Training Cognitive Functions Using DUAL-REHAB, a New Dual-Task Application in MCI and SMC: A Study Protocol of a Randomized Control Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Protocol: DUAL-REHAB Training
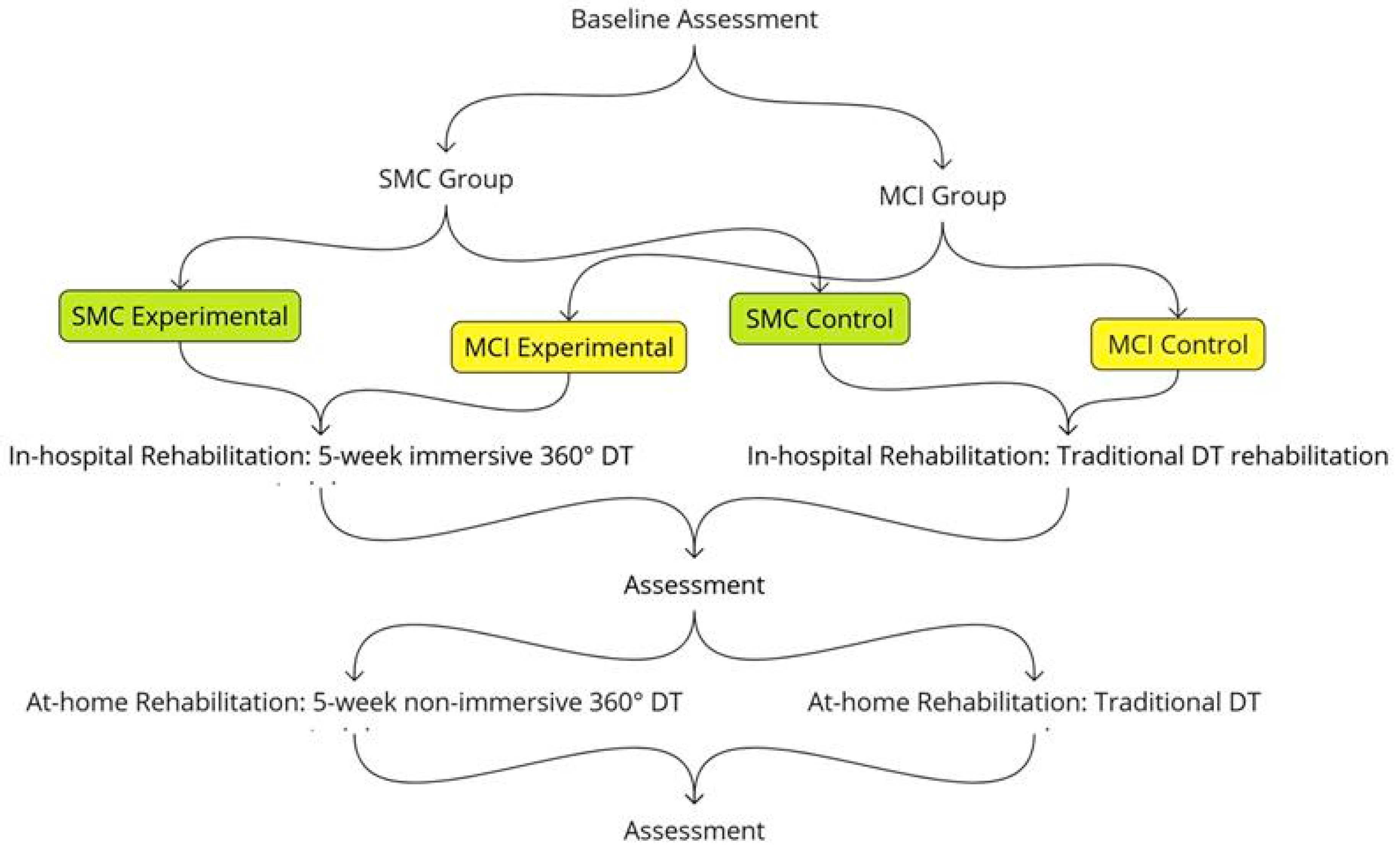
2.2. Study Design
2.3. Sample and Recruitment
2.4. Assessment Instruments
2.4.1. Measure of Cognitive Functions
- The Mini-Mental State Examination [41], a screening battery used to obtain information about general cognitive performance;
- The Digit Span [44], where participants listen to a series of digits read aloud and must repeat them in the same order;
- The Digit Span Backward [44], where the researcher reads a series of digits aloud to the participants, who are requested to repeat the same series of digits in the opposite sequence;
- The Corsi Span Test [44], measuring short-term spatial memory, where the researcher taps a sequence of blocks that the patient needs to reproduce afterwards, in the same order;
- Rey-Osterrieth complex figure [45], where the researcher asks the patient to copy a complex figure, then the patient has to recall the same one after 20 min;
- The Trail Making Test [46], which measures attention and ability of set-shifting and is composed of two parts: the first part (A) is a searching task, and the second part (B) requires shifting in alternatively searching numbers and letters;
- The Clock Drawing Test [47], a valid screening tool for evaluating cognitive decline including visuospatial abilities, executive function, and conceptual understanding, wherein, in this version of the test, participants are presented with a pre-drawn clock face and instructed to insert the numbers and draw the clock hands to indicate a specified time;
- The oral naming of nouns and verbs of E.N.P.A [48], where participants have to name the drawn element or action in tasks that each consist of 10 pictures;
- The Phonological, Semantic verbal fluency [49], a measure of language production;
- The Attentional Matrices Test [50], which is used for measuring selective attention, that is, the patient’s ability to detect visual targets among distractors;
- The Frontal Assessment Battery [51], a short cognitive and behavioral six-subtest battery for the screening of global executive dysfunction;
- Short-Story recall [45], which requires participants to memorize and recall a short story after a period of 20 min;
- The Stroop Colour Word Test [52], which is used in clinical practice to assess several abilities linked to the frontal lobe, such as selective attention, cognitive flexibility, and sensitivity to interference. It consists of three different parts: (i) Participants read color-word names printed in black ink, (ii) they name the color of neutral stimuli, and (iii) they must name the ink color of incongruent color words. This last condition generates the “Stroop effect,” a slowing in response times and increased errors due to interference between automatic reading and the required color-naming task;
- Dual-task performance [53], a test consisting of performing digit recall and tracking tasks separately and then simultaneously.
2.4.2. Measure of Motor Functioning
- The cognitive Timed Up & Go Test [54]: This is a test of balance traditionally used to evaluate functional mobility in frail older adults. The cognitive part requires participants to complete the test while counting backward by threes; in the motor part, patients have to stand up from a chair, walk for 3 m quickly, turn around, walk back, and sit down.
- The Timed 10-Meter Walk [55]: This is used for evaluating gait speed and functional mobility. The test measures the time required for participants to walk 10 m at their preferred walking speed along a straight path.
2.5. Statistical Analysis
3. Expected Results
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCI | Mild cognitive impairment |
| SMC | Subjective memory complaint |
| AD | Alzheimer’s Disease |
| DT | Dual-task |
| HMD | Head-mounted display |
| CDR | Clinical dementia rating |
References
- Aguzzoli, C.S.; Anstey, K.J.; Atri, A.; Barbarino, P.; Benoist, C.; Brijnath, B.; Bruno, M.A.; Cose, L.; Darge, D.; Dean, W.; et al. World Alzheimer Report 2024 Global Changes in Attitudes to Dementia Contributors: Survey Translators; Alzheimer’s Disease International: London, UK, 2024. [Google Scholar]
- Moustafa, A.A.; Tindle, R.; Alashwal, H.; Diallo, T.M.O. A Longitudinal Study Using Latent Curve Models of Groups with Mild Cognitive Impairment and Alzheimer’s Disease. J. Neurosci. Methods 2021, 350, 109040. [Google Scholar] [CrossRef] [PubMed]
- Moustafar, A. Alzheimer’s Disease Understanding Biomarkers, Big Data, and Therapy, 1st ed.; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Aisen, P.S.; Cummings, J.; Jack, C.R.J.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the Path to 2025: Understanding the Alzheimer’s Disease Continuum. Alzheimers Res Ther 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.J.; Holtzman, D.M. Biomarker Modeling of Alzheimer’s Disease. Neuron 2013, 80, 1347–1358. [Google Scholar] [CrossRef]
- Weintraub, S.; Carrillo, M.C.; Farias, S.T.; Goldberg, T.E.; Hendrix, J.A.; Jaeger, J.; Knopman, D.S.; Langbaum, J.B.; Park, D.C.; Ropacki, M.T.; et al. Measuring Cognition and Function in the Preclinical Stage of Alzheimer’s Disease. Alzheimers Dement. 2018, 4, 64–75. [Google Scholar] [CrossRef]
- Park, J.-H. Effects of Cognitive-Physical Dual-Task Training on Executive Function and Activity in the Prefrontal Cortex of Older Adults with Mild Cognitive Impairment. Brain NeuroRehabil. 2021, 14, e23. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Chen, T.-F.; Chiu, M.-J. From Mild Cognitive Impairment to Subjective Cognitive Decline: Conceptual and Methodological Evolution. Neuropsychiatr. Dis. Treat. 2017, 13, 491–498. [Google Scholar] [CrossRef]
- Tuena, C.; Mancuso, V.; Stramba-Badiale, C.; Pedroli, E.; Stramba-Badiale, M.; Riva, G.; Repetto, C. Egocentric and Allocentric Spatial Memory in Mild Cognitive Impairment with Real-World and Virtual Navigation Tasks: A Systematic Review. J. Alzheimers Dis. 2021, 79, 95–116. [Google Scholar] [CrossRef]
- Gallou-Guyot, M.; Mandigout, S.; Combourieu-Donnezan, L.; Bherer, L.; Perrochon, A. Cognitive and Physical Impact of Cognitive-Motor Dual-Task Training in Cognitively Impaired Older Adults: An Overview. Neurophysiol. Clin. 2020, 50, 441–453. [Google Scholar] [CrossRef]
- Cappa, S.F.; Ribaldi, F.; Chicherio, C.; Frisoni, G.B. Subjective Cognitive Decline: Memory Complaints, Cognitive Awareness, and Metacognition. Alzheimer’s Dement. 2024, 20, 6622–6631. [Google Scholar] [CrossRef]
- Warren, S.L.; Reid, E.; Whitfield, P.; Moustafa, A.A. Subjective Memory Complaints as a Predictor of Mild Cognitive Impairment and Alzheimer’s Disease. Discov. Psychol. 2022, 2, 13. [Google Scholar] [CrossRef]
- Metternich, B.; Kosch, D.; Kriston, L.; Härter, M.; Hüll, M. The Effects of Nonpharmacological Interventions on Subjective Memory Complaints: A Systematic Review and Meta-Analysis. Psychother. Psychosom. 2010, 79, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Zapater-Fajarí, M.; Crespo-Sanmiguel, I.; Pérez, V.; Hidalgo, V.; Salvador, A. Subjective Memory Complaints in Young and Older Healthy People: Importance of Anxiety, Positivity, and Cortisol Indexes. Pers. Individ. Dif. 2022, 197, 111768. [Google Scholar] [CrossRef]
- Smith, L.; Shin, J.I.; Song, T.-J.; Underwood, B.R.; Jacob, L.; López Sánchez, G.F.; Schuch, F.; Oh, H.; Veronese, N.; Soysal, P.; et al. Association between Depression and Subjective Cognitive Complaints in 47 Low- and Middle-Income Countries. J. Psychiatr. Res. 2022, 154, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Pérez Palmer, N.; Trejo Ortega, B.; Joshi, P. Cognitive Impairment in Older Adults: Epidemiology, Diagnosis, and Treatment. Psychiatr. Clin. N. Am. 2022, 45, 639–661. [Google Scholar] [CrossRef]
- Sukontapol, C.; Kemsen, S.; Chansirikarn, S.; Nakawiro, D.; Kuha, O.; Taemeeyapradit, U. The Effectiveness of a Cognitive Training Program in People with Mild Cognitive Impairment: A Study in Urban Community. Asian J. Psychiatr. 2018, 35, 18–23. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, X.-X.; Chen, L.; Liu, Y.; Li, Y.-R. A Systematic Review and Network Meta-Analysis Comparing Various Non-Pharmacological Treatments for Older People with Mild Cognitive Impairment. Asian J. Psychiatr. 2023, 86, 103635. [Google Scholar] [CrossRef]
- Pereira Oliva, H.N.; Mansur Machado, F.S.; Rodrigues, V.D.; Leão, L.L.; Monteiro-Júnior, R.S. The Effect of Dual-Task Training on Cognition of People with Different Clinical Conditions: An Overview of Systematic Reviews. IBRO Rep. 2020, 9, 24–31. [Google Scholar] [CrossRef]
- Ali, N.; Tian, H.; Thabane, L.; Ma, J.; Wu, H.; Zhong, Q.; Gao, Y.; Sun, C.; Zhu, Y.; Wang, T. The Effects of Dual-Task Training on Cognitive and Physical Functions in Older Adults with Cognitive Impairment; A Systematic Review and Meta-Analysis. J. Prev. Alzheimers Dis. 2022, 9, 359–370. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.-H. Does Cognitive-Physical Dual-Task Training Have Better Clinical Outcomes than Cognitive Single-Task Training Does? A Single-Blind, Randomized Controlled Trial. Healthcare 2023, 11, 1544. [Google Scholar] [CrossRef]
- Fritz, N.E.; Cheek, F.M.; Nichols-Larsen, D.S. Motor-Cognitive Dual-Task Training in Persons With Neurologic Disorders: A Systematic Review. J. Neurol. Phys. Ther. 2015, 39, 142–153. [Google Scholar] [CrossRef]
- Norouzi, E.; Vaezmosavi, M.; Gerber, M.; Pühse, U.; Brand, S. Dual-Task Training on Cognition and Resistance Training Improved Both Balance and Working Memory in Older People. Phys. Sportsmed. 2019, 47, 471–478. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, T.L.; Lamberg, E.M.; Muratori, L.M. Building a Framework for a Dual Task Taxonomy. Biomed. Res. Int. 2015, 2015, 591475. [Google Scholar] [CrossRef] [PubMed]
- Gallou-Guyot, M.; Mandigout, S.; Bherer, L.; Perrochon, A. Effects of Exergames and Cognitive-Motor Dual-Task Training on Cognitive, Physical and Dual-Task Functions in Cognitively Healthy Older Adults: An Overview. Ageing Res. Rev. 2020, 63, 101135. [Google Scholar] [CrossRef]
- Gheysen, F.; Poppe, L.; DeSmet, A.; Swinnen, S.; Cardon, G.; De Bourdeaudhuij, I.; Chastin, S.; Fias, W. Physical Activity to Improve Cognition in Older Adults: Can Physical Activity Programs Enriched with Cognitive Challenges Enhance the Effects? A Systematic Review and Meta-Analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 63. [Google Scholar] [CrossRef]
- Versi, N.; Murphy, K.; Robinson, C.; Franklin, M. Simultaneous Dual-Task Interventions That Improve Cognition in Older Adults: A Scoping Review of Implementation-Relevant Details. J. Aging Res. 2022, 2022, 6686910. [Google Scholar] [CrossRef]
- Franzen, M.D.; Wilhelm, K.L. Conceptual Foundations of Ecological Validity in Neuropsychological Assessment. In Ecological Validity of Neuropsychological Testing; Sbordone, R.J., Long, C.J., Eds.; Gr Press/St Lucie Press, Inc.: Delray Beach, FL, USA, 1996; pp. 91–112. [Google Scholar]
- Mellinger, C.D.; Hanson, T.A. Considerations of Ecological Validity in Cognitive Translation and Interpreting Studies. Transl. Cogn. Behav. 2022, 5, 1–26. [Google Scholar] [CrossRef]
- Burgess, P.W.; Alderman, N.; Forbes, C.; Costello, A.; Coates, L.M.-A.; Dawson, D.R.; Anderson, N.D.; Gilbert, S.J.; Dumontheil, I.; Channon, S. The Case for the Development and Use of “Ecologically Valid” Measures of Executive Function in Experimental and Clinical Neuropsychology. J. Int. Neuropsychol. Soc. 2006, 12, 194–209. [Google Scholar] [CrossRef]
- Borghesi, F.; Mancuso, V.; Pedroli, E.; Cipresso, P. From virtual reality to 360 videos: Upgrade or downgrade? The multidimensional healthcare VR technology. In Handbook of Research on Implementing Digital Reality and Interactive Technologies to Achieve Society 5.0; Cipresso, P., Ed.; IGI Global: Hershey, PA, USA, 2022; pp. 549–572. [Google Scholar] [CrossRef]
- Riva, G.; Mancuso, V.; Cavedoni, S.; Stramba-Badiale, C. Virtual Reality in Neurorehabilitation: A Review of Its Effects on Multiple Cognitive Domains. Expert Rev. Med. Devices 2020, 17, 1035–1061. [Google Scholar] [CrossRef]
- Mancuso, V.; Bruni, F.; Stramba-Badiale, C.; Riva, G.; Cipresso, P.; Pedroli, E. How Do Emotions Elicited in Virtual Reality Affect Our Memory? A Systematic Review. Comput. Hum. Behav. 2023, 146, 107812. [Google Scholar] [CrossRef]
- Parsons, C.E.; Crane, C.; Parsons, L.J.; Fjorback, L.O.; Kuyken, W. Home Practice in Mindfulness-Based Cognitive Therapy and Mindfulness-Based Stress Reduction: A Systematic Review and Meta-Analysis of Participants’ Mindfulness Practice and Its Association with Outcomes. Behav. Res. Ther. 2017, 95, 29–41. [Google Scholar] [CrossRef]
- Serino, S.; Repetto, C. New Trends in Episodic Memory Assessment: Immersive 360° Ecological Videos. Front. Psychol. 2018, 9, 1878. [Google Scholar] [CrossRef] [PubMed]
- Horr, T.; Messinger-Rapport, B.; Pillai, J.A. Systematic Review of Strengths and Limitations of Randomized Controlled Trials for Non-Pharmacological Interventions in Mild Cognitive Impairment: Focus on Alzheimer’s Disease. J. Nutr. Health Aging 2015, 19, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Ribarič, S. Physical Exercise, a Potential Non-Pharmacological Intervention for Attenuating Neuroinflammation and Cognitive Decline in Alzheimer’s Disease Patients. Int. J. Mol. Sci. 2022, 23, 3245. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.S.; Mauser, J.; Nuno, M.; Sherzai, D. The Efficacy of Cognitive Intervention in Mild Cognitive Impairment (MCI): A Meta-Analysis of Outcomes on Neuropsychological Measures. Neuropsychol. Rev. 2017, 27, 440–484. [Google Scholar] [CrossRef]
- Chan, W.C.; Yeung, J.W.F.; Wong, C.S.M.; Lam, L.C.W.; Chung, K.F.; Luk, J.K.H.; Lee, J.S.W.; Law, A.C.K. Efficacy of Physical Exercise in Preventing Falls in Older Adults with Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 149–154. [Google Scholar] [CrossRef]
- Abdulrab, K.; Heun, R. Subjective Memory Impairment. A Review of Its Definitions Indicates the Need for a Comprehensive Set of Standardised and Validated Criteria. Eur. Psychiatry 2008, 23, 321–330. [Google Scholar] [CrossRef]
- Magni, E.; Binetti, G.; Bianchetti, A.; Rozzini, R.; Trabucchi, M. Study in Italian Elderly Population. Eur. J. Neurol. 1996, 3, 198–202. [Google Scholar] [CrossRef]
- Morris, J.C. Clinical Dementia Rating: A Reliable and Valid Diagnostic and Staging Measure for Dementia of the Alzheimer Type. Int. Psychogeriatr. 1997, 9 (Suppl. S1), 173–178. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and Backward Span for Verbal and Visuo-Spatial Data: Standardization and Normative Data from an Italian Adult Population. Neurol. Sci. 2013, 34, 749–754. [Google Scholar] [CrossRef]
- Carlesimo, G.; Buccione, I.; Fadda, L.; Graceffa, A.; Mauri, M.; Lorusso, S.; Bevilacqua, G.; Caltagirone, C. Normative Data of Two Memory Tasks: Short-Story Recall and Rey’s Figure. Nuova Riv. Neurol. 2002, 12, 1–13. [Google Scholar]
- Siciliano, M.; Chiorri, C.; Battini, V.; Sant’Elia, V.; Altieri, M.; Trojano, L.; Santangelo, G. Regression-Based Normative Data and Equivalent Scores for Trail Making Test (TMT): An Updated Italian Normative Study. Neurol. Sci. 2019, 40, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Caffarra, P.; Gardini, S.; Zonato, F.; Concari, L.; Dieci, F.; Copelli, S.; Freedman, M.; Stracciari, A.; Venneri, A. Italian Norms for the Freedman Version of the Clock Drawing Test. J. Clin. Exp. Neuropsychol. 2011, 33, 982–988. [Google Scholar] [CrossRef]
- Capasso, R.; Miceli, G. Esame Neuropsicologico per l’Afasia: ENPA; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; Volume 4. [Google Scholar]
- Costa, A.; Bagoj, E.; Monaco, M.; Zabberoni, S.; De Rosa, S.; Papantonio, A.M.; Mundi, C.; Caltagirone, C.; Carlesimo, G.A. Standardization and Normative Data Obtained in the Italian Population for a New Verbal Fluency Instrument, the Phonemic/Semantic Alternate Fluency Test. Neurol. Sci. 2014, 35, 365–372. [Google Scholar] [CrossRef]
- Sala, S.D.; Laiacona, M.; Spinnler, H.; Ubezio, C. A Cancellation Test: Its Reliability in Assessing Attentional Deficits in Alzheimer’s Disease. Psychol. Med. 1992, 22, 885–901. [Google Scholar] [CrossRef]
- Appollonio, I.; Piamarta, M.L.; Isella, V.; Villa, F.; Consoli, T.; Nichelli, M.L.; Russo, A. The Frontal Assessment Battery (FAB): Normative Values in an Italian Population Sample. Neurol. Sci. 2005, 26, 108–116. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Francesca, D.; Zonato, F.; Venneri, A. A Short Version of the Stroop Test: Normative Data in an Italian Population Sample. Nuova Riv. Neurol. 2002, 12, 111–115. [Google Scholar]
- Della Sala, S.; Foley, J.A.; Beschin, N.; Allerhand, M.; Logie, R.H. Assessing Dual-Task Performance Using a Paper-and-Pencil Test: Normative Data. Arch. Clin. Neuropsychol. 2010, 25, 410–419. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Bohannon, R.W. Comfortable and Maximum Walking Speed of Adults Aged 20-79 Years: Reference Values and Determinants. Age Ageing 1997, 26, 15–19. [Google Scholar] [CrossRef]
- De Girolamo, G.; Rucci, P.; Scocco, P.; Becchi, A.; Coppa, F.; D’Addario, A.; Daru, E.; De Leo, D.; Galassi, L.; Mangelli, L. Quality of Life Assessment: Validation of the Italian Version of the WHOQOL-Brief. Epidemiol. Psychiatr. Sci. 2000, 9, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Optale, G.; Urgesi, C.; Busato, V.; Marin, S.; Piron, L.; Priftis, K.; Gamberini, L.; Capodieci, S.; Bordin, A. Controlling Memory Impairment in Elderly Adults Using Virtual Reality Memory Training: A Randomized Controlled Pilot Study. Neurorehabil. Neural Repair. 2010, 24, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, V.; Borghesi, F.; Bruni, F.; Pedroli, E.; Cipresso, P. Mapping the Landscape of Research on 360-Degree Videos and Images: A Network and Cluster Analysis. Virtual Real. 2024, 28, 101. [Google Scholar] [CrossRef]
- Pedroli, E.; Greci, L.; Colombo, D.; Serino, S.; Cipresso, P.; Arlati, S.; Mondellini, M.; Boilini, L.; Giussani, V.; Goulene, K.; et al. Characteristics, Usability, and Users Experience of a System Combining Cognitive and Physical Therapy in a Virtual Environment: Positive Bike. Sensors 2018, 18, 2343. [Google Scholar] [CrossRef]
- Parsons, T.D. Virtual Reality for Enhanced Ecological Validity and Experimental Control in the Clinical, Affective and Social Neurosciences. Front. Hum. Neurosci. 2015, 9, 660. [Google Scholar] [CrossRef]
- Bruni, F.; Mancuso, V.; Greci, L.; Arlati, S.; Cavallo, M.; Riva, G.; Goulene, K.; Stramba-Badiale, M.; Pedroli, E. A Cross-Platform Application for the Ecological and Remote Assessment of Memory Impairment in Aging: ECO-MEMORY. Virtual Real. 2023, 27, 2757–2767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroli, E.; Bruni, F.; Mancuso, V.; Cavedoni, S.; Bigotto, F.; Panigada, J.; Rossi, M.; Boilini, L.; Goulene, K.; Stramba-Badiale, M.; et al. Training Cognitive Functions Using DUAL-REHAB, a New Dual-Task Application in MCI and SMC: A Study Protocol of a Randomized Control Trial. Technologies 2025, 13, 96. https://doi.org/10.3390/technologies13030096
Pedroli E, Bruni F, Mancuso V, Cavedoni S, Bigotto F, Panigada J, Rossi M, Boilini L, Goulene K, Stramba-Badiale M, et al. Training Cognitive Functions Using DUAL-REHAB, a New Dual-Task Application in MCI and SMC: A Study Protocol of a Randomized Control Trial. Technologies. 2025; 13(3):96. https://doi.org/10.3390/technologies13030096
Chicago/Turabian StylePedroli, Elisa, Francesca Bruni, Valentina Mancuso, Silvia Cavedoni, Francesco Bigotto, Jonathan Panigada, Monica Rossi, Lorenzo Boilini, Karine Goulene, Marco Stramba-Badiale, and et al. 2025. "Training Cognitive Functions Using DUAL-REHAB, a New Dual-Task Application in MCI and SMC: A Study Protocol of a Randomized Control Trial" Technologies 13, no. 3: 96. https://doi.org/10.3390/technologies13030096
APA StylePedroli, E., Bruni, F., Mancuso, V., Cavedoni, S., Bigotto, F., Panigada, J., Rossi, M., Boilini, L., Goulene, K., Stramba-Badiale, M., & Serino, S. (2025). Training Cognitive Functions Using DUAL-REHAB, a New Dual-Task Application in MCI and SMC: A Study Protocol of a Randomized Control Trial. Technologies, 13(3), 96. https://doi.org/10.3390/technologies13030096





