Characterization of Triplet State of Cyanine Dyes with Two Chromophores Effect of Molecule Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of the T1 State Quantum Yield
2.2. Determination of the BCD T1 State Energy
3. Results and Discussion
3.1. Determination of the BCD T1 State Quantum Yields (φT)
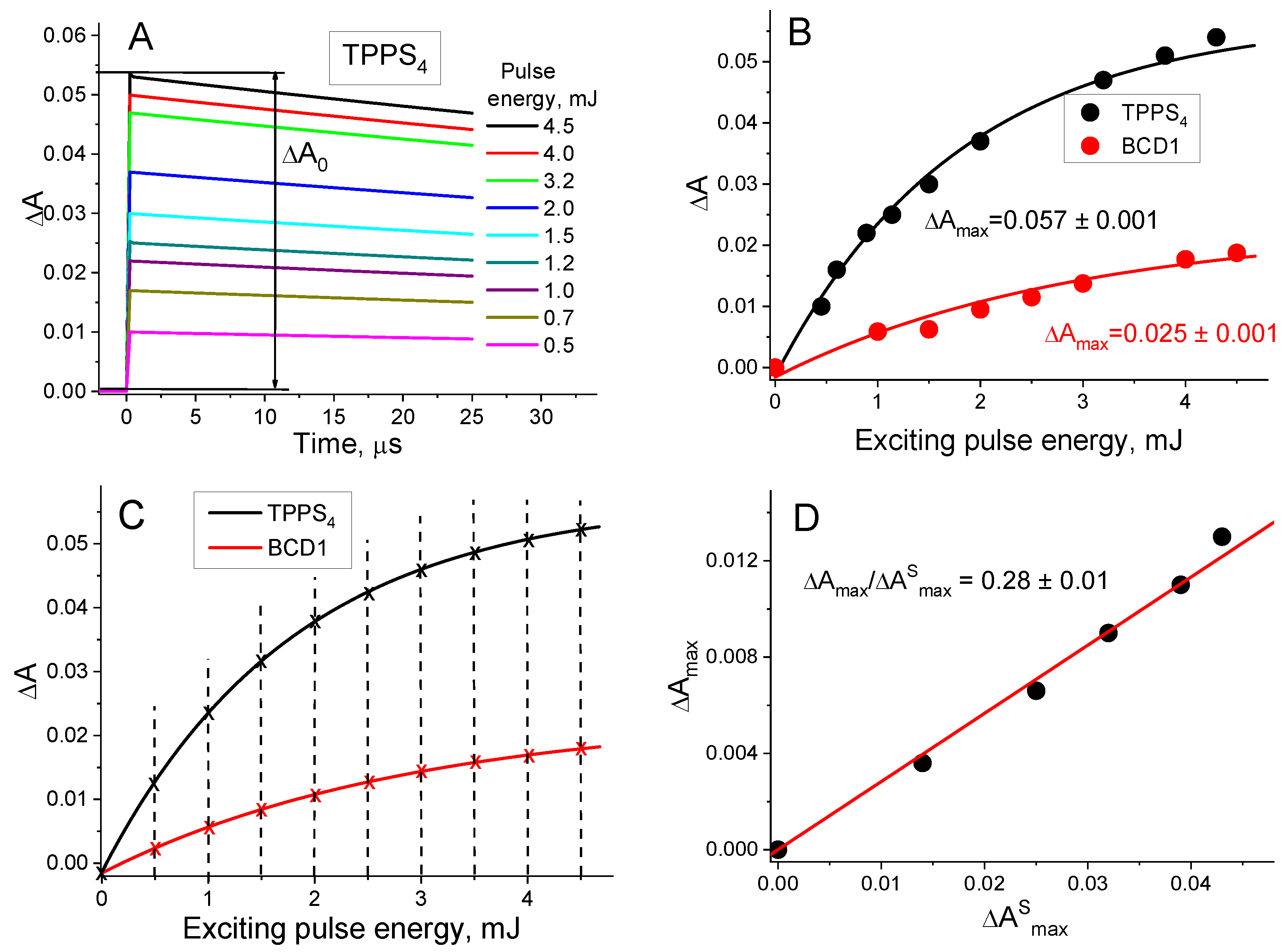
- In the first step, the amplitudes of the T1 state decay kinetic curves of the BCD and of the standard compound (ΔA0 and ) were measured for various exciting pulse energies (E) (Figure 2A).
- In the second step, the dependences of ΔA0 and on E were fitted in accordance with the mono-exponential equation (Figure 2B). From this fitting, the and values were obtained.
- In the third step, the obtained fittings were used to determine ΔA0 and for the same E values (Figure 2C).
- In the fourth step, the dependence of ΔA0 on was constructed to determine the average value (Figure 2D).
3.2. Determination of the BCD T1 State Energy (ET)
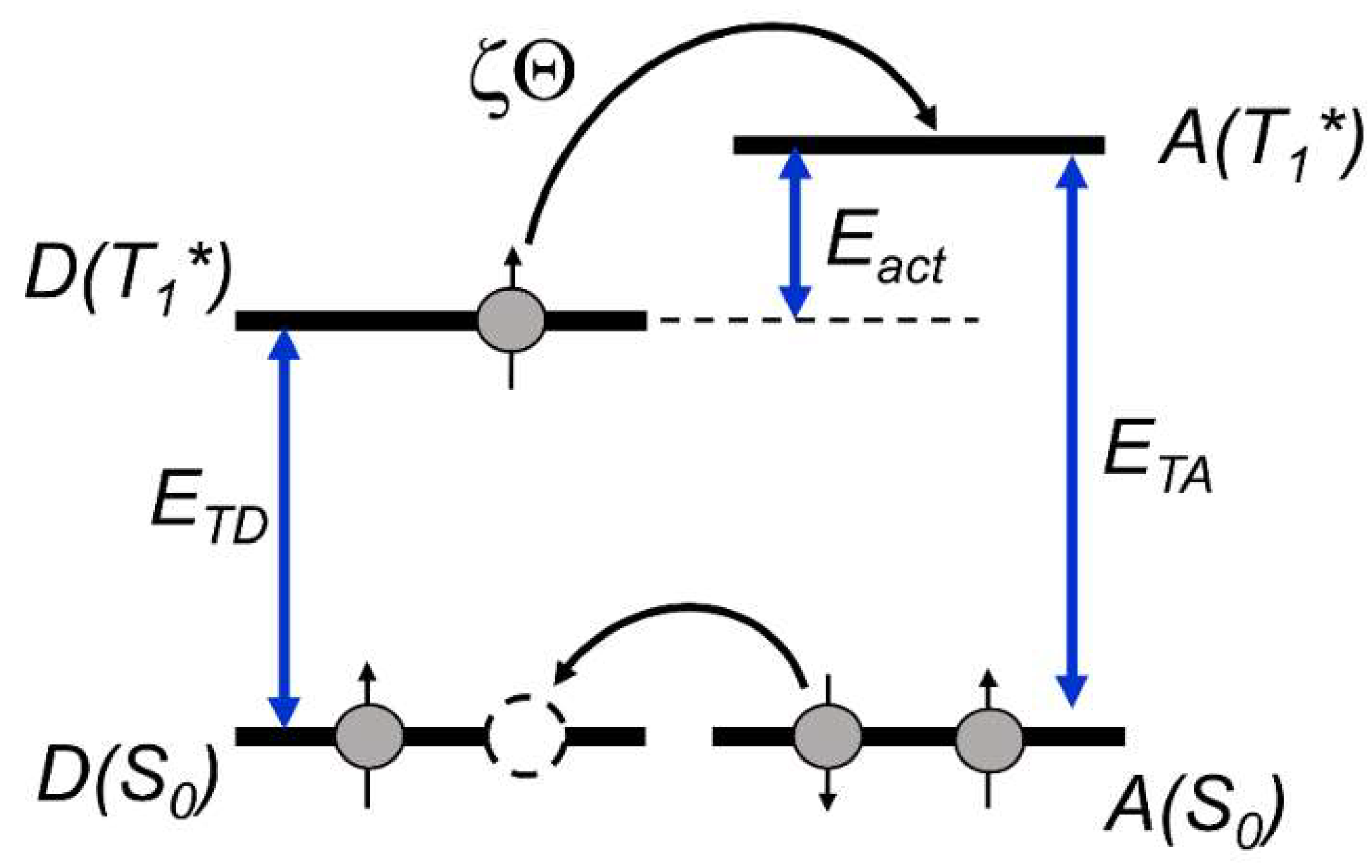
- In the first step, the quenching constants of the BCD T1 state by azulene (kq) at different temperatures were determined (Figure 3A).
- In the second step, the dependence of ln(kq) on the reverse absolute temperature (1/Θ) was constructed to determine the activation energy (Eact), which is necessary to realize the energy transfer from the BCD T1 state to azulene (Figure 3B).
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shindy, H.A. Fundamentals in the chemistry of cyanine dyes: A review. Dyes Pigm. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Mustroph, H.; Reiner, K.; Mistol, J.; Ernst, S.; Keil, D.; Hennig, L. Relationship between the Molecular Structure of Cyanine Dyes and the Vibrational Fine Structure of their Electronic Absorption Spectra. Chem. Phys. Chem. 2009, 10, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shi, L.; Zhang, B.; Liu, L.; Fu, Y.; Zhang, X. Recent advances in bioprobes and biolabels based on cyanine dyes. Anal. Bioanal. Chem. 2022, 414, 4551–4573. [Google Scholar] [CrossRef] [PubMed]
- Levitus, M.; Ranjit, S. Cyanine dyes in biophysical research: The photophysics of polymethine fluorescent dyes in biomolecular environments. Quart. Ver. Biophys. 2011, 44, 123–151. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Rönicke, F.; Schepers, U.; Wagenknecht, H.A. A new structure-activity relationship for cyanine dyes to improve photostability and fluorescence properties for live cell imaging. Chem. Sci. 2018, 9, 6557–6563. [Google Scholar] [CrossRef]
- Lange, N.; Szlasa, W.; Saczko, J.; Chwiłkowska, A. Potential of Cyanine Derived Dyes in Photodynamic Therapy. Pharmaceutics 2021, 13, 818. [Google Scholar] [CrossRef]
- Kiprianov, A.I. Absorption Spectra of Organic Dyes Containing Two Chromophores. Russ. Chem. Ver. 1971, 40, 594–607. [Google Scholar] [CrossRef]
- Kiprianov, A.I.; Dyadyusha, G.G. Absorption spectra of organic dyes containing two conjugated chromophores in molecule. Ukr. Chem. J. 1969, 35, 608. (In Russian) [Google Scholar]
- Borisevich, Y.E. Influence of resonance interaction on absorption spectra of dyes with two chromophores. Proc. Acad. Sci. USSR 1978, 4–6, 743–746. [Google Scholar]
- Schaberle, F.A.; Galembeck, S.E.; Borissevitch, I.E. Computational study of steric and spectroscopic characteristics of bi-chromophoric cyanine dyes: Comparison with experimental data. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 72, 863–867. [Google Scholar] [CrossRef]
- Borisevich, Y.E.; Kuz´min, V.A.; Mikhailenko, F.A.; Dyadyusha, G.G. Triplet state of bis-cyanine dyes. Proc. Acad. Sci. USSR 1976, 228, 448–451. [Google Scholar]
- Egorov, A.E.; Kostyukov, A.A.; Borissevitch, I.E.; Kuzmin, V.A. Photoinduced electron transfer from electron donor to bis-carbocyanine dye in excited triplet state. Mend. Commun. 2021, 31, 68–69. [Google Scholar] [CrossRef]
- Murakami, L.S.; Ferreira, L.P.; Santos, J.S.; da Silva, R.S.; Nomizo, A.; Kuz’min, V.A.; Borissevitch, I.E. Photocytotoxicity of a cyanine dye with two chromophores toward melanoma and normal cells. Biochim. Biophys. Acta (BBA) Gen. Sub. 2015, 1850, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Silva, É.R.; Pavanelli, A.L.S.; Mostaço, L.B.; Schaberle, F.A.; Galembeck, S.E.; Gonçalves, P.J.; Silva, R.C.E.; Ferreira, L.P.; Nekipelova, T.D.; Kostyukov, A.A.; et al. Phototransformation of cyanine dye with two chromophores. Effects of oxygen and dye concentration. J. Photochem. Photobiol. A Chem. 2017, 349, 42–48. [Google Scholar] [CrossRef]
- Borisevich, Y.E.; Kuzmin, V.A.; Dyadyusha, G.G.; Mikhailenko, F.A. Effect of splitting the triplet levels of biscyanin dyes. Proc. Acad. Sci. USSR 1976, 229, 616–619. [Google Scholar]
- Kiprianov, A.I.; Mikhailenko, F.A.; Mushkalo, I.L. Synthesis of centre-symmetrical dimethylbenzobisthiazole. Khim. Geterotsikl. Soedin. 1970, 10, 1435–1443. [Google Scholar]
- Borissevitch, I.E.; Silveira-Alves, E., Jr.; Almeida, C.G.L.; Souza, G.R.L.; Sokolov, S.S.; Gonçalves, P.J. An Alternative Method to Determine the Quantum Yield of the Excited Triplet State Using Laser Flash Photolysis. Photonics 2023, 10, 409. [Google Scholar] [CrossRef]
- Gonçalves, P.J.; Aggarwal, L.P.F.; Marquezin, C.A.; Ito, A.S.; De Boni, L.; Barbosa Neto, N.M.; Rodrigues, J.J.; Zílio, S.C.; Borissevitch, I.E. Effects of interaction with CTAB micelles on photophysical characteristics of meso-tetrakis(sulfonatophenyl) porphyrin. J. Photochem. Photobiol. A Chem. 2006, 181, 378–384. [Google Scholar] [CrossRef]
- Grewer, C.; Brauer, H.D. Mechanism of the Triplet-State Quenching by Molecular Oxygen in Solution. J. Phys. Chem. 1994, 98, 4230–4235. [Google Scholar] [CrossRef]
- Wilkinson, F.; McGarvey, D.J.; Olea, A.F. Excited Triplet State Interactions with Molecular Oxygen: Influence of Charge Transfer on the Bimolecular Quenching Rate Constants and the Yields of Singlet Oxygen () for Substituted Naphthalenes in Various Solvents. J. Phys. Chem. 1994, 98, 3762–3769. [Google Scholar] [CrossRef]
- Porter, G.; Topp, M.R. Nanosecond flash photolysis. Proc. R. Soc. Lond. 1970, A315, 163–184. [Google Scholar] [CrossRef]
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Modern Molecular Photochemistry of Organic Molecules; University Science Books: Sausalito, CA, USA, 2010. [Google Scholar] [CrossRef]
- Skourtis, S.S.; Liu, C.; Antoniou, P.; Beratan, D.N. Dexter energy transfer pathways. Proc. Nat. Acad. Sci. USA 2016, 113, 8115–8120. [Google Scholar] [CrossRef]
- Herkstroeter, W.G. Triplet energies of azulene,.beta.-carotene, and ferrocene. J. Am. Chem. Soc. 1975, 97, 4161–4167. [Google Scholar] [CrossRef]
- Kray, H.-J.; Nickel, B. Triplet lifetime and delayed fluorescence of azulene. Chem. Phys. 1980, 53, 235–241. [Google Scholar] [CrossRef]
- McRae, E.G.; Kasha, M. Enhancement of Phosphorescence Ability upon Aggregation of Dye Molecules. J. Chem. Phys. 1958, 28, 721–722. [Google Scholar] [CrossRef]
- Kasha, M. Molecular Excitons in Small Aggregates. In Spectroscopy of the Excited State; Di Bartolo, B., Pacheco, D., Goldberg, V., Eds.; NATO Advanced Study Institutes Series; Springer: Boston, MA, USA, 1976; Volume 12. [Google Scholar] [CrossRef]
- Kasha, M. Energy Transfer, Charge Transfer, and Proton Transfer in Molecular Composite Systems. In Physical and Chemical Mechanisms in Molecular Radiation Biology. Basic Life Sciences; Glass, W.A., Varma, M.N., Eds.; Springer: Boston, MA, USA, 1991; Volume 58. [Google Scholar]
- Dietz, F.; Rentsch, S.K. On the mechanism of photoisomerization and the structure of the photoisomers of cyanine dyes. Chem. Phys. 1985, 96, 145–151. [Google Scholar] [CrossRef]
- Sundstroem, V.; Gillbro, T. Viscosity-dependent isomerization yields of some cyanine dyes. A picosecond laser spectroscopy study. J. Phys. Chem. 1982, 86, 1788–1794. [Google Scholar] [CrossRef]
- Schaberle, F.A.; Kuz’min, V.A.; Borissevitch, I.E. Spectroscopic studies of the interaction of bichromophoric cyanine dyes with DNA. Effect of ionic strength. Biochim. Biophys. Acta (BBA) Gen. Sub. 2003, 1621, 183–191. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy and the development of metal-based photosensitisers. Met. Based Drugs 2008, 2008, 276109. [Google Scholar] [CrossRef] [PubMed]
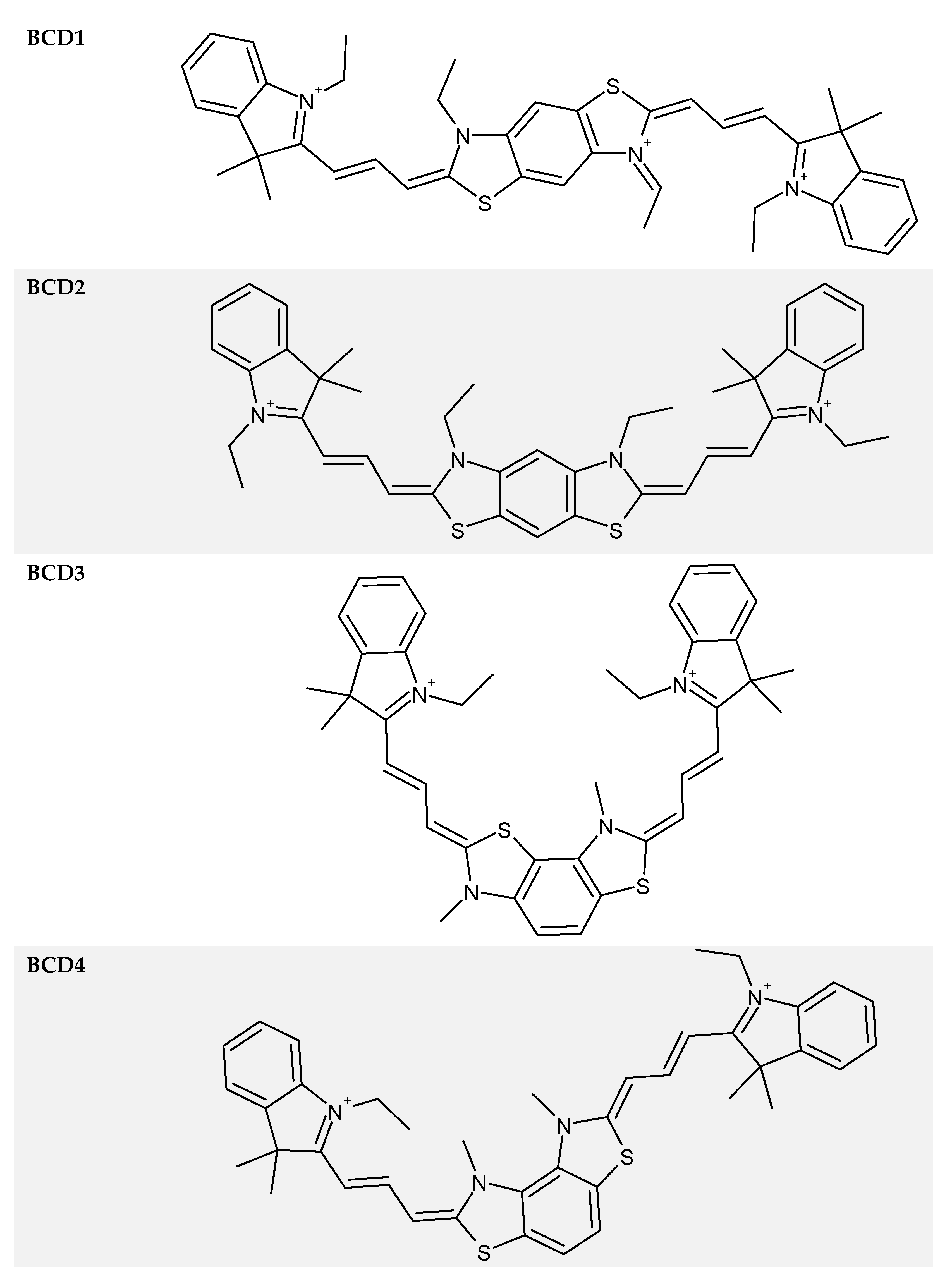

| Dye | α | φT | ET, cm−1 | ΔET, cm−1 [8] | ES, cm−1 [7] | ΔES-T, cm−1 |
|---|---|---|---|---|---|---|
| BCD1 | 180 | 0.21 ± 0.02 | 11,800 ± 200 | 3700 ± 100 | 15,270 ± 10 | 3500 ± 100 |
| BCD2 | 151 ± 2 | 0.18 ± 0.02 | 12,000 ± 200 | 3600 ± 100 | 15,630 ± 10 | 3600 ± 100 |
| BCD3 | 91 ± 1 | 0.08 ± 0.02 | 12,400 ± 300 | 3500 ± 100 | 15,720 ± 10 | 3300 ± 100 |
| BCD4 | 123 ± 4 | 0.04 ± 0.03 | 12,900 ± 400 | 3300 ± 100 | 16,530 ± 10 | 3600 ± 100 |
| Dye | α° | λ1, nm | ε1 × 10−5, M−1cm−1 | λ2, nm | ε2 × 10−5, M−1cm−1 |
|---|---|---|---|---|---|
| BCD1 | 180 | -- | -- | 655 | 2.8 |
| BCD2 | 151 ± 2 | 525 | 0.2 | 640 | 2.4 |
| BCD3 | 91 ± 1 | 520 | 1.5 | 634 | 1.3 |
| BCD4 | 123 ± 4 | 525 | 0.6 | 605 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borissevitch, I.E.; Gonçalves, P.J.; Ferreira, L.P.; Kostyukov, A.A.; Kuzmin, V.A. Characterization of Triplet State of Cyanine Dyes with Two Chromophores Effect of Molecule Structure. Technologies 2023, 11, 90. https://doi.org/10.3390/technologies11040090
Borissevitch IE, Gonçalves PJ, Ferreira LP, Kostyukov AA, Kuzmin VA. Characterization of Triplet State of Cyanine Dyes with Two Chromophores Effect of Molecule Structure. Technologies. 2023; 11(4):90. https://doi.org/10.3390/technologies11040090
Chicago/Turabian StyleBorissevitch, Iouri E., Pablo J. Gonçalves, Lucimara P. Ferreira, Alexey A. Kostyukov, and Vladimir A. Kuzmin. 2023. "Characterization of Triplet State of Cyanine Dyes with Two Chromophores Effect of Molecule Structure" Technologies 11, no. 4: 90. https://doi.org/10.3390/technologies11040090
APA StyleBorissevitch, I. E., Gonçalves, P. J., Ferreira, L. P., Kostyukov, A. A., & Kuzmin, V. A. (2023). Characterization of Triplet State of Cyanine Dyes with Two Chromophores Effect of Molecule Structure. Technologies, 11(4), 90. https://doi.org/10.3390/technologies11040090






