The Role of Vegetable Genetic Resources in Nutrition Security and Vegetable Breeding
Abstract
1. Introduction
2. The Importance of Vegetables for Nutrition Security
2.1. The Commodity Group “Vegetables”
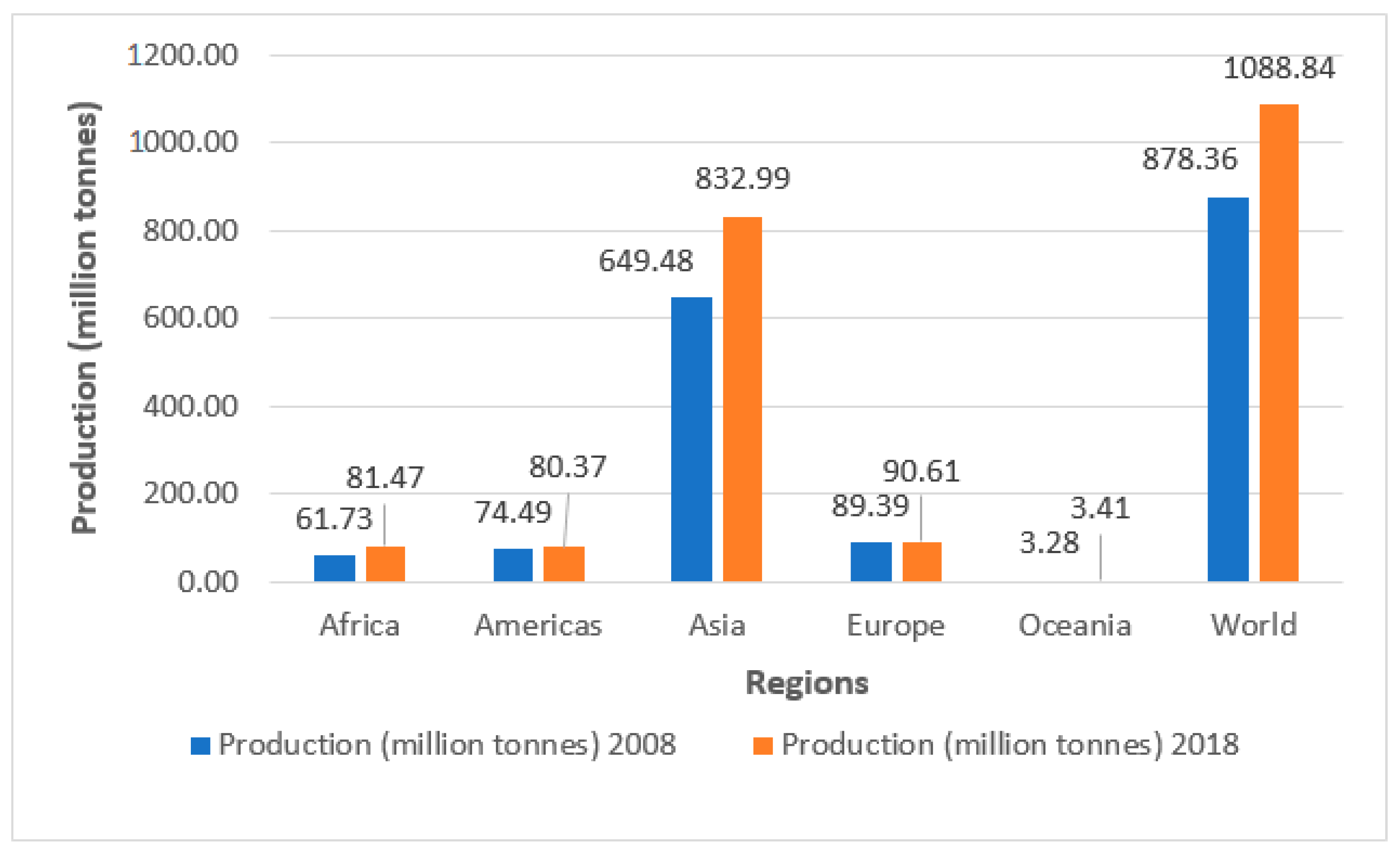
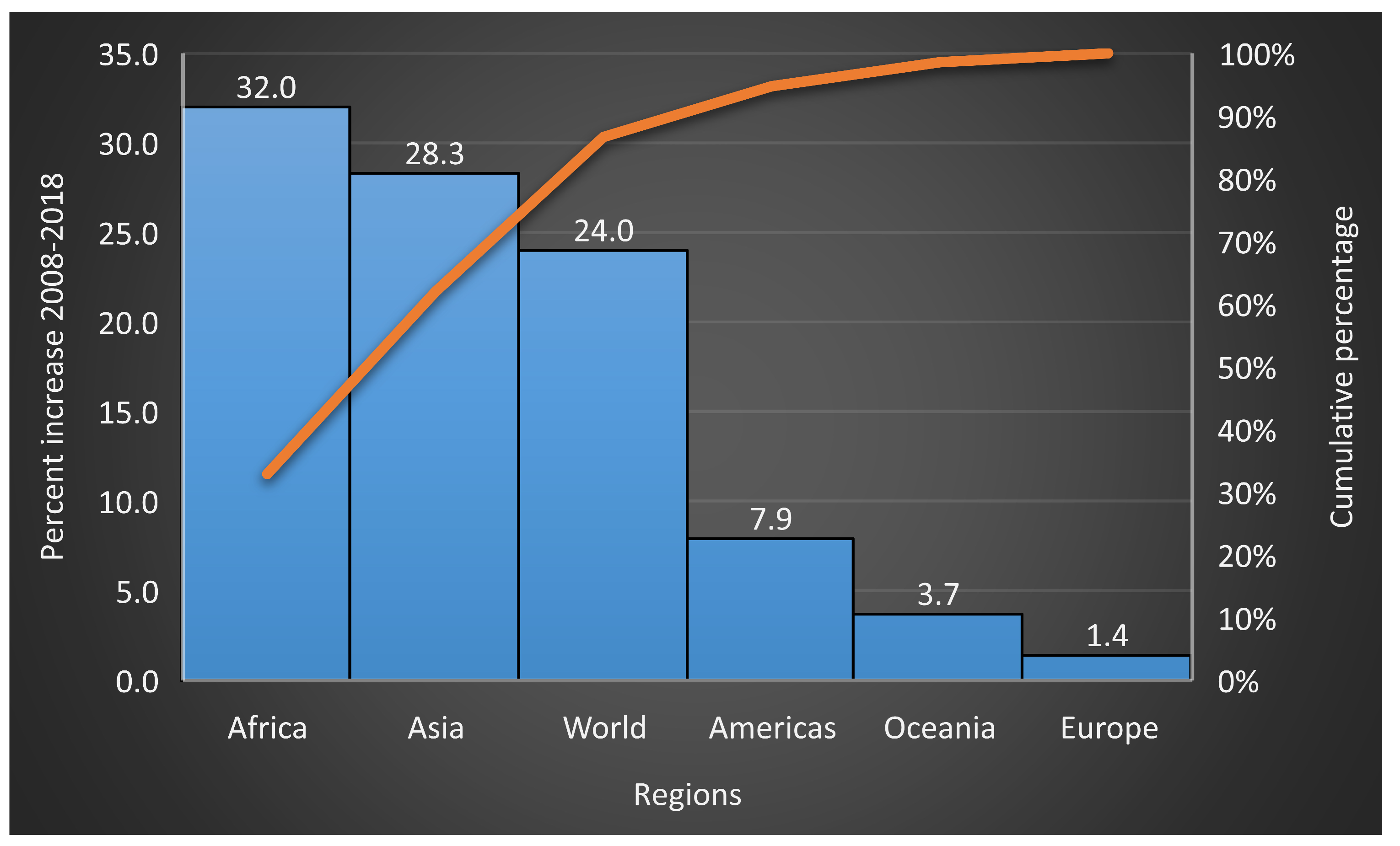
2.2. Global Vegetable Production
2.3. Research and Development Efforts Focusing on Vegetables
2.4. Diversifying Production Systems and the Role of Home and Household Gardens for Nutrition Security
- Improved food security,
- Increased availability of food and better nutrition through food diversity,
- Income and enhanced rural employment through additional or off-season production,
- Decreased risk through diversification,
- Environmental benefits from recycling water and waste nutrients, controlling shade, dust, and erosion, and maintaining or increasing local biodiversity.
3. Vegetable Genetic Resources Conservation and Linkages with the Farming Community
3.1. Ex Situ Conservation of Vegetable Genetic Resources and Collecting Needs
3.2. Linking Genebanks with the Farming Communities
3.2.1. Community Seedbanks for Locally Important Crop Diversity
3.2.2. Variety Introduction of Agricultural Crops in General and Vegetable Crops in Particular
- (1)
- Only those accessions and breeding lines were selected for the vegetable seed kits which had undergone screening for yield, disease resistance, and consumer preference under local conditions in Tanzania.
- (2)
- Distributed accessions and breeding lines were open-pollinated so that farmers could save seed for the next cropping cycle or for sharing extra seed with other famers in the community.
- (3)
- Seed distribution channels were international NGOs, farmer groups, and local government units and WorldVeg development projects. Seed kit distribution was not intended as emergency seed aid. The distribution through projects was related to home garden projects intended for improving dietary diversity and diversifying incomes.
- (4)
- Seed kits were distributed together with capacity building in vegetable production and seed saving provided by NGOs or project staff. Seed kits also contained instructions for good agricultural practices for the successful cultivation of the crops and information on the nutritional value of the crops included in the seed kits.
- (5)
- Seed kits were distributed only once to the same household. A regular supply of seed was not envisaged to avoid damaging local seed enterprises which later picked up seed production and sale of some traditional vegetables.
4. Vegetable Genetic Resources as Building Blocks for Vegetable Breeding
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Pingali, P.L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- FAO-Food and Agriculture Organization of the United Nations. The State of Food and Agriculture 2003–2004; FAO: Rome, Italy, 2004; p. 222. [Google Scholar]
- Evenson, R.E.; Gollin, D. Assessing the impact of the Green Revolution, 1960 to 2000. Science 2003, 300, 758–762. [Google Scholar] [CrossRef] [PubMed]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2019. Safeguarding Against Economic Slowdowns and Downturns; FAO: Rome, Italy, 2019; p. 239. [Google Scholar]
- United Nations Development Programme (UNDP). Sustainable Development Goals. Available online: https://www.undp.org/content/undp/en/home/sustainable-development-goals.html (accessed on 1 March 2020).
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Valin, H.; Sands, R.D.; Van der Mensbrugghe, D.; Nelson, G.C.; Ahammad, H.; Blanc, E.; Bodirsky, B.; Fujimori, S.; Hasegawa, T.; Havlik, P.; et al. The future of food demand: Understanding differences in global economic models. Agric. Econ. 2014, 45, 51–67. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Paper No. 12-03; FAO: Rome, Italy, 2012; p. 147. [Google Scholar]
- Depenbusch, L.; Klasen, S. The effect of bigger human bodies on the future global calorie requirements. PLoS ONE 2019, 14, e0223188. [Google Scholar] [CrossRef]
- Development Initiatives. 2018 Global Nutrition Report: Shining a Light to Spur Action on Nutrition; Development Initiatives: Bristol, UK, 2018; Available online: https://globalnutritionreport.org/reports/global-nutrition-report-2018/ (accessed on 2 March 2020).
- Centers for Disease Control and Prevention (CDC). Overweight & Obesity. Available online: https://www.cdc.gov/obesity/index.html (accessed on 2 June 2020).
- Bennett, J.E.; Stevens, G.A.; Mathers, C.D.; Bonita, R.; Rehm, J.; Kruk, M.E.; Riley, L.M.; Dain, K.; Kengne, A.P.; Chalkidou, K.; et al. NCD countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet 2018, 392, 1072–1088. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the anthropocene: The EAT-lancet commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Fischer, G.C.; Garnett, T. Plates, Pyramids, and Planets: Developments in National Healthy and Sustainable Dietary Guidelines: A State of Play Assessment; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Gepts, P. Plant genetic resources conservation and utilization: The accomplishments and future of a societal insurance policy. Crop Sci. 2006, 46, 2278–2292. [Google Scholar] [CrossRef]
- Harlan, J.R. Crops and man. Am. Soc. Agron. Crop Sci. Soc. Am. 1992, 16, 63–262. [Google Scholar]
- Doughty, J. Dangers of reducing the range of food choice in developing countries. Ecol. Food Nutr. 1979, 8, 275–283. [Google Scholar] [CrossRef]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Reardon, T.; Echeverria, R.; Berdegué, J.; Minten, B.; Liverpool-Tasie, S.; Tschirley, D.; Zilberman, D. Rapid transformation of food systems in developing regions: Highlighting the role of agricultural research & innovations. Agric. Syst. 2019, 172, 47–59. [Google Scholar] [CrossRef]
- Pingali, P. Westernization of Asian diets and the transformation of food systems: Implications for research and policy. Food Policy 2007, 32, 281–298. [Google Scholar] [CrossRef]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Khoury, C.K.; Bjorkman, A.D.; Dempewolf, H.; Ramirez-Villegas, J.; Guarino, L.; Jarvis, A.; Rieseberg, L.H.; Struik, P.C. Increasing homogeneity in global food supplies and the implications for food security. Proc. Nat. Acad. Sci. USA 2014, 111, 4001–4006. [Google Scholar] [CrossRef]
- Padulosi, S.; Sthapit, B.; Lamers, H.; Kennedy, G.; Hunter, D. Horticultural biodiversity to attain sustainable food and nutrition security. Acta Hortic. 2018, 1205, 21–34. [Google Scholar] [CrossRef]
- Kahane, R.; Hodgkin, T.; Jaenicke, H.; Hoogendoorn, C.; Hermann, M.; Hughes, J.D.A.; Padulosi, S.; Looney, N. Agrobiodiversity for food security, health and income. Agron. Sustain. Dev. 2013, 33, 671–693. [Google Scholar] [CrossRef]
- The Royal Society. Reaping the Benefits: Science and the Sustainable Intensification of Global Agriculture; The Royal Society: London, UK, 2009; p. 72. [Google Scholar]
- World Health Organization (WHO). Promoting Fruit and Vegetable Consumption. Available online: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/activities/technical-support-to-member-states/promoting-fruit-and-vegetable-consumption (accessed on 2 March 2020).
- Oyebode, O.; Gordon-Dseagu, V.; Walker, A.; Mindell, J.S. Fruit and vegetable consumption and all-cause, cancer and CVD mortality: Analysis of Health Survey for England data. J. Epidemiol. Community Health 2014, 68, 856–862. [Google Scholar] [CrossRef]
- Olney, D.K.; Pedehombga, A.; Ruel, M.T.; Dillon, A. A 2-year integrated agriculture and nutrition and health behavior change communication program targeted to women in Burkina Faso reduces anemia, wasting, and diarrhea in children 3–12.9 months of age at baseline: A cluster-randomized controlled trial. J. Nutr. 2015, 145, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, K.K.C.; Dias, G.M.; Veeramani, A.; Swanton, C.J.; Fraser, D.; Steinke, D.; Lee, E.; Wittman, H.; Farber, J.M.; Dunfield, K.; et al. When too much isn’t enough: Does current food production meet global nutritional needs? PLoS ONE 2018, 13, e0205683. [Google Scholar] [CrossRef]
- Siegel, K.R.; Ali, M.K.; Srinivasiah, A.; Nugent, R.A.; Narayan, K.V. Do we produce enough fruits and vegetables to meet global health need? PLoS ONE 2014, 9, e104059. [Google Scholar] [CrossRef] [PubMed]
- Mason-D’Croz, D.; Sulser, T.B.; Wiebe, K.; Rosegrant, M.W.; Lowder, S.K.; Nin-Pratt, A.; Willenbockel, D.; Robinson, S.; Zhu, T.; Cenacchi, N.; et al. Agricultural investments and hunger in Africa modeling potential contributions to SDG2–zero hunger. World Dev. 2019, 116, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Keatinge, J.D.H.; Yang, R.Y.; Hughes, J.D.A.; Easdown, W.J.; Holmer, R. The importance of vegetables in ensuring both food and nutritional security in attainment of the millennium development goals. Food Secur. 2011, 3, 491–501. [Google Scholar] [CrossRef]
- Ellison, B.; Muth, M.K.; Golan, E. Opportunities and challenges in conducting economic research on food loss and waste. Appl. Econ. Perspect. Policy 2019, 41, 1–19. [Google Scholar] [CrossRef]
- Tenkouano, A. The nutritional and economic potential of vegetables. In State of the World’s Food and Agriculture; Worldwatch Institute, WW Norton & Company, Inc.: New York, NY, USA, 2011; pp. 27–38. [Google Scholar]
- Schreinemachers, P.; Simmons, E.B.; Wopereis, M.C. Tapping the economic and nutritional power of vegetables. Glob. Food Secur. 2018, 16, 36–45. [Google Scholar] [CrossRef]
- Harris, D.R. Vavilov’s concept of centres of origin of cultivated plants: Its genesis and its influence on the study of agricultural origins. Biol. J. Linn. Soc. 1990, 39, 7–16. [Google Scholar] [CrossRef]
- Lin, L.J.; Hsiao, Y.Y.; Kuo, C.G. Discovering Indigenous Treasures: Promising Indigenous Vegetables from Around the World; AVRDC-The World Vegetable Center: Shanhua, Taiwan, 2009; p. 317. [Google Scholar]
- Keatinge, J.D.H.; Holmer, R.J.; Ebert, A.W.; Hughes, J.D.A. Less visible but yet vital for human health: Nutrient-dense indigenous vegetables and their need for urgent promotion in balanced diets. In Promotion of Underutilized Indigenous Food Resources for Food Security and Nutrition in Asia and the Pacific; Durst, P., Bayasgalanbat, N., Eds.; Food and Agriculture Organization of the United Nations: Bangkok, Thailand, 2014; pp. 36–45. [Google Scholar]
- World Vegetable Center. Annual Report 2019; World Vegetable Center: Shanhua, Taiwan, 2020; p. 72. [Google Scholar]
- FAO-Food and Agriculture Organization of the United Nations. FAOSTAT-Crops. 2020. Available online: http://www.fao.org/faostat/en/#data/QC/ (accessed on 14 March 2020).
- Alston, J.M.; Pardey, P.G. Public funding for research into specialty crops. HortScience 2008, 43, 1461–1470. [Google Scholar] [CrossRef]
- Haddad, L.; Hawkes, C.; Webb, P.; Thomas, S.; Beddington, J.; Waage, J.; Flynn, D. A new global research agenda for food. Nature 2016, 540, 30–32. [Google Scholar] [CrossRef]
- Pingali, P. Agricultural policy and nutrition outcomes-getting beyond the preoccupation with staple grains. Food Secur. 2015, 7, 583–591. [Google Scholar] [CrossRef]
- Fischer, R.A.; Byerlee, D.; Edmeades, G.O. Crop Yields and Global Food Security: Will Yield Increase Continue to Feed the World? Australian Centre for International Agricultural Research: Canberra, Australia, 2014; Volume 22, p. 634. [Google Scholar]
- Sriwichai, W.; Berger, J.; Picq, C.; Avallone, S. Determining factors of lipophilic micronutrient bioaccessibility in several leafy vegetables. J. Agric. Food Chem. 2016, 64, 1695–1701. [Google Scholar] [CrossRef]
- Lipinski, B.; Hanson, C.; Lomax, J.; Kitinoja, L.; Waite, R.; Searchinger, T. Reducing Food Loss and Waste. World Resources Institute Working Paper. 2013. Available online: http://www.worldresourcesreport.org (accessed on 6 April 2020).
- Salim, N.S.M.; Singh, A.; Raghavan, V. Potential utilization of fruit and vegetable wastes for food through drying or extraction techniques. Nov. Tech. Nutr. Food Sci. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Herforth, A.; Arimond, M.; Álvarez-Sánchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A global review of food-based dietary guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef]
- Beed, F.; Dubois, T.; Yang, R.Y. Nutrition implications of urban and peri-urban agriculture. Agric. Dev. 2015, 26, 7–11. [Google Scholar]
- Kurgat, B.K.; Ngenoh, E.; Bett, H.K.; Stöber, S.; Mwonga, S.; Lotze-Campen, H.; Rosenstock, T.S. Drivers of sustainable intensification in Kenyan rural and peri-urban vegetable production. Int. J. Agric. Sustain. 2018, 16, 385–398. [Google Scholar] [CrossRef]
- Waha, K.; Müller, C.; Bondeau, A.; Dietrich, J.P.; Kurukulasuriya, P.; Heinke, J.; Lotze-Campen, H. Adaptation to climate change through the choice of cropping system and sowing date in sub-Saharan Africa. Glob. Environ. Chang. 2013, 23, 130–143. [Google Scholar] [CrossRef]
- Malézieux, E.; Crozat, Y.; Dupraz, C.; Laurans, M.; Makowski, D.; Ozier-Lafontaine, H.; Rapidel, B.; De Tourdonnet, S.; Valantin-Morison, M. Mixing plant species in cropping systems: Concepts, tools and models. A review. Agron. Sustain. Dev. 2009, 29, 43–62. [Google Scholar] [CrossRef]
- Ebert, A.W. Potential of underutilized traditional vegetables and legume crops to contribute to food and nutritional security, income and more sustainable production systems. Sustainability 2014, 6, 319–335. [Google Scholar] [CrossRef]
- Timler, C.; Alvarez, S.; DeClerck, F.; Remans, R.; Raneri, J.; Carmona, N.E.; Mashingaidze, N.; Chatterjee, S.A.; Chiang, T.W.; Termote, C.; et al. Exploring solution spaces for nutrition-sensitive agriculture in Kenya and Vietnam. Agric. Syst. 2020, 180, 102774. [Google Scholar] [CrossRef]
- World Vegetable Center. The World Vegetable Center’s Approach to Household Gardening for Nutrition; World Vegetable Center: Shanhua, Taiwan, 2016; p. 35. [Google Scholar]
- Schreinemachers, P.; Brown, S.; Roothaert, R.; Sobgui, M.C.; Toure, S.H. Research to impact: The World Vegetable Center’s household garden model. Acta Hortic. 2018, 1205, 305–314. [Google Scholar] [CrossRef]
- Landon-Lane, C. Livelihoods Grow in Gardens-Diversifying Rural Income through Home Garden, 2nd ed.; FAO: Rome, Italy, 2011; p. 80. [Google Scholar]
- Patalagsa, M.A.; Schreinemachers, P.; Begum, S.; Begum, S. Sowing seeds of empowerment: Effect of women’s home garden training in Bangladesh. Agric. Food Secur. 2015, 4, 24. [Google Scholar] [CrossRef]
- Hughes, J.D.A.; Keatinge, J.D.H. The nourished Millennium: How vegetables put global goals for healthy, balanced diets within reach. In High Value Vegetables in Southeast Asia: Production, Supply and Demand; Proceedings of the SEAVEG 2012 Regional Symposium; Holmer, R., Linwattana, G., Nath, P., Keatinge, J.D.H., Eds.; AVRDC-The World Vegetable Center: Tainan, Taiwan, 2013; pp. 11–26. [Google Scholar]
- Yang, R.-Y.; Keding, G.B. Nutritional contributions of important African indigenous vegetables. In African Indigenous Vegetables in Urban Agriculture; Shackleton, C.M., Pasquini, M., Drescher, A.W., Eds.; Earthscan: London, UK, 2009; pp. 105–143. [Google Scholar]
- FAO-Food and Agriculture Organization of the United Nations. The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2010; p. 399. [Google Scholar]
- Ebert, A.W. Chapter 16-ex situ conservation of plant genetic resources of major vegetables. In Conservation of Tropical Plant Species; Normah, M.N., Chin, H.F., Reed, B.M., Eds.; Springer Science Business Media: New York, NY, USA, 2013; pp. 373–417. [Google Scholar] [CrossRef]
- FAO-Food and Agriculture Organization of the United Nations. Future Smart Food-Rediscovering Hidden Treasures of Neglected and Underutilized Species for Zero Hunger in Asia, Executive Summary; FAO: Bangkok, Thailand, 2018; p. 36. [Google Scholar]
- Challinor, A.J.; Koehler, A.-K.; Ramirez-Villegas, J.; Whitfield, S.; Das, B. Current warming will reduce yields unless maize breeding and seed systems adapt immediately. Nat. Clim. Chang. 2016, 6, 954–958. [Google Scholar] [CrossRef]
- FAO-Food and Agriculture Organization of the United Nations. Genebank Standards for Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2014; p. 182. [Google Scholar]
- Westengen, O.T.; Skarbø, K.; Mulesa, T.H.; Berg, T. Access to genes: Linkages between genebanks and farmers’ seed systems. Food Secur. 2018, 10, 9–25. [Google Scholar] [CrossRef]
- Vernooy, R.; Sthapit, B.; Galluzzi, G.; Shrestha, P. The multiple functions and services of community seedbanks. Resources 2014, 3, 636–656. [Google Scholar] [CrossRef]
- Vernooy, R.; Shrestha, P.; Sthapit, B. Community Seed Banks: Origins, Evolution and Prospects; Routledge: Abingdon, UK, 2015. [Google Scholar]
- Andersen, R.; Shrestha, P.; Otieno, G.; Nishikawa, Y.; Kasasa, P.; Mushita, A. Community Seed Banks: Sharing Experiences from North and South; Diversifood: Paris, France, 2018; p. 44. [Google Scholar]
- Ebert, A.W.; de los Santos, E.B.; San Buenaventura, A.; Imperial, R.M. Community-based seed production of traditional vegetables to enhance nutrition security and empower farmers in the Philippines. Acta Hortic. 2015, 1102, 135–142. [Google Scholar] [CrossRef]
- Clancy, E.; Vernooy, R. Realizing Farmers’ Rights through Community-Based Agricultural Biodiversity Management; Bioversity International: Rome, Italy, 2016; p. 8. [Google Scholar]
- Van Etten, J. Crowdsourcing crop improvement in sub-Saharan Africa: A proposal for a scalable and inclusive approach to food security. IDS Bull. 2011, 42, 102–110. [Google Scholar] [CrossRef]
- Bioversity International. The Crowdsourcing Approach: Seeds for Needs; Bioversity International: Rome, Italy, 2016; p. 2. [Google Scholar]
- Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA). Iniciativa de colaboración en Conservación de Recursos Fitogenéticos con el CRF: Sinergias con la Conservación en Finca de Variedades locales. Available online: http://wwwsp.inia.es/Comunicacion/Notasdeprensa/Lists/Notas%20Prensa/Attachments/310/NP%20CRF%20Sinergias%20conservaci%C3%B3n%20.pdf?utm_source=Agro.biodiver.se+subscribers&utm_campaign=3c430ad69f-RSS_EMAIL_CAMPAIGN&utm_medium=email&utm_term=0_949cf01306-3c430ad69f-44963817 (accessed on 2 May 2020).
- Schreinemachers, P.; Mavlyanova, R. Utilization of WorldVeg Vegetable Germplasm in Central Asia and the Caucasus; World Vegetable Center: Shanhua, Taiwan, 2019; p. 27. [Google Scholar]
- Mavlyanova, R. Personal Communication; World Vegetable Center, Central Asia and the Caucasus: Tashkent, Uzbekistan, 2020. [Google Scholar]
- Stoilova, T.; van Zonneveld, M.; Roothaert, R.; Schreinemachers, P. Connecting genebanks to farmers in east Africa through the distribution of vegetable seed kits. Plant Genet. Resour. 2019, 17, 306–309. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Ortiz, R. Landrace germplasm for improving yield and abiotic stress adaptation. Trends Plant Sci. 2016, 21, 31–42. [Google Scholar] [CrossRef]
- Hanson, P.M.; Yang, R.Y. Genetic improvement of tomato (Solanum lycopersicum L.) for phytonutrient content at AVRDC-the world vegetable center. Ekin J. Crop Breed. Genet. 2016, 2, 1–10. [Google Scholar]
- Zhang, Q.I.; Yu, E.; Medina, A. Development of advanced interspecific-bridge lines among Cucurbita pepo, C. maxima, and C. moschata. HortScience 2012, 47, 452–458. [Google Scholar] [CrossRef]
- Da Silva Dias, J.C. Guiding strategies for breeding vegetable cultivars. Agric. Sci. 2014, 5, 9–32. [Google Scholar] [CrossRef][Green Version]
- Business Wire. Global Vegetable Seed Market 2019–2024: Growth, Trends and Forecasts-Research and Markets. Available online: https://www.businesswire.com/news/home/20190430005782/en/Global-Vegetable-Seed-Market-2019-2024-Growth-Trends (accessed on 4 May 2020).
- Reddy, M.K.; Srivastava, A.; Lin, S.W.; Kumar, R.; Shieh, H.C.; Ebert, A.W.; Chawda, N.; Kumar, S. Exploitation of AVRDC’s chili pepper (Capsicum spp.) germplasm in India. J. Taiwan Soc. Hortic. Sci. 2015, 61, 1–9. [Google Scholar]
- Ebert, A.W.; Schafleitner, R. Chapter 9. Utilization of wild relatives in the breeding of tomato and other major vegetables. In Crop Wild Relatives and Climate Change, 1st ed.; Redden, R., Yadav, S.S., Maxted, N., Dulloo, M.E., Guarino, L., Smith, P., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 2015; pp. 141–172. [Google Scholar]
- Zamir, D. Improving plant breeding with exotic genetic libraries. Nat. Rev. Genet. 2001, 2, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Prohens, J.; Gramazio, P.; Plazas, M.; Dempewolf, H.; Kilian, B.; Díez, M.J.; Fita, A.; Herraiz, F.J.; Rodríguez-Burruezo, A.; Soler, S.; et al. Introgressiomics: A new approach for using crop wild relatives in breeding for adaptation to climate change. Euphytica 2017, 213, 158. [Google Scholar] [CrossRef]
- Herzog, E.; Falke, K.C.; Presterl, T.; Scheuermann, D.; Ouzunova, M.; Frisch, M. Selection strategies for the development of maize introgression populations. PLoS ONE 2014, 9, e92429. [Google Scholar] [CrossRef]
- Gur, A.; Zamir, D. Mendelizing all components of a pyramid of three yield QTL in tomato. Front. Plant Sci. 2015, 6, 1096. [Google Scholar] [CrossRef]
- Eshed, Y.; Zamir, D. An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 1995, 141, 1147–1162. [Google Scholar]
- Lippman, Z.B.; Semel, Y.; Zamir, D. An integrated view of quantitative trait variation using tomato interspecific introgression lines. Curr. Opin. Genet. Dev. 2007, 17, 545–552. [Google Scholar] [CrossRef]
- Gramazio, P.; Prohens, J.; Plazas, M.; Mangino, G.; Herraiz, F.J.; Vilanova, S. Development and genetic characterization of advanced backcross materials and an introgression line population of Solanum incanum in a S. melongena background. Front. Plant Sci. 2017, 8, 1477. [Google Scholar] [CrossRef]
- Prohens, J.; Whitaker, B.D.; Plazas, M.; Vilanova, S.; Hurtado, M.; Blasco, M.; Gramazio, P.; Stommel, J.R. Genetic diversity in morphological characters and phenolic acids content resulting from an interspecific cross between eggplant, Solanum melongena, and its wild ancestor (S. incanum). Ann. Appl. Biol. 2013, 162, 242–257. [Google Scholar] [CrossRef]
- Taher, D.; Solberg, S.Ø.; Prohens, J.; Chou, Y.Y.; Rakha, M.; Wu, T.H. World vegetable center eggplant collection: Origin, composition, seed dissemination and utilization in breeding. Front. Plant Sci. 2017, 8, 1484. [Google Scholar] [CrossRef] [PubMed]
- Mangino, G.; Plazas, M.; Vilanova, S.; Prohens, J.; Gramazio, P. Performance of a set of eggplant (Solanum melongena) lines with introgressions from its wild relative, S. incanum under open field and screenhouse conditions and detection of QTLs. Agronomy 2020, 10, 467. [Google Scholar] [CrossRef]
- Meldrum, G.; Padulosi, S.; Lochetti, G.; Robitaille, R.; Diulgheroff, S. Issues and prospects for the sustainable use and conservation of cultivated vegetable diversity for more nutrition-sensitive agriculture. Agriculture 2018, 8, 112. [Google Scholar] [CrossRef]
- Stamp, P.; Messmer, R.; Walter, A. Competitive underutilized crops will depend on the state funding of breeding programmes: An opinion on the example of Europe. Plant Breed. 2012, 131, 461–464. [Google Scholar] [CrossRef]
- Frison, E.A.; Cherfas, J.; Hodgkin, T. Agricultural biodiversity is essential for a sustainable improvement in food and nutrition security. Sustainability 2011, 3, 238–253. [Google Scholar] [CrossRef]
- Jackson, L.E.; Pascual, U.; Hodgkin, T. Utilizing and conserving agrobiodiversity in agricultural landscapes. Agric. Ecosyst. Environ. 2007, 121, 196–210. [Google Scholar] [CrossRef]
- McCouch, S.; Baute, G.J.; Bradeen, J.; Bradeen, J.; Bramel, P.; Bretting, P.K.; Buckler, E.; Burke, J.M.; Charest, D.; Cloutier, S. Feeding the future. Nature 2013, 499, 23–24. [Google Scholar] [CrossRef]
- Keatinge, J.D.H.; Waliyar, F.; Jamnadass, R.H.; Moustafa, A.; Andrade, M.; Drechsel, P.; Hughes, J.D.A.; Palchamy, K.; Luther, K. Re-learning old lessons for the future of food-by bread alone no longer: Diversifying diets with fruit and vegetables. Crop Sci. 2010, 50, 51–62. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebert, A.W. The Role of Vegetable Genetic Resources in Nutrition Security and Vegetable Breeding. Plants 2020, 9, 736. https://doi.org/10.3390/plants9060736
Ebert AW. The Role of Vegetable Genetic Resources in Nutrition Security and Vegetable Breeding. Plants. 2020; 9(6):736. https://doi.org/10.3390/plants9060736
Chicago/Turabian StyleEbert, Andreas W. 2020. "The Role of Vegetable Genetic Resources in Nutrition Security and Vegetable Breeding" Plants 9, no. 6: 736. https://doi.org/10.3390/plants9060736
APA StyleEbert, A. W. (2020). The Role of Vegetable Genetic Resources in Nutrition Security and Vegetable Breeding. Plants, 9(6), 736. https://doi.org/10.3390/plants9060736




