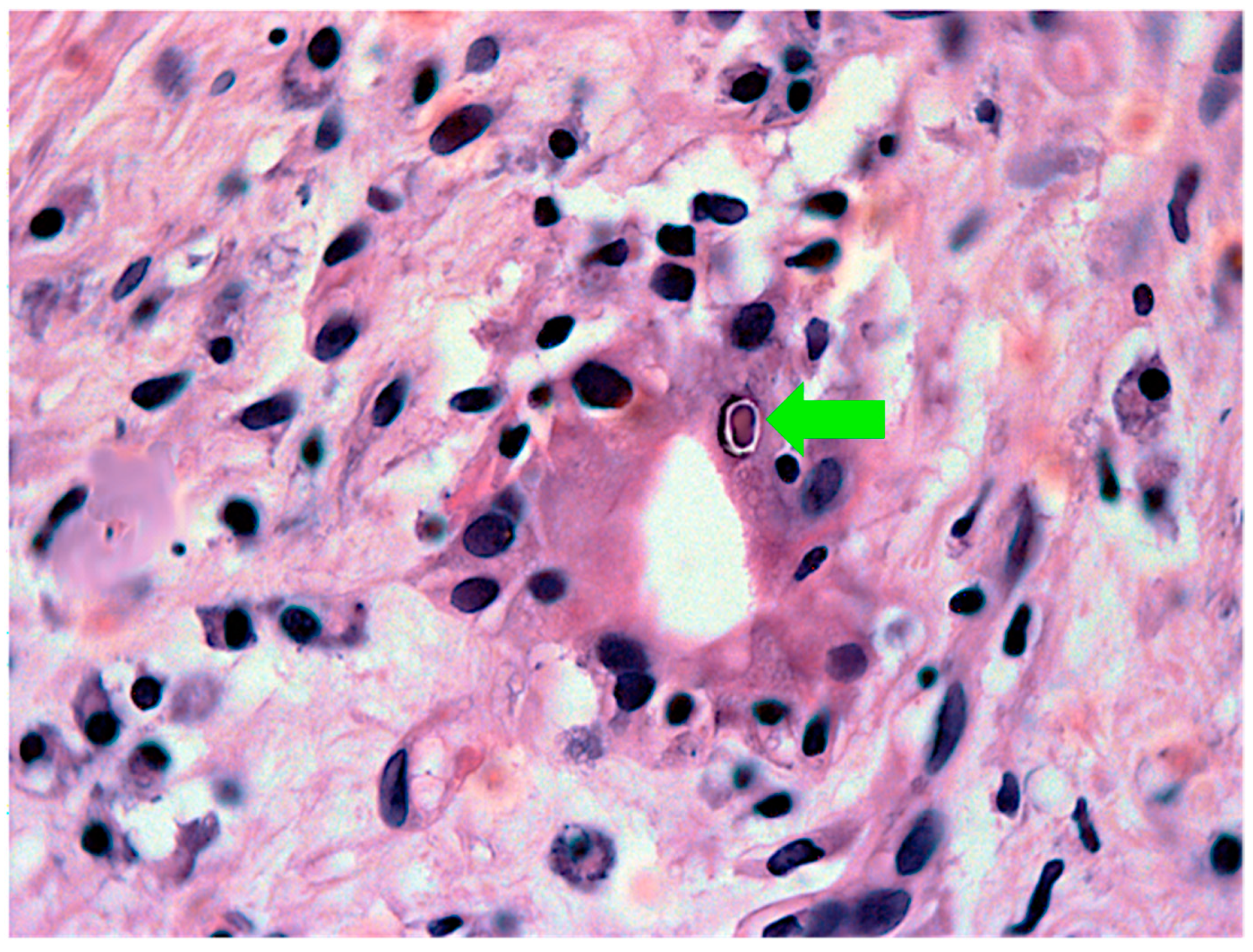
| Intrahepatic Cholestasis |
|---|
| 1. Idiopathic Neonatal Hepatitis |
| 2. Disorders in Embryogenesis of Biliary Structures Alagille syndrome (JAG1; NOTCH2); Cholestasis lymphedema syndrome (Aagenaes syndrome). Ciliopathies:
|
| 3.Disorders in Primary Bile Acids Synthesis and Conjugation Synthesis: 3β-Hydroxy-Δ5-C27-steroid oxidoreductase deficiency (HSD3B7); Oxosteroid 5β-reductase deficiency (AKR1D1); Sterol 27-hydroxylase deficiency – Cerebrotendinous xanthomatosis (CYP27A1); Oxysterol 7α-hydroxylase (CYP7B1) deficiency; 2-Methylacyl-CoA racemase deficiency (AMACR). Conjugation: Bile acid-CoA ligase deficiency—Familial hypercholanemia (BAAT and TJP2). |
| 4. Transport and Secretion of Cholephilic Compounds Nuclear receptors regulation: PFIC5 (functional defect of FXR, gene NR1H4). Bile salts intracellular traffic: PFIC6 (myosin VB protein, gene MYO5B); Arthrogryposis, Renal Dysfunction, and Cholestasis (apical polarity maintenance, genes VPS33B, VIPAS39). Canalicular membrane secretion: bile salts—BSEP protein (PFIC2, BRIC2, gene ABCB11); phospholipids—MDR3 protein (gene ABCB4); cholesterol—sitosterolemia (ABCG5, ABCG8). |
| 5. Hepatocellular Junctional Complexes PFIC4 (TJP2); NISCH syndrome (CLDN1). |
| 6. Complex or Multi Organic Disorders Phosphatidylserine translocation disorder: PFIC1, BRIC1 (ATP8B1); Arthrogryposis, Renal Dysfunction, and Cholestasis (VPS33B, VIPAS39); Cerebrotendinous xanthomatosis (CYP27A1); Congenital Defects of Glycosylation (CDG) (ALG3, ALG8, GLS1, PMM2, MPI, COG1, COG7, ATP6AP1). Peroxisomal disorders—Zellweger spectrum disorder/neonatal adrenoleukodystrophy/neonatal Refsum disease/Heimler syndrome (PEX gene family). Neonatal intrahepatic cholestasis caused by CITRIN deficiency (NICCD)—Citrullinemia type II (SLC25A13). Mitochondrial Respiratory chain disorder—Mitochondrial depletion syndrome (DGK, POLG, MPV17, DGUOK); Respiratory chain complex III deficiency—GRACILE syndrome (BSC1L); Long-chain 3-hydroxy acyl-CoA dehydrogenase (LCHADD) deficiency (HADHA). |
| 7. Metabolic Liver Diseases Involving biliary system—Alpha 1-antitrypsin disease (SERPINA 1); Cystic fibrosis (CFTR, ∆F508 variant). Not involving biliary system—Glycogen storage disease type IV (GBE1). Metabolic Intoxication—Galactosemia (GALT); Hereditary Fructose Intolerance (ALDOB); Tyrosinemia 1 (FAH). Endocrine Disease—Hypothyroidism; Hypopituitarism. Lipid metabolism disorder (storage)—Wolman syndrome (LIPA); Niemann-Pick disease (NPC 1 and 2); Gaucher disease type II (GBA); Farber disease type IV (ASAH1). |
| 8. Environmental: Congenital Infections Bacterial—Syphilis; bacterial sepsis; urinary tract infection; Tuberculosis; Listeriosis. Viral—Cytomegalovirus; Rubella; Herpes Simplex; Hepatitis (A, B, C); HIV; Parvovirus B19; Varicella zoster; Paramyxovirus; Enteric viral sepsis; Echovirus; Coxsackievirus; Adenovirus. Parasitic—Toxoplasmosis. |
| 9. Immune Disorders Neonatal Lupus erythematosus; Neonatal Hepatitis with Autoimmune hemolytic anemia; Gestational alloimmune liver disease (GALD). |
| 10. Others Transient cholestasis. Parenteral nutrition-associated liver disease (PNALD). Liver cirrhosis; Histiocytosis X; Fibrosing Hepatitis with Transient Leukemia (Trisomy 21); Shock and Hypoperfusion; Neonatal asphyxia; Intestinal obstruction; Drug induced-liver injury (DILI). |
| Extrahepatic Obstructive Cholestasis Biliary atresia. Choledochal cyst; Spontaneous perforation of the common bile duct; Biliary sludge/mucous plug; Cholelithiasis. |
| Source of genetic information: https://panelapp.genomicsengland.co.uk/ Additional References—[21,22] |
(1) Suggestive findings of BA
Blood count, platelet count, reticulocytes, Coombs test. Coagulation: PT/INR, aPTT, fibrinogen, antithrombin III. Blood electrolytes, calcium, phosphate, magnesium. Lactate, pyruvate, ketones, urea, uric acid, creatinine, ferritin, iron level, transferrin saturation, creatine kinase, lipase, alpha-fetoprotein. Pulse oximetry, blood gases. Protein electrophoresis.
Reducing substances in urine (baby under normal diet).
|
| References: [7,24,25,26,27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quelhas, P.; Jacinto, J.; Cerski, C.; Oliveira, R.; Oliveira, J.; Carvalho, E.; dos Santos, J. Protocols of Investigation of Neonatal Cholestasis—A Critical Appraisal. Healthcare 2022, 10, 2012. https://doi.org/10.3390/healthcare10102012
Quelhas P, Jacinto J, Cerski C, Oliveira R, Oliveira J, Carvalho E, dos Santos J. Protocols of Investigation of Neonatal Cholestasis—A Critical Appraisal. Healthcare. 2022; 10(10):2012. https://doi.org/10.3390/healthcare10102012
Chicago/Turabian StyleQuelhas, Patricia, Joana Jacinto, Carlos Cerski, Rui Oliveira, Jorge Oliveira, Elisa Carvalho, and Jorge dos Santos. 2022. "Protocols of Investigation of Neonatal Cholestasis—A Critical Appraisal" Healthcare 10, no. 10: 2012. https://doi.org/10.3390/healthcare10102012
APA StyleQuelhas, P., Jacinto, J., Cerski, C., Oliveira, R., Oliveira, J., Carvalho, E., & dos Santos, J. (2022). Protocols of Investigation of Neonatal Cholestasis—A Critical Appraisal. Healthcare, 10(10), 2012. https://doi.org/10.3390/healthcare10102012









