Sex-Specific Association between Underlying Diseases and the Severity and Mortality Due to COVID-19 Infection: A Retrospective Observational Cohort Analysis of Clinical Epidemiological Information Collected by the Korea Disease Control and Prevention Agency
Abstract
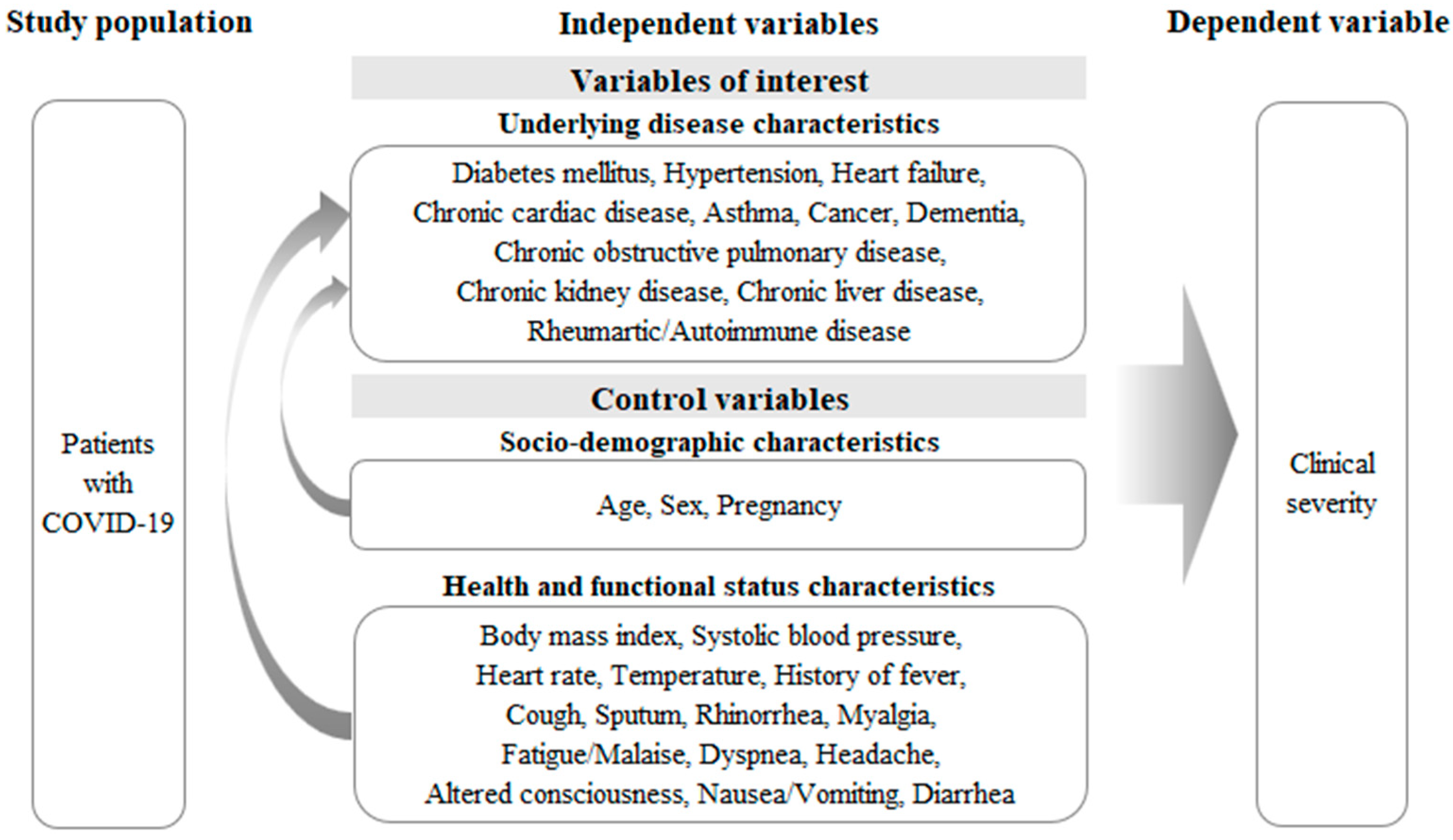
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Sample
2.2. Measurements
2.2.1. Dependent Variable: Clinical Severity
2.2.2. Variables of Interest: Underlying Diseases
2.2.3. Control Variables
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Coronavirus Disease (COVID-19). Available online: https://www.who.int/health-topics/coronavirus#tab=tab_1 (accessed on 15 September 2022).
- Ministry of Health and Welfare (MOHW). What is COVID-19? Available online: http://ncov.mohw.go.kr/baroView.do (accessed on 15 September 2022).
- World Health Organization (WHO). WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 (accessed on 15 September 2022).
- World Health Organization (WHO). WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—30 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---30-march-2020 (accessed on 15 September 2022).
- Ministry of Health and Welfare (MOHW). Domestic Occurrences. Available online: http://ncov.mohw.go.kr/bdBoardList_Real.do?brdId=1&brdGubun=11&ncvContSeq=&contSeq=&board_id=&gubun= (accessed on 2 May 2022).
- World Health Organization (WHO). Health Emergency Dashboard. Available online: https://covid19.who.int/ (accessed on 2 May 2022).
- Borio, C. The Covid-19 economic crisis: Dangerously unique. Bus. Econ. 2020, 55, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Behrman, P.; Dulin, A.; Baskin, M.L.; Buscemi, J.; Alcaraz, K.I.; Goldstein, C.M.; Carson, T.L.; Shen, M.; Fitzgibbon, M. Addressing inequities in COVID-19 morbidity and mortality: Research and policy recommendations. Transl. Behav. Med. 2020, 10, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Moo-Sik, L. Fragmentary thoughts about code of conduct and risk communication to prevent and control COVID-19 in Korea, 2020. Korean J. Health Educ. Promot. 2020, 37, 103–107. [Google Scholar]
- Rosenthal, N.; Cao, Z.; Gundrum, J.; Sianis, J.; Safo, S. Risk Factors Associated With In-Hospital Mortality in a US National Sample of Patients With COVID-19. JAMA Netw. Open 2020, 3, e2029058. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Kim, Y.; Nam, S.; Kim, S. Impact Factors and Validity of Blood Variables on Death in COVID-19 patient: Using Data of Korea Disease Control and Prevention Agency. J. Korea Soc. Comput. Inf. 2020, 25, 179–185. [Google Scholar]
- Lee, J.; Lim, D.; Hong, S.; Park, M.; Kim, G.; Lim, N.; Lee, S.; Park, J.; Song, D.; Chai, H. The primary report of clinical data analysis on the COVID-19 in the Republic of Korea. Public Health Wkly. Rep. 2020, 13, 2054–2058. [Google Scholar]
- Choi, Y.J.; Park, J.-Y.; Lee, H.S.; Suh, J.; Song, J.Y.; Byun, M.-K.; Cho, J.H.; Kim, H.J.; Park, H.J. Variable effects of underlying diseases on the prognosis of patients with COVID-19. PLoS ONE 2021, 16, e0254258. [Google Scholar] [CrossRef]
- Ji, W.; Huh, K.; Kang, M.; Hong, J.; Bae, G.H.; Lee, R.; Na, Y.; Choi, H.; Gong, S.Y.; Choi, Y.H.; et al. Effect of Underlying Comorbidities on the Infection and Severity of COVID-19 in Korea: A Nationwide Case-Control Study. J. Korean Med. Sci. 2020, 35, e237. [Google Scholar] [CrossRef]
- Moon, S.-S.; Lee, K.; Park, J.; Yun, S.; Lee, Y.S.; Lee, D.S. Clinical Characteristics and Mortality Predictors of COVID-19 Patients Hospitalized at Nationally-Designated Treatment Hospitals. J. Korean Med. Sci. 2020, 35, 1146170. [Google Scholar] [CrossRef]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020, 2020, e3319. [Google Scholar] [CrossRef]
- Kyoung, D.-S.; Lee, J.; Nam, H.; Park, M.H. Dementia and COVID-19 Mortality in South Korea. Dement. Neurocogn. Disord. 2021, 20, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chai, P.; Yu, J.; Fan, X. Effects of cancer on patients with COVID-19: A systematic review and meta-analysis of 63,019 participants. Cancer Biol. Med. 2021, 18, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Shin, J.I.; Moon, S.Y.; Jin, H.Y.; Kim, S.Y.; Yang, J.M.; Cho, S.H.; Kim, S.; Lee, M.; Park, Y.; et al. Autoimmune inflammatory rheumatic diseases and COVID-19 outcomes in South Korea: A nationwide cohort study. Lancet Rheumatol. 2021, 3, e698–e706. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Wang, X.; Jia, X.; Li, J.; Lei, H.; Liu, Z.; Liao, F.; Ji, M.; Lv, X.; et al. Do underlying cardiovascular diseases have any impact on hospitalised patients with COVID-19? Heart 2020, 106, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhou, N.; Zha, W.; Lv, Y. Hypertension is a clinically important risk factor for critical illness and mortality in COVID-19: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Dadson, P.; Tetteh, C.D.; Rebelos, E.; Badeau, R.M.; Moczulski, D. Underlying Kidney Diseases and Complications for COVID-19: A Review. Front. Med. 2020, 7, 600144. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Pennington, A.F.; Goodman, A.B.; Rosenblum, H.G.; Belay, B.; Ko, J.Y.; Chevinsky, J.R.; Schieber, L.Z.; Summers, A.D.; Lavery, A.M.; et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020–March 2021. Prev. Chronic Dis. 2021, 18, E66. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare (MOHW). Press Release. Available online: http://ncov.mohw.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6451&contSeq=6451&board_id=312&gubun=ALL (accessed on 13 September 2022).
- Driggin, E.; Maddox Thomas, M.; Ferdinand Keith, C.; Kirkpatrick James, N.; Ky, B.; Morris Alanna, A.; Mullen, J.B.; Parikh Sahil, A.; Philbin Daniel, M.; Vaduganathan, M. ACC Health Policy Statement on Cardiovascular Disease Considerations for COVID-19 Vaccine Prioritization: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 1938–1948. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Agathis, N.T.; Nelson, J.M.; Preston, L.E.; Ko, J.Y.; Belay, B.; Pennington, A.F.; Danielson, M.L.; DeSisto, C.L.; Chevinsky, J.R.; et al. Underlying Medical Conditions Associated With Severe COVID-19 Illness Among Children. JAMA Netw. Open 2021, 4, e2111182. [Google Scholar] [CrossRef]
- Tharakan, S.; Nomoto, K.; Miyashita, S.; Ishikawa, K. Body temperature correlates with mortality in COVID-19 patients. Crit. Care 2020, 24, 298. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Lu, L.; Zhang, B.; Luo, J.; Li, W.; He, L.; Sander, J.W.; Mu, J.; Zhu, C.; Zhou, D. Association of consciousness impairment and mortality in people with COVID-19. Acta Neurol. Scand. 2021, 144, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Bolay, H.; Gül, A.; Baykan, B. COVID-19 is a Real Headache! Headache 2020, 60, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Carod-Artal, F.J. Neurological complications of coronavirus and COVID-19. Rev. Neurol. 2020, 70, 311–322. [Google Scholar] [CrossRef]
- Allali, G.; Marti, C.; Grosgurin, O.; Morélot-Panzini, C.; Similowski, T.; Adler, D. Dyspnea: The vanished warning symptom of COVID-19 pneumonia. J. Med. Virol. 2020, 92, 2272–2273. [Google Scholar] [CrossRef]
- Matta, S.; Rajpal, S.; Chopra, K.K.; Arora, V.K. Covid-19 vaccines and new mutant strains impacting the pandemic. Indian J. Tuberc. 2021, 68, 171–173. [Google Scholar] [CrossRef]
- Yi, S.; Choe, Y.J.; Kim, J.; Kim, Y.Y.; Kim, R.K.; Jang, E.J.; Lim, D.S.; Byeon, H.R.; Lee, S.; Park, E.; et al. SARS-CoV-2 Breakthrough Infections after introduction of 4 COVID-19 Vaccines, South Korea, 2021. Emerg. Infect. Dis. 2022, 28, 753–756. [Google Scholar] [CrossRef]
- Li, Y.; Fang, L.; Zhu, S.; Xie, Y.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; Wu, C.; et al. Echocardiographic Characteristics and Outcome in Patients With COVID-19 Infection and Underlying Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 642973. [Google Scholar] [CrossRef]
- Farshidfar, F.; Koleini, N.; Ardehali, H. Cardiovascular complications of COVID-19. JCI Insight 2021, 6, e148980. [Google Scholar] [CrossRef]
- Khorrami, Z.; Nili, S.; Sharifi, H.; Eybpoosh, S.; Shokoohi, M. Association of cigarette smoking, obesity, and underlying medical conditions with COVID-19 hospitalization and mortality in Iran: A nationwide retrospective ecological study. Med. J. Islam. Repub. Iran 2020, 34, 133. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, D.C.; Mackey, D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: A systematic review and meta-analysis. Respir. Med. 2020, 171, 106096. [Google Scholar] [CrossRef] [PubMed]

| Variable | Category | Males (N = 2106) | Females (N = 2971) | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Socio-demographic characteristics | |||||
| Age (years) | 0–39 | 853 | (40.5) | 848 | (3.5) |
| 40–69 | 929 | (44.1) | 1604 | (54.0) | |
| ≥70 | 324 | (15.4) | 519 | (17.5) | |
| Pregnancy | No | - | - | 2952 | (99.4) |
| Yes | - | - | 19 | (0.6) | |
| Health and functional status characteristics | |||||
| Body mass index | Underweight | 73 | (3.5) | 155 | (5.2) |
| Normal | 564 | (26.8) | 1111 | (37.4) | |
| Overweight | 452 | (21.4) | 493 | (16.6) | |
| Obese | 610 | (29.0) | 535 | (18.0) | |
| No-answer | 407 | (19.3) | 677 | (22.8) | |
| Systolic blood pressure | Normal | 361 | (17.2) | 874 | (29.4) |
| Pre-hypertension | 902 | (42.8) | 1162 | (39.1) | |
| Hypertension | 843 | (40.0) | 935 | (31.5) | |
| Heart rate | Bradycardia | 49 | (2.3) | 59 | (2.0) |
| Normal | 1748 | (83.0) | 2510 | (84.5) | |
| Tachycardia | 309 | (14.7) | 402 | (13.5) | |
| Fever | No | 1621 | (77.0) | 2248 | (75.7) |
| Yes | 485 | (23.0) | 723 | (24.3) | |
| Cough | No | 1275 | (60.5) | 1671 | (56.2) |
| Yes | 831 | (39.5) | 1300 | (43.8) | |
| Sputum | No | 1565 | (74.3) | 2053 | (69.1) |
| Yes | 541 | (25.7) | 918 | (30.9) | |
| Sore throat | No | 1849 | (87.8) | 2451 | (82.5) |
| Yes | 257 | (12.2) | 520 | (17.5) | |
| Rhinorrhea | No | 1905 | (90.5) | 2666 | (89.7) |
| Yes | 201 | (9.5) | 305 | (10.3) | |
| Myalgia | No | 1817 | (86.3) | 2438 | (82.1) |
| Yes | 289 | (13.7) | 533 | (17.9) | |
| Fatigue Malaise | No | 2007 | (95.3) | 2843 | (95.7) |
| Yes | 99 | (4.7) | 128 | (4.3) | |
| Dyspnea | No | 1866 | (88.6) | 2596 | (87.4) |
| Yes | 240 | (11.4) | 375 | (12.6) | |
| Headache | No | 1835 | (87.1) | 2397 | (80.7) |
| Yes | 271 | (12.9) | 574 | (19.3) | |
| Altered consciousness | No | 2094 | (99.4) | 2954 | (99.4) |
| Yes | 12 | (0.6) | 17 | (0.6) | |
| Nausea Vomiting | No | 2040 | (96.9) | 2802 | (94.3) |
| Yes | 66 | (3.1) | 169 | (5.7) | |
| Diarrhea | No | 1931 | (91.7) | 2707 | (91.1) |
| Yes | 175 | (8.3) | 264 | (8.9) | |
| Underlying diseases characteristics | |||||
| Diabetes mellitus | No | 1794 | (85.2) | 2622 | (88.2) |
| Yes | 312 | (14.8) | 349 | (11.8) | |
| Hypertension | No | 1633 | (77.5) | 2308 | (77.7) |
| Yes | 473 | (22.5) | 663 | (22.3) | |
| Heart failure | No | 2086 | (99.1) | 2935 | (98.8) |
| Yes | 20 | (0.9) | 36 | (1.2) | |
| Chronic cardiac disease | No | 2020 | (95.9) | 2885 | (97.1) |
| Yes | 86 | (4.1) | 86 | (2.9) | |
| Asthma | No | 2060 | (97.8) | 2896 | (97.5) |
| Yes | 46 | (2.2) | 75 | (2.5) | |
| Chronic obstructive pulmonary disease | No | 2082 | (98.9) | 2956 | (99.5) |
| Yes | 24 | (1.1) | 15 | (0.5) | |
| Chronic kidney disease | No | 2080 | (98.8) | 2943 | (99.1) |
| Yes | 26 | (1.2) | 28 | (0.9) | |
| Chronic liver disease | No | 2060 | (97.8) | 2937 | (98.9) |
| Yes | 46 | (2.2) | 34 | (1.1) | |
| Cancer | No | 2057 | (97.7) | 2880 | (96.9) |
| Yes | 49 | (2.3) | 91 | (3.1) | |
| Rheumatic Autoimmune disease | No | 2094 | (99.4) | 2946 | (99.2) |
| Yes | 12 | (0.6) | 25 | (0.8) | |
| Dementia | No | 2038 | (96.8) | 2820 | (94.9) |
| Yes | 68 | (3.2) | 151 | (5.1) | |
| Variable | Category | Males | p-Value | Females | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Non-Severity (N = 1956) | Clinical Severity (N = 150) | Clinical Non-Severity (N = 2838) | Clinical Severity (N = 133) | ||||||||
| Socio-demographic characteristics | |||||||||||
| Age (years) | 0–39 | 850 | (99.6) | 3 | (0.4) | <0.001 | 844 | (99.5) | 4 | (0.5) | <0.001 |
| 40–69 | 874 | (94.1) | 55 | (5.9) | 1578 | (98.4) | 26 | (1.6) | |||
| ≥70 | 232 | (71.6) | 92 | (28.4) | 416 | (80.2) | 103 | (19.8) | |||
| Pregnancy | No | 2819 | (95.5) | 133 | (4.5) | 0.677 | |||||
| Yes | 19 | (100.0) | - | - | |||||||
| Health and functional status characteristics | |||||||||||
| Body mass index | Underweight | 67 | (91.8) | 6 | (8.2) | <0.001 | 143 | (92.3) | 12 | (7.7) | <0.001 |
| Normal | 533 | (94.5) | 31 | (5.5) | 1078 | (97.0) | 33 | (3.0) | |||
| Overweight | 430 | (95.1) | 22 | (4.9) | 483 | (98.0) | 10 | (2.0) | |||
| Obese | 568 | (93.1) | 42 | (6.9) | 514 | (96.1) | 21 | (3.9) | |||
| No-answer | 358 | (88.0) | 49 | (12.0) | 620 | (91.6) | 57 | (8.4) | |||
| Systolic blood pressure | Normal | 332 | (92.0) | 29 | (8.0) | 0.032 | 832 | (95.2) | 42 | (4.8) | <0.001 |
| Pre-hypertension | 853 | (94.6) | 49 | (5.4) | 1130 | (97.2) | 32 | (2.8) | |||
| Hypertension | 771 | (91.5) | 72 | (8.5) | 876 | (93.7) | 59 | (6.3) | |||
| Heart rate | Bradycardia | 48 | (98.0) | 1 | (2.0) | <0.001 | 54 | (91.5) | 5 | (8.5) | 0.173 |
| Normal | 1640 | (93.8) | 108 | (6.2) | 2404 | (95.8) | 106 | (4.2) | |||
| Tachycardia | 268 | (86.7) | 41 | (13.3) | 380 | (94.5) | 22 | (5.5) | |||
| Fever | No | 1538 | (94.9) | 83 | (5.1) | <0.001 | 2163 | (96.2) | 85 | (3.8) | 0.001 |
| Yes | 418 | (86.2) | 67 | (13.8) | 675 | (93.4) | 48 | (6.6) | |||
| Cough | No | 1184 | (92.9) | 96 | (7.1) | 0.974 | 1589 | (95.1) | 82 | (4.9) | 0.198 |
| Yes | 772 | (92.9) | 59 | (7.1) | 1249 | (96.1) | 51 | (3.9) | 0.198 | ||
| Sputum | No | 1469 | (93.9) | 96 | (6.1) | 0.003 | 1958 | (95.4) | 95 | (4.6) | 0.552 |
| Yes | 487 | (90.0) | 54 | (10.0) | 880 | (95.9) | 38 | (4.1) | |||
| Sore throat | No | 1709 | (92.4) | 140 | (7.6) | 0.032 | 2321 | (94.7) | 130 | (5.3) | <0.001 |
| Yes | 247 | (96.1) | 10 | (3.9) | 517 | (99.4) | 3 | (0.6) | |||
| Rhinorrhea | No | 1759 | (92.3) | 130 | (7.2) | 0.886 | 2537 | (95.2) | 129 | (4.8) | 0.005 |
| Yes | 197 | (98.0) | 20 | (6.9) | 301 | (98.7) | 4 | (1.3) | |||
| Myalgia | No | 1687 | (92.8) | 130 | (7.2) | 0.002 | 2315 | (95.0) | 123 | (5.0) | 0.001 |
| Yes | 269 | (93.1) | 20 | (6.9) | 523 | (98.1) | 10 | (1.9) | |||
| Fatigue Malaise | No | 1872 | (93.3) | 135 | (6.7) | 0.002 | 2717 | (95.6) | 126 | (4.4) | 0.579 |
| Yes | 84 | (84.8) | 15 | (15.2) | 121 | (94.5) | 7 | (5.5) | |||
| Dyspnea | No | 1794 | (96.1) | 72 | (3.9) | <0.001 | 2524 | (97.2) | 72 | (2.8) | <0.001 |
| Yes | 162 | (67.5) | 78 | (32.5) | 314 | (83.7) | 61 | (16.3) | |||
| Headache | No | 1699 | (92.6) | 136 | (7.4) | 0.180 | 2272 | (94.8) | 125 | (5.2) | <0.001 |
| Yes | 257 | (94.8) | 14 | (5.2) | 566 | (98.6) | 8 | (1.4) | |||
| Altered consciousness | No | 1955 | (93.4) | 139 | (6.6) | <0.001 | 2831 | (95.8) | 123 | (4.2) | <0.001 |
| Yes | 1 | (83.3) | 11 | (91.7) | 7 | (41.2) | 10 | (58.8) | |||
| Nausea Vomiting | No | 1901 | (93.2) | 139 | (6.8) | 0.006 | 2677 | (95.5) | 125 | (4.5) | 0.868 |
| Yes | 55 | (83.3) | 11 | (16.7) | 161 | (95.3) | 8 | (4.7) | |||
| Diarrhea | No | 1792 | (92.8) | 139 | (7.2) | 0.653 | 2585 | (95.5) | 122 | (4.5) | 0.799 |
| Yes | 164 | (93.7) | 11 | (6.3) | 253 | (95.8) | 11 | (4.2) | |||
| Underlying diseases characteristics | |||||||||||
| Diabetes mellitus | No | 1702 | (94.9) | 92 | (5.1) | <0.001 | 2536 | (96.7) | 86 | (3.3) | <0.001 |
| Yes | 254 | (81.4) | 58 | (18.6) | 302 | (86.5) | 47 | (13.5) | |||
| Hypertension | No | 1560 | (95.5) | 73 | (4.5) | <0.001 | 2263 | (98.1) | 45 | (1.9) | <0.001 |
| Yes | 396 | (83.7) | 77 | (16.3) | 575 | (86.7) | 88 | (13.3) | |||
| Heart failure | No | 1942 | (93.1) | 144 | (6.9) | 0.002 | 2814 | (95.9) | 121 | (4.1) | <0.001 |
| Yes | 14 | (70.0) | 6 | (30.0) | 24 | (66.7) | 12 | (33.3) | |||
| Chronic cardiac disease | No | 1888 | (93.5) | 132 | (6.5) | <0.001 | 2762 | (95.7) | 123 | (4.3) | 0.004 |
| Yes | 68 | (79.1) | 18 | (20.9) | 76 | (88.4) | 10 | (11.6) | |||
| Asthma | No | 1914 | (92.9) | 146 | (7.1) | 0.565 | 2772 | (95.7) | 124 | (4.3) | 0.006 |
| Yes | 42 | (91.3) | 4 | (8.7) | 66 | (88.0) | 9 | (12.0) | |||
| Chronic obstructive pulmonary disease | No | 1938 | (93.1) | 144 | (6.9) | 0.005 | 2.827 | (95.9) | 122 | (4.1) | 0.004 |
| Yes | 18 | (75.0) | 6 | (25.0) | 17 | (60.7) | 11 | (39.3) | |||
| Chronic kidney disease | No | 1937 | (93.1) | 143 | (6.9) | 0.002 | 2805 | (95.5) | 132 | (4.5) | <0.001 |
| Yes | 19 | (73.1) | 7 | (26.9) | 33 | (97.1) | 1 | (2.9) | |||
| Chronic liver disease | No | 1916 | (93.0) | 144 | (7.0) | 0.137 | 2751 | (95.5) | 129 | (4.5) | 0.498 |
| Yes | 40 | (8.7) | 6 | (13.0) | 87 | (95.6) | 4 | (4.4) | |||
| Cancer | No | 1921 | (93.4) | 136 | (6.6) | <0.001 | 2751 | (95.5) | 129 | (4.5) | 0.858 |
| Yes | 35 | (71.4) | 14 | (28.6) | 87 | (95.6) | 4 | (4.4) | |||
| Rheumatic Autoimmune disease | No | 1947 | (93.0) | 147 | (7.0) | 0.048 | 2813 | (95.5) | 133 | (4.5) | 0.626 |
| Yes | 9 | (75.0) | 3 | (25.0) | 25 | (100.0) | - | - | |||
| Dementia | No | 1917 | (94.1) | 121 | (5.9) | <0.001 | 2734 | (97.0) | 86 | (3.0) | <0.001 |
| Yes | 39 | (57.4) | 29 | (42.6) | 104 | (68.9) | 47 | (31.1) | |||
| Variables | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude a | Adjusted b | Crude a | Adjusted b | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Underlying diseases (ref: No disease) | ||||||||
| Diabetes mellitus | 2.59 *** | (1.72–3.89) | 1.81 ** | (1.18–2.77) | 1.93 ** | (1.24–2.99) | 1.87 * | (1.15–3.02) |
| Hypertension | 2.59 *** | (1.76–3.81) | 1.20 | (0.79–1.82) | 4.06 *** | (2.66–6.20) | 1.73 * | (1.08–2.78) |
| Heart failure | 3.57 * | (1.20–10.61) | 1.78 | (0.55–5.74) | 3.19 ** | (1.34–7.59) | 2.04 | (0.82–5.04) |
| Chronic cardiac disease | 2.11 * | (1.14–3.92) | 1.36 | (0.68–2.72) | 1.09 | (0.48–2.45) | 1.01 | (0.42–2.46) |
| Asthma | 0.94 | (0.30–2.92) | 0.68 | (0.18–2.64) | 2.88 * | (1.22–6.76) | 1.78 | (0.70–4.48) |
| Chronic obstructive pulmonary disease | 3.31 * | (1.08–10.09) | 2.55 | (0.84–7.78) | 1.51 | (0.34–6.74) | 1.27 | (0.29–5.59) |
| Chronic kidney disease | 1.73 | (0.60–4.98) | 1.77 | (0.60–5.26) | 5.62 *** | (2.25–14.06) | 5.09 *** | (1.87–13.86) |
| Chronic liver disease | 1.09 | (0.41–2.88) | 1.22 | (0.45–3.32) | 0.54 | (0.07–4.19) | 0.56 | (0.07–4.46) |
| Cancer | 5.47 *** | (2.71–11.04) | 2.69 ** | (1.27–5.69) | 0.89 | (0.29–2.70) | 0.86 | (0.25–2.90) |
| Rheumatic/Autoimmune disease | 4.81 * | (1.16–19.98) | 6.69 ** | (1.60–27.98) | - | - | - | - |
| Dementia | 10.44 *** | (5.99–18.20) | 4.09 *** | (2.14–7.82) | 7.33 *** | (4.67–11.51) | 3.08 *** | (1.81–5.23) |
| Age (ref: 0–39) | ||||||||
| 40–69 | 8.80 *** | (13.81–127.38) | 2.21 | (0.74–6.56) | ||||
| ≥70 | 41.94 *** | 11.84 *** | (3.97–35.31) | |||||
| Body mass index (ref: Normal) | ||||||||
| Underweight | 1.32 | (0.43–4.07) | - | - | ||||
| Overweight | 1.08 | (0.57–2.04) | - | - | ||||
| Obese | 1.73 | (0.98–3.07) | - | - | ||||
| No-answer | 1.89* | (1.07–3.34) | - | - | ||||
| Heart rate (ref: Normal) | ||||||||
| Bradycardia | 0.55 | (0.10–3.11) | - | - | ||||
| Tachycardia | 2.21 ** | (1.37–3.57) | - | - | ||||
| Fever (Ref: No) | 2.53 *** | (1.68–3.82) | 2.27 *** | (1.43–3.60) | ||||
| Cough (Ref: No) | 0.88 | (0.54–1.42) | - | - | ||||
| Sputum (Ref: No) | 1.74 * | (1.06–2.86) | - | - | ||||
| According to our | - | - | 0.17 ** | (0.05–0.59) | ||||
| Rhinorrhea (Ref: No) | 0.35 * | (0.13–0.93) | - | - | ||||
| Myalgia (Ref: No) | - | - | 0.51 | (0.24–1.07) | ||||
| Dyspnea (Ref: No) | - | - | 6.43 *** | (4.07–10.15) | ||||
| Headache (Ref: No) | 0.90 | (0.47–1.72) | 0.36 * | (0.16–0.83) | ||||
| Altered consciousness (Ref: No) | 86.15 *** | (7.66–969.16) | 20.03 *** | (5.40–74.30) | ||||
| Nausea/Vomiting (Ref: No) | - | - | 0.70 | (0.28–1.76) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.; Kim, R.; Chung, W. Sex-Specific Association between Underlying Diseases and the Severity and Mortality Due to COVID-19 Infection: A Retrospective Observational Cohort Analysis of Clinical Epidemiological Information Collected by the Korea Disease Control and Prevention Agency. Healthcare 2022, 10, 1846. https://doi.org/10.3390/healthcare10101846
Oh H, Kim R, Chung W. Sex-Specific Association between Underlying Diseases and the Severity and Mortality Due to COVID-19 Infection: A Retrospective Observational Cohort Analysis of Clinical Epidemiological Information Collected by the Korea Disease Control and Prevention Agency. Healthcare. 2022; 10(10):1846. https://doi.org/10.3390/healthcare10101846
Chicago/Turabian StyleOh, Hwayeong, Roeul Kim, and Woojin Chung. 2022. "Sex-Specific Association between Underlying Diseases and the Severity and Mortality Due to COVID-19 Infection: A Retrospective Observational Cohort Analysis of Clinical Epidemiological Information Collected by the Korea Disease Control and Prevention Agency" Healthcare 10, no. 10: 1846. https://doi.org/10.3390/healthcare10101846
APA StyleOh, H., Kim, R., & Chung, W. (2022). Sex-Specific Association between Underlying Diseases and the Severity and Mortality Due to COVID-19 Infection: A Retrospective Observational Cohort Analysis of Clinical Epidemiological Information Collected by the Korea Disease Control and Prevention Agency. Healthcare, 10(10), 1846. https://doi.org/10.3390/healthcare10101846






