Effectiveness of Dry Needling of Myofascial Trigger Points in the Triceps Surae Muscles: Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
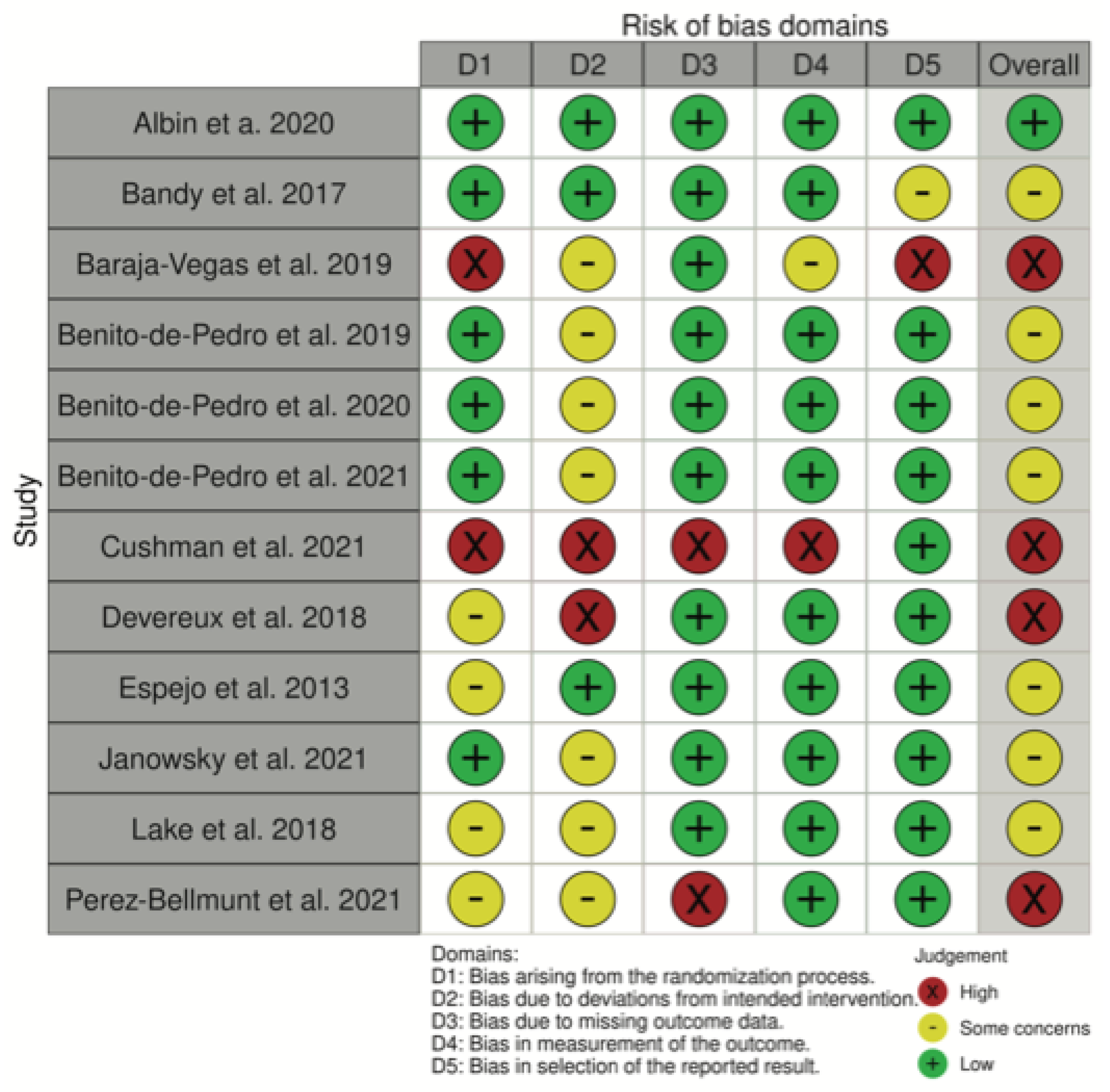
2.3. Methodological Quality and Risk of Bias Assessment
2.4. Data Extraction
3. Results
3.1. Study Selection
3.2. Methodological Quality and Risk of Bias Assessment
3.3. Population Characteristics
3.4. Intervention Characteristics
3.5. Outcome Measures
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Weller, J.L.; Comeau, D.; Otis, J.A.D. Myofascial Pain. Semin. Neurol. 2018, 38, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Barbero, M.; Schneebeli, A.; Koetsier, E.; Maino, P. Myofascial Pain Syndrome and Trigger Points: Evaluation and Treatment in Patients with Musculoskeletal Pain. Curr. Opin. Support. Palliat. Care 2019, 13, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Celik, D.; Mutlu, E.K. Clinical Implication of Latent Myofascial Trigger Point Topical Collection on Myofascial Pain. Curr. Pain Headache Rep. 2013, 17, 353. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P.; Thaker, N.; Heimur, J.; Aredo, J.v.; Sikdar, S.; Gerber, L. Myofascial Trigger Points Then and Now: A Historical and Scientific Perspective. PM R 2015, 7, 746–761. [Google Scholar] [CrossRef]
- Li, L.; Stoop, R.; Clijsen, R.; Hohenauer, E.; Fernández-De-Las-Peñas, C.; Huang, Q.; Barbero, M. Criteria Used for the Diagnosis of Myofascial Trigger Points in Clinical Trials on Physical Therapy: Updated Systematic Review. Clin. J. Pain 2020, 36, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, C.; Larsen, A.H.; Hartvigsen, J. A Systematic, Critical Review of Manual Palpation for Identifying Myofascial Trigger Points: Evidence and Clinical Significance. Arch. Phys. Med. Rehabil. 2008, 89, 1169–1176. [Google Scholar] [CrossRef]
- Chen, Q.; Basford, J.; An, K.N. Ability of Magnetic Resonance Elastography to Assess Taut Bands. Clin. Biomech. 2008, 23, 623–629. [Google Scholar] [CrossRef]
- Kuan, T.S.; Hsieh, Y.L.; Chen, S.M.; Chen, J.T.; Yen, W.C.; Hong, C.Z. The Myofascial Trigger Point Region: Correlation between the Degree of Irritability and the Prevalence of Endplate Noise. Am. J. Phys. Med. Rehabil. 2007, 86, 183–189. [Google Scholar] [CrossRef]
- Ge, H.-Y.; Arendt-Nielsen, L.; Madeleine, P. Accelerated Muscle Fatigability of Latent Myofascial Trigger Points in Humans. Pain Med. 2012, 13, 957–964. [Google Scholar] [CrossRef]
- Ibarra, J.M.; Ge, H.-Y.; Wang, C.; Martínez Vizcaíno, V.; Graven-Nielsen, T.; Arendt-Nielsen, L. Latent Myofascial Trigger Points Are Associated With an Increased Antagonistic Muscle Activity During Agonist Muscle Contraction. J. Pain 2011, 12, 1282–1288. [Google Scholar] [CrossRef]
- Lucas, K.R.; Polus, B.I.; Rich, P.A. Latent Myofascial Trigger Points: Their Effects on Muscle Activation and Movement Efficiency. J. Bodyw. Mov. Ther. 2004, 8, 160–166. [Google Scholar] [CrossRef]
- Ishøi, L.; Krommes, K.; Husted, R.S.; Juhl, C.B.; Thorborg, K. Diagnosis, Prevention and Treatment of Common Lower Extremity Muscle Injuries in Sport–Grading the Evidence: A Statement Paper Commissioned by the Danish Society of Sports Physical Therapy (DSSF). Br. J. Sports Med. 2020, 54, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Fernández Sanchis, D.; Cuenca Zaldívar, J.N.; Calvo, S.; Herrero, P.; Gómez Barrera, M. Cost-Effectiveness of Upper Extremity Dry Needling in the Rehabilitation of Patients with Stroke. Acupunct. Med. J. Br. Med. Acupunct. Soc. 2021, 40, 160–168. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Nijs, J. Trigger Point Dry Needling for the Treatment of Myofascial Pain Syndrome: Current Perspectives within a Pain Neuroscience Paradigm. J. Pain Res. 2019, 12, 1899–1911. [Google Scholar] [CrossRef]
- Dunning, J.; Butts, R.; Mourad, F.; Young, I.; Flannagan, S.; Perreault, T. Dry Needling: A Literature Review with Implications for Clinical Practice Guidelines. Phys. Ther. Rev. 2014, 19, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Lew, J.; Kim, J.; Nair, P. Comparison of Dry Needling and Trigger Point Manual Therapy in Patients with Neck and Upper Back Myofascial Pain Syndrome: A Systematic Review and Meta-Analysis. J. Man. Manip. Ther. 2021, 29, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Chaffee, K.; Alsarraj, J. Trigger Point Injections and Dry Needling Can Be Effective in Treating Long COVID Syndrome-Related Myalgia: A Case Report. J. Med. Case Rep. 2022, 16, 1–4. [Google Scholar] [CrossRef]
- Perreault, T.; Dunning, J.; Butts, R. The Local Twitch Response during Trigger Point Dry Needling: Is It Necessary for Successful Outcomes? J. Bodyw. Mov. Ther. 2017, 21, 940–947. [Google Scholar] [CrossRef]
- Kietrys, D.M.; Palombaro, K.M.; Azzaretto, E.; Hubler, R.; Schaller, B.; Schlussel, J.M.; Tucker, M. Effectiveness of Dry Needling for Upper-Quarter Myofascial Pain: A Systematic Review and Meta-Analysis. J. Orthop. Sport Phys. Ther. 2013, 43, 620–634. [Google Scholar] [CrossRef]
- Gattie, E.; Cleland, J.A.; Snodgrass, S. The Effectiveness of Trigger Point Dry Needling for Musculoskeletal Conditions by Physical Therapists: A Systematic Review and Meta-Analysis. J. Orthop. Sport Phys Ther. 2017, 47, 133–149. [Google Scholar] [CrossRef]
- Espejo-Antúnez, L.; Tejeda, J.F.-H.; Albornoz-Cabello, M.; Rodríguez-Mansilla, J.; de la Cruz-Torres, B.; Ribeiro, F.; Silva, A.G. Dry Needling in the Management of Myofascial Trigger Points: A Systematic Review of Randomized Controlled Trials. Complement. Ther. Med. 2017, 33, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ahmad, A.; Sadiq, S.; Asim, H.M. Effects of Dry Needling in Lower Extremity Myofascial Trigger Points: Systematic Review. J. Pak. Med. Assoc. 2021, 71, 2596–2603. [Google Scholar] [CrossRef] [PubMed]
- Llurda-Almuzara, L.; Labata-Lezaun, N.; Meca-Rivera, T.; Navarro-Santana, M.J.; Cleland, J.A.; Fernández-de-las-Peñas, C.; Pérez-Bellmunt, A. Is Dry Needling Effective for the Management of Plantar Heel Pain or Plantar Fasciitis? An Updated Systematic Review and Meta-Analysis. Pain Med. 2021, 22, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, S.; Guidi, M.; Marcotulli, G. The Effectiveness of Conservative, Non-Pharmacological Treatment, of Plantar Heel Pain: A Systematic Review with Meta-Analysis. Foot 2017, 33, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.A.; Lenz, A.L.; Lenhart, R.L.; Thelen, D.G. The Modulation of Forward Propulsion, Vertical Support, and Center of Pressure by the Plantarflexors during Human Walking. Gait Posture 2013, 38, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Macklin, K.; Healy, A.; Chockalingam, N. The Effect of Calf Muscle Stretching Exercises on Ankle Joint Dorsiflexion and Dynamic Foot Pressures, Force and Related Temporal Parameters. Foot 2012, 22, 10–17. [Google Scholar] [CrossRef]
- Caruso, J.F.; Coday, M.A.; Ramsey, C.A.; Griswold, S.H.; Polanski, D.W.; Drummond, J.L.; Walker, R.H. Leg and Calf Press Training Modes and Their Impact on Jump Performance Adaptations. J. Strength Cond. Res. 2008, 22, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Santos, C.M.d.C.; Pimenta, C.A.d.M.; Nobre, M.R.C. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Foley, N.C.; Teasell, R.W.; Bhogal, S.K.; Speechley, M.R. Stroke Rehabilitation Evidence-Based Review: Methodology. Top Stroke Rehabil. 2003, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Albin, S.; Koppenhaver, S.; MacDonald, C.; Capoccia, S.; Ngo, D.; Phippen, S.; Pineda, R.; Wendlandt, A.; Hoffman, L. The Effect of Dry Needling on Gastrocnemius Muscle Stiffness and Strength in Participants with Latent Trigger Points. J. Electromyogr. Kinesiol. 2020, 55, 102479. [Google Scholar] [CrossRef]
- Bandy, W.D.; Nelson, R.; Beamer, L. Comparison of Dry Needling Vs. Sham on the Performance of Vertical Jump. Int. J. Sports Phys. Ther. 2017, 12, 747–752. [Google Scholar] [CrossRef]
- Baraja-Vegas, L.; Martin-Rodriguez, S.; Piqueras-Sanchiz, F.; Faundez-Aguilera, J.; Bautista, I.J.; Barrios, C.; Fernandez-de-las-Penas, C. Localization of Muscle Edema and Changes on Muscle Contractility After Dry Needling of Latent Trigger Points in the Gastrocnemius Muscle. Pain Med. 2019, 20, 1387–1394. [Google Scholar] [CrossRef]
- Benito-De-Pedro, M.; Becerro-De-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Rodríguez-Sanz, D.; López-López, D.; Cosín-Matamoros, J.; Martínez-Jiménez, E.M.; Calvo-Lobo, C. Effectiveness between Dry Needling and Ischemic Compression in the Triceps Surae Latent Myofascial Trigger Points of Triathletes on Pressure Pain Threshold and Thermography: A Single Blinded Randomized Clinical Trial. J. Clin. Med. 2019, 8, 1632. [Google Scholar] [CrossRef]
- Benito-De-Pedro, M.; Becerro-De-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Rodrıguez-Sanz, D.; Lopez-L opez, D.; Palomo-Lopez, P.; Mazoteras-Pardo, V.; Calvo-Lobo, C. Effectiveness of Deep Dry Needling vs Ischemic Compression in the Latent Myofascial Trigger Points of the Shortened Triceps Surae from Triathletes on Ankle Dorsiflexion, Dynamic, and Static Plantar Pressure Distribution: A Clinical Trial. Pain Med. 2020, 21, E172–E181. [Google Scholar] [CrossRef]
- Benito-De-Pedro, M.; Calvo-Lobo, C.; López-López, D.; Benito-De-Pedro, A.I.; Romero-Morales, C.; San-Antolín, M.; Vicente-Campos, D.; Rodríguez-Sanz, D. Electromyographic Assessment of the Efficacy of Deep Dry Needling versus the Ischemic Compression Technique in Gastrocnemius of Medium-Distance Triathletes. Sensors 2021, 21, 2906. [Google Scholar] [CrossRef]
- Cushman, D.M.; Cummings, K.; Skinner, L.; Holman, A.; Haight, P.; Brobeck, M.; Teramoto, M.; Tang, C. Postrace Dry Needling Does Not Reduce Subsequent Soreness and Cramping-A Randomized Controlled Trial. Clin. J. Sport Med. 2021, 31, 225–231. [Google Scholar] [CrossRef]
- Devereux, F.; O’Rourke, B.; Byrne, P.J.; Byrne, D.; Kinsella, S. Effects of Myofascial Trigger Point Release on Power and Force Production in the Lower Limb Kinetic Chain. J. Strength Cond. Res. 2019, 33, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Espejo Antúnez, L.; Gacimartín García, A.; Pérez Cardeñosa, M.R.; Cardero Durán, M.A.; De la Cruz-Torres, B.; Albornoz-Cabello, M. Effects on Adverse Neural Tension by Slump Test after Dry Needling of Myofascial Trigger Point of the Gastrocnemius Muscle. Fisioterapia 2014, 36, 127–134. [Google Scholar] [CrossRef]
- Janowski, J.A.; Phelan-Smith, D.M.L.; Brady, M.N.K.; Michels, K.L.; Timm, A.H.; Boucher, N.M.; Casteen, K.D.; Village, D.; Sleeper, M.D. Acute Effects of Dry Needling on Myofascial Trigger Points in the Triceps Surae of Ballet Dancers: A Pilot Randomized Controlled Trial. Int. J. Sports Phys. Ther. 2021, 16, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.D.; Myers, H.; Aefsky, B.; Butler, R. Immediate and Short Term Effect of Dry Needling on Triceps Surae Range of Motion and Functional Movement: A Randomized Trial. Int. J. Sports Phys. Ther. 2018, 13, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bellmunt, A.; Casasayas-Cos, O.; López-De-Celis, C.; Rodríguez-Sanz, J.; Rodríguez-Jiménez, J.; Ortiz-Miguel, S.; Meca-Rivera, T.; Fernández-De-las-Peñas, C. Effects of Dry Needling of Latent Trigger Points on Viscoelastic and Muscular Contractile Properties: Preliminary Results of a Randomized within-Participant Clinical Trial. J. Clin. Med. 2021, 10, 3848. [Google Scholar] [CrossRef]
- Braithwaite, F.A.; Walters, J.L.; Li, L.S.K.; Moseley, G.L.; Williams, M.T.; Mcevoy, M.P.; Fa, B.; Jl, W.; Lsk, L.; Gl, M.; et al. Blinding Strategies in Dry Needling Trials: Systematic Review and Meta-Analysis Background. Blinding of Participants and Therapists in Trials of Physical Interventions. Phys. Ther. 2019, 99, 1461–1480. [Google Scholar] [CrossRef]
- Rodríguez-Mansilla, J.; González-Sánchez, B.; De Toro García, Á.; Valera-Donoso, E.; Garrido-Ardila, E.M.; Jiménez-Palomares, M.; López-Arza, M.V.G. Effectiveness of Dry Needling on Reducing Pain Intensity in Patients with Myofascial Pain Syndrome: A Meta-Analysis. J. Tradit. Chin. Med. 2016, 36, 1–13. [Google Scholar] [CrossRef]
- Navarro-Santana, M.J.; Gómez-Chiguano, G.F.; Cleland, J.A.; Arias-Buría, J.L.; Fernández-de-las-Peñas, C.; Plaza-Manzano, G. Effects of Trigger Point Dry Needling for Nontraumatic Shoulder Pain of Musculoskeletal Origin: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzaa216. [Google Scholar] [CrossRef]
- Sánchez-Infante, J.; Navarro-Santana, M.J.; Bravo-Sánchez, A.; Jiménez-Diaz, F.; Abián-Vicén, J. Is Dry Needling Applied by Physical Therapists Effective for Pain in Musculoskeletal Conditions? A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzab070. [Google Scholar] [CrossRef]
- Navarro-Santana, M.J.; Sanchez-Infante, J.; Fernández-de-las-Peñas, C.; Cleland, J.A.; Martín-Casas, P.; Plaza-Manzano, G. Effectiveness of Dry Needling for Myofascial Trigger Points Associated with Neck Pain Symptoms: An Updated Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 3300. [Google Scholar] [CrossRef]
- Arias-Buría, J.L.; Franco-Hidalgo-Chacón, M.M.; Cleland, J.A.; Palacios-Ceña, M.; Fuensalida-Novo, S.; Fernández-de-las-Peñas, C. Effects of Kinesio Taping on Post-Needling Induced Pain After Dry Needling of Active Trigger Point in Individuals With Mechanical Neck Pain. J. Manip. Physiol. Ther. 2020, 43, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pintado-Zugasti, A.; Mayoral del Moral, O.; Gerwin, R.D.; Fernández-Carnero, J. Post-Needling Soreness after Myofascial Trigger Point Dry Needling: Current Status and Future Research. J. Bodyw. Mov. Ther. 2018, 22, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Domingo, A.; Mayoral, O.; Monterde, S.; Santafé, M.M. Neuromuscular Damage and Repair after Dry Needling in Mice. Evid.-Based Complement. Altern. Med. 2013, 2013, 260806. [Google Scholar] [CrossRef] [PubMed]
- Cagnie, B.; Dewitte, V.; Barbe, T.; Timmermans, F.; Delrue, N.; Meeus, M. Physiologic Effects of Dry Needling. Curr. Pain Headache Rep. 2013, 17, 348. [Google Scholar] [CrossRef]
- Ziaeifar, M.; Arab, A.M.; Nourbakhsh, M.R. Clinical Effectiveness of Dry Needling Immediately After Application on Myofascial Trigger Point in Upper Trapezius Muscle. J. Chiropr. Med. 2016, 15, 252–258. [Google Scholar] [CrossRef]
- Morihisa, R.; Eskew, J.; McNamara, A.; Young, J. Dry Needling in Subjects with Muscular Trigger Points in the Lower Quarter: A Systematic Review. Int. J. Sports Phys. Ther. 2016, 11, 1–14. [Google Scholar]
- Huguenin, L. Effect of Dry Needling of Gluteal Muscles on Straight Leg Raise: A Randomised, Placebo Controlled, Double Blind Trial. Br. J. Sport Med. 2005, 39, 84–90. [Google Scholar] [CrossRef]
- Mayoral, O.; Salvat, I.; Martín, M.T.; Martín, S.; Santiago, J.; Cotarelo, J.; Rodríguez, C. Efficacy of Myofascial Trigger Point Dry Needling in the Prevention of Pain after Total Knee Arthroplasty: A Randomized, Double-Blinded, Placebo-Controlled Trial. Evid.-Based Complement. Altern. Med. 2013, 2013, 694941. [Google Scholar] [CrossRef]
- Blanco-Díaz, M.; Ruiz-Redondo, R.; Escobio-Prieto, I.; De la Fuente-Costa, M.; Albornoz-Cabello, M.; Casaña, J. A Systematic Review of the Effectiveness of Dry Needling in Subacromial Syndrome. Biology 2022, 11, 243. [Google Scholar] [CrossRef]
- Jayaseelan, D.J.; Faller, B.T.; Avery, M.H. The Utilization and Effects of Filiform Dry Needling in the Management of Tendinopathy: A Systematic Review. Physiother. Theory Pr. 2021, 1–13. [Google Scholar] [CrossRef]
- Valencia-Chulián, R.; Heredia-Rizo, A.M.; Moral-Munoz, J.A.; Lucena-Anton, D.; Luque-Moreno, C. Dry Needling for the Management of Spasticity, Pain, and Range of Movement in Adults after Stroke: A Systematic Review. Complement. Ther. Med. 2020, 52, 102515. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, C.J.; Vanetten, L.; Willy, R.; di Stasi, S.; Magnussen, R.; Briggs, M. The Effects of Needling Therapies on Muscle Force Production: A Systematic Review and Meta-Analysis. J. Orthop. Sport Phys. Ther. 2019, 49, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Laita, L.; Jiménez-del-Barrio, S.; Marín-Zurdo, J.; Moreno-Calvo, A.; Marín-Boné, J.; Albarova-Corral, M.I.; Estébanez-de-Miguel, E. Effectiveness of Dry Needling Therapy on Pain, Hip Muscle Strength, and Physical Function in Patients With Hip Osteoarthritis: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2021, 102, 959–966. [Google Scholar] [CrossRef]
- Ceballos-Laita, L.; Jiménez-del-Barrio, S.; Marín-Zurdo, J.; Moreno-Calvo, A.; Marín-Boné, J.; Albarova-Corral, M.I.; Estébanez-de-Miguel, E. Effects of Dry Needling in Hip Muscles in Patients with Hip Osteoarthritis: A Randomized Controlled Trial. Musculoskelet. Sci. Pr. 2019, 43, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Zarei, H.; Bervis, S.; Piroozi, S.; Motealleh, A. Added Value of Gluteus Medius and Quadratus Lumborum Dry Needling in Improving Knee Pain and Function in Female Athletes With Patellofemoral Pain Syndrome: A Randomized Clinical Trial. Arch. Phys. Med. Rehabil. 2020, 101, 265–274. [Google Scholar] [CrossRef]
- Sánchez-Infante, J.; Bravo-Sánchez, A.; Jiménez, F.; Abián-Vicén, J. Effects of Dry Needling on Muscle Stiffness in Latent Myofascial Trigger Points: A Randomized Controlled Trial. J. Pain 2021, 22, 817–825. [Google Scholar] [CrossRef]
- Kelly, J.P.; Koppenhaver, S.L.; Michener, L.A.; Kolber, M.J.; Cleland, J.A. Immediate Decrease of Muscle Biomechanical Stiffness Following Dry Needling in Asymptomatic Participants. J. Bodyw. Mov. Ther. 2021, 27, 605–611. [Google Scholar] [CrossRef]
- Mullins, J.F.; Nitz, A.J.; Hoch, M.C. Dry Needling Equilibration Theory: A Mechanistic Explanation for Enhancing Sensorimotor Function in Individuals with Chronic Ankle Instability. Physiother. Theory Pr. 2021, 37, 672–681. [Google Scholar] [CrossRef]
- Martin, R.L.; Chimenti, R.; Cuddeford, T.; Houck, J.; Matheson, J.W.; McDonough, C.M.; Paulseth, S.; Wukich, D.K.; Carcia, C.R. Achilles Pain, Stiffness, and Muscle Power Deficits: Midportion Achilles Tendinopathy Revision 2018. J. Orthop. Sport Phys. Ther. 2018, 48, A1–A38. [Google Scholar] [CrossRef]
- Kumagai, H.; Miyamoto-Mikami, E.; Hirata, K.; Kikuchi, N.; Kamiya, N.; Hoshikawa, S.; Zempo, H.; Naito, H.; Miyamoto, N.; Fuku, N. ESR1 Rs2234693 Polymorphism Is Associated with Muscle Injury and Muscle Stiffness. Med. Sci. Sport Exerc. 2019, 51, 19–26. [Google Scholar] [CrossRef]
- Lucas, K.R.; Rich, P.A.; Polus, B.I. Muscle Activation Patterns in the Scapular Positioning Muscles during Loaded Scapular Plane Elevation: The Effects of Latent Myofascial Trigger Points. Clin. Biomech. 2010, 25, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Tesarz, J.; Schuster, A.K.; Hartmann, M.; Gerhardt, A.; Eich, W. Pain Perception in Athletes Compared to Normally Active Controls: A Systematic Review with Meta-Analysis. Pain 2012, 153, 1253–1262. [Google Scholar] [CrossRef] [PubMed]


| Author/Year | Country | Population/Age | Type of Intervention | Intervention Dose/Method of Intervention | Outcome Measures | Measuring Instruments | Results |
|---|---|---|---|---|---|---|---|
| Albin et al. [34] 2020 | United States | Non-Athletes G1: n = 52/25.1 ± 3.6 G2: n = 50/27.0 ± 5.0 N = 102/18–50 years | G1: DN G2: Sham needling | 2 sessions of DN pistoning technique for 5–10 s in 3 latent MTrPs of gastrocnemius to elicit as many LTR as possible | -Muscle stiffness (resting and contracted gastrocnemius) -Muscle strength (triceps surae) | -Myoton PRO -Hand-held dynamometer | Significant improvements were found in the resting muscle stiffness at the site of the MTrPs for the DN group (p = 0.03). |
| Bandy et al. [35] 2017 | United States | Non-Athletes G1: n = 18/ND G2: n = 17/ND N = 35/22.7 ± 2.4 | G1: DN G2: Sham needling | 1 session of DN of latent MTrPS in four sites on bilateral gastrocnemius (two at the medial head and two at the lateral head). The needles were tapped and inserted, one right after the other | -Vertical Jump height | -Chalk marks on the wall | The DN group significantly increased vertical jump eight 1.2 inches over the sham group (p = 0.038). |
| Baraja-Vegas et al. [36] 2019 | Spain | Non-Athletes G1: n = 18 (target leg) G2: n = 18 (contralateral leg) N = 18/25.5 ± 5.0 | G1: DN G2: Not intervention | 1 session of DN in gastrocnemius latent MTrPs using the fast-in and fast-out technique during 8–10 insertions to elicit LTR | -Intramuscular edema -Muscle contractile properties -Pain | -Magnetic Resonance Imaging -Tensiomyography -11-point Numerical Pain Rating Scale | Significant changes between groups were found in the intramuscular edema for the DN group (p < 0.001). Significant changes between groups were found in the resting muscle stiffness with an improvement in contraction time for the DN group (p < 0.001). |
| Benito-de-Pedro et al. [37] 2019 | Spain | Athletes (Triathlon) G1: n = 17/35.3 ± 5.4 G2: n = 17/33.7 ± 5.7 N = 34 (18–75 years) | G1: DN G2: Ischemic compression | 1 session of deep DN in triceps surae, on latent MTrPs using the fast-in and fast-out technique to elicit LTR until the limit of tolerance of the patient or reaching a maximum number of 8 to 10 insertions | -Pressure pain thresholds -Thermographic measurement | -Wagner analog algometer -Thermographic camera with MSX technology | Statistically significant differences between groups were found in the Pressure pain threshold reduction in favor of the DN group (p < 0.05). |
| Benito-de-Pedro et al. [38] 2020 | Spain | Athletes (Triathlon) G1: n = 17/ND G2: n = 17/ND N = 34 (18–75 years) | G1: DN G2: Ischemic compression | 1 session of deep DN in gastrocnemius, on latent MTrPs using the fast-in and fast-out technique to elicit LTR until the LTR were exhausted, up to the limit of tolerance of the patient or reaching a maximum number of 8 to 10 insertions | -Ankle dorsiflexion ROM -Dynamic plantar pressures -Static plantar pressures | -Goniometer -Plantar pressure sensor platform with T-plate software | No significant changes between groups were found in any outcome. |
| Benito-de-Pedro et al. [39] 2021 | Spain | Athletes (Triathlon) G1: n = 17/35.3 ± 5.4 G2: n = 17/33.7 ± 5.7 N = 34 (18–75 years) | G1: DN G2: Ischemic compression | 1 session of deep DN in gastrocnemius, on latent MTrPs using the fast-in and fast-out technique to elicit LTR until the LTR were exhausted, up to the limit of tolerance of the patient or reaching a maximum number of 8 to 10 insertions | -Superficial electromyographic activity | -Electromyography | Statistically significant differences between groups were found for a reduction of superficial EMG measurements differences (%) in triathletes who train at a speed lower than 1 m/s, in favor of the DN group (p = 0.037). |
| Cushman et al. [40] 2021 | United States | Athletes (Runners) G1: n = 28/42.1 ± 11.8 G2: n = 33/41.2 ± 13.1 N = 61 (>18 years) | G1: DN G2: Sham needling | 1 session of DN in soleus to elicit LTR until the LTR was extinguished or reaching a maximum number of 10 insertions | -Pain (soreness) -Postrace cramps -Subjective improvement of soreness | -Numeric Pain Rating Scale -Survey | Objective pain scores showed an increase in pain of the soleus muscles at days 1 and 2 in the DN group (p ≤ 0.003 and p ≤ 0.041, respectively). |
| Devereux et al. [41] 2018 | Ireland | Athletes (Any sport competitively in which jumping, sprinting, twisting, turning, acceleration, and deceleration were important components) G1: n = 10/ND G2: n = 10/ND G3: n = 10/ND G4: n = 10/ND N = 40/25.6 ± 5.5 | G1: DN rectus femoris G2: DN medial gastrocnemius G3: DN rectus femoris + gastrocnemius G4: Not intervention | 1 session of Deep DN of latent MTrPS to elicit LTR | -Jump height -Power output -Optimal force -Optimal velocity | My Jump App (iOS) | Significant improvements were found in jump height for DN medial gastrocnemius group from immediately to 48 h post-DN (p = 0.01). |
| Espejo Antúnez et al. [42] 2014 | Spain | Non-Athletes G1: n = 23/22.4 ± 1.5 G2: n = 22/21.1 ± 1.3 N = 45 (>18 years) | G1: DN G2: Sham needling | 1 session of DN in gastrocnemius latent MTrPs using the fast-in and fast-out technique to elicit LTR | -Adverse neural tension -Pain | -Slump neurodynamic test -Visual Analogue Scale | Significant differences were found between groups for the perceived pain in favor of the DN group (p < 0.01). |
| Janowski et al. [43] 2021 | United States | Athletes (Professional ballet dancers) G1: ND G2: ND N = 11 ND | G1: DN + stretching G2: sham needling + stretching | 1 session of DN in triceps surae MTrPs repeatedly moved up and down in order to elicit LTR | -Pain -Temperature -Ankle dorsiflexion ROM -Maximum muscular torque of plantar flexion | -Visual Analogue Scale -Surface thermometer -Goniometer -Biodex | No statistically significant differences between groups were found in any outcome. |
| Lake et al. [44] 2018 | United States | Non-Athletes G1: n = 10/25.1 ± 2.4 G2: n = 10/27.1 ± 4.9 G3: n = 10/23.3 ± 4.8 N = 30/26.4 ± 3.1 | G1: DN G2: Stretching G3: DN + stretching | G1: 1 session of DN pistoning technique (eliciting LTR) in gastrocnemius and soleus G2: 1 session of 30 s 3 times each leg G3: G1 + G2 interventions | -ROM (passive dorsiflexion, closed chain half kneeling and standing dorsiflexion) -Deep squat -Functional dorsiflexion and dynamic balance | -Inclinometer -Deep squat score -Y-Balance Test of the Lower Quarter (YBT-LQ) | Significant differences were found between groups for deep squat performance in favor of the DN group (p < 0.01). |
| Pérez-Bellmunt et al. [45] 2021 | Spain | Non-Athletes G1: n = 25/ND G2: n = 25/ND N = 50/22.4 ± 8.4 | G1: DN G2: Not intervention | 1 session of DN in gastrocnemius latent MTrPs using the fast-in and fast-out technique to elicit LTR | -Viscoelastic properties and contractile properties -Pressure pain sensitivity -Ankle dorsiflexion ROM -Muscle strength | -MyotonPro instrument -Manual algometer/11-point Numerical Pain Rating Scale -Goniometer during lunge test -Dynamometer | Significant differences between groups were found in the lateral gastrocnemius viscoelastic properties: stiffness (p = 0.02), relaxation (p = 0.045), and creep (p = 0.03), in favor of the DN group. The control group showed a higher increase in pressure pain thresholds than the experimental group (p = 0.03). |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Albin et al. [34] 2020 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 9 |
| Bandy et al. [35] 2017 | Yes | Yes | No | Yes | Yes | No | No | No | No | Yes | Yes | 5 |
| Baraja-Vegas et al. [36] 2019 | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 5 |
| Benito-de-Pedro et al. [37] 2019 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Benito-de-Pedro et al. [38] 2020 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Benito-de-Pedro et al. [39] 2021 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Cushman et al. [40] 2021 | Yes | Yes | No | Yes | Yes | No | Yes | No | No | Yes | No | 5 |
| Devereux et al. [41] 2018 | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | 6 |
| Espejo Antúnez et al. [42] 2014 | No | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | 7 |
| Janowski et al. [43] 2021 | Yes | Yes | No | No | Yes | No | Yes | No | No | Yes | Yes | 5 |
| Lake et al. [44] 2018 | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | 6 |
| Pérez-Bellmunt et al. [45] 2021 | Yes | Yes | No | Yes | No | No | Yes | No | No | Yes | Yes | 5 |
| Author/Year | Population | Study Design | Pain | Pressure Pain Thresholds | ROM | Muscle Strength | Muscle Stiffness | Functional Performance | Overall PEDro | Overall RoB 2.0 |
|---|---|---|---|---|---|---|---|---|---|---|
| Albin et al. [34] 2020 | Non-athletes | RCT | N/A | N/A | N/A | = | + (Resting muscle stiffness) | N/A | Excellent | Low risk |
| = (Contracted muscle stiffness) | ||||||||||
| Bandy et al. [35] 2017 | Non-athletes | RCT | N/A | N/A | N/A | N/A | N/A | + (Jump height) | Fair | Some concerns |
| Baraja-Vegas et al. [36] 2019 | Non-athletes | CT | N/A | N/A | N/A | N/A | + | N/A | Fair | High risk |
| Benito-de-Pedro et al. [37] 2019 | Athletes | RCT | N/A | - | N/A | N/A | N/A | N/A | Good | Some concerns |
| Benito-de-Pedro et al. [38] 2020 | Athletes | RCT | N/A | N/A | = | N/A | N/A | N/A | Good | Some concerns |
| Benito-de-Pedro et al. [39] 2021 | Athletes | RCT | N/A | N/A | N/A | N/A | N/A | N/A | Good | Some concerns |
| Cushman et al. [40] 2021 | Athletes | RCT | N/A | N/A | N/A | N/A | N/A | N/A | Fair | High risk |
| Devereux et al. [41] 2018 | Athletes | RCT | N/A | N/A | N/A | N/A | N/A | + (Jump height) | Good | High risk |
| = (Jump power output, optimal force, and velocity) | ||||||||||
| Espejo Antúnez et al. [42] 2014 | Non-athletes | RCT | + | N/A | N/A | N/A | N/A | N/A | Good | Some concerns |
| Janowski et al. [43] 2021 | Athletes | Pilot RCT | = | N/A | = | = | N/A | N/A | Fair | Some concerns |
| Lake et al. [44] 2018 | Non-athletes | RCT | N/A | N/A | = | N/A | N/A | + (Deep squat) | Good | Some concerns |
| Pérez-Bellmunt et al. [45] 2021 | Non-athletes | Within-participant RCT | N/A | - | = | = | + | N/A | Fair | High risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucena-Anton, D.; Luque-Moreno, C.; Valencia-Medero, J.; Garcia-Munoz, C.; Moral-Munoz, J.A. Effectiveness of Dry Needling of Myofascial Trigger Points in the Triceps Surae Muscles: Systematic Review. Healthcare 2022, 10, 1862. https://doi.org/10.3390/healthcare10101862
Lucena-Anton D, Luque-Moreno C, Valencia-Medero J, Garcia-Munoz C, Moral-Munoz JA. Effectiveness of Dry Needling of Myofascial Trigger Points in the Triceps Surae Muscles: Systematic Review. Healthcare. 2022; 10(10):1862. https://doi.org/10.3390/healthcare10101862
Chicago/Turabian StyleLucena-Anton, David, Carlos Luque-Moreno, Jesus Valencia-Medero, Cristina Garcia-Munoz, and Jose A. Moral-Munoz. 2022. "Effectiveness of Dry Needling of Myofascial Trigger Points in the Triceps Surae Muscles: Systematic Review" Healthcare 10, no. 10: 1862. https://doi.org/10.3390/healthcare10101862
APA StyleLucena-Anton, D., Luque-Moreno, C., Valencia-Medero, J., Garcia-Munoz, C., & Moral-Munoz, J. A. (2022). Effectiveness of Dry Needling of Myofascial Trigger Points in the Triceps Surae Muscles: Systematic Review. Healthcare, 10(10), 1862. https://doi.org/10.3390/healthcare10101862










