Identification and Molecular Characterization of Geranyl Diphosphate Synthase (GPPS) Genes in Wintersweet Flower
Abstract
1. Introduction
2. Results
2.1. Isolation of cDNAs Encoding CpGPPS/GGPPS
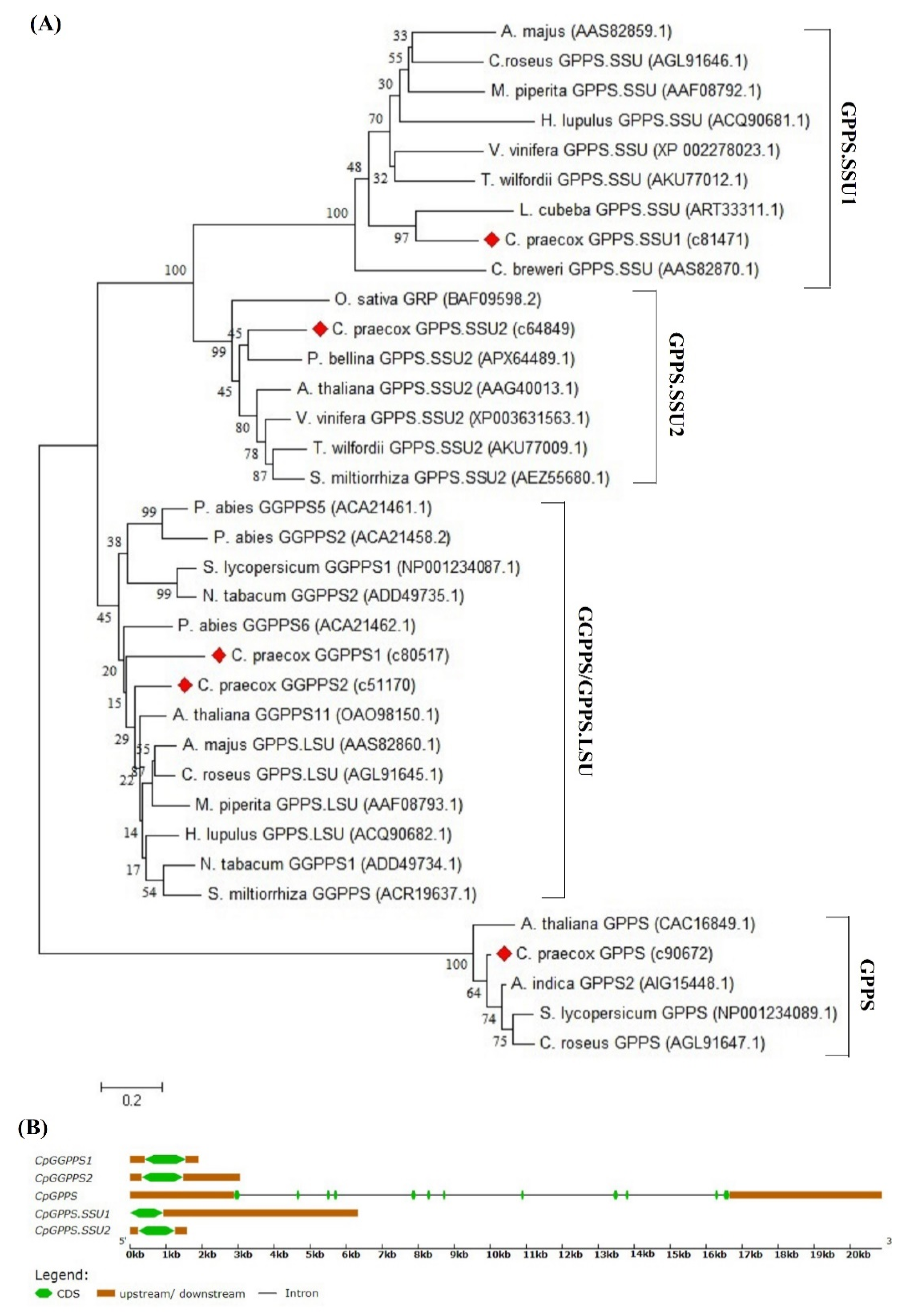
2.2. Sequence Comparison, Gene Structure, and Phylogenetic Analysis
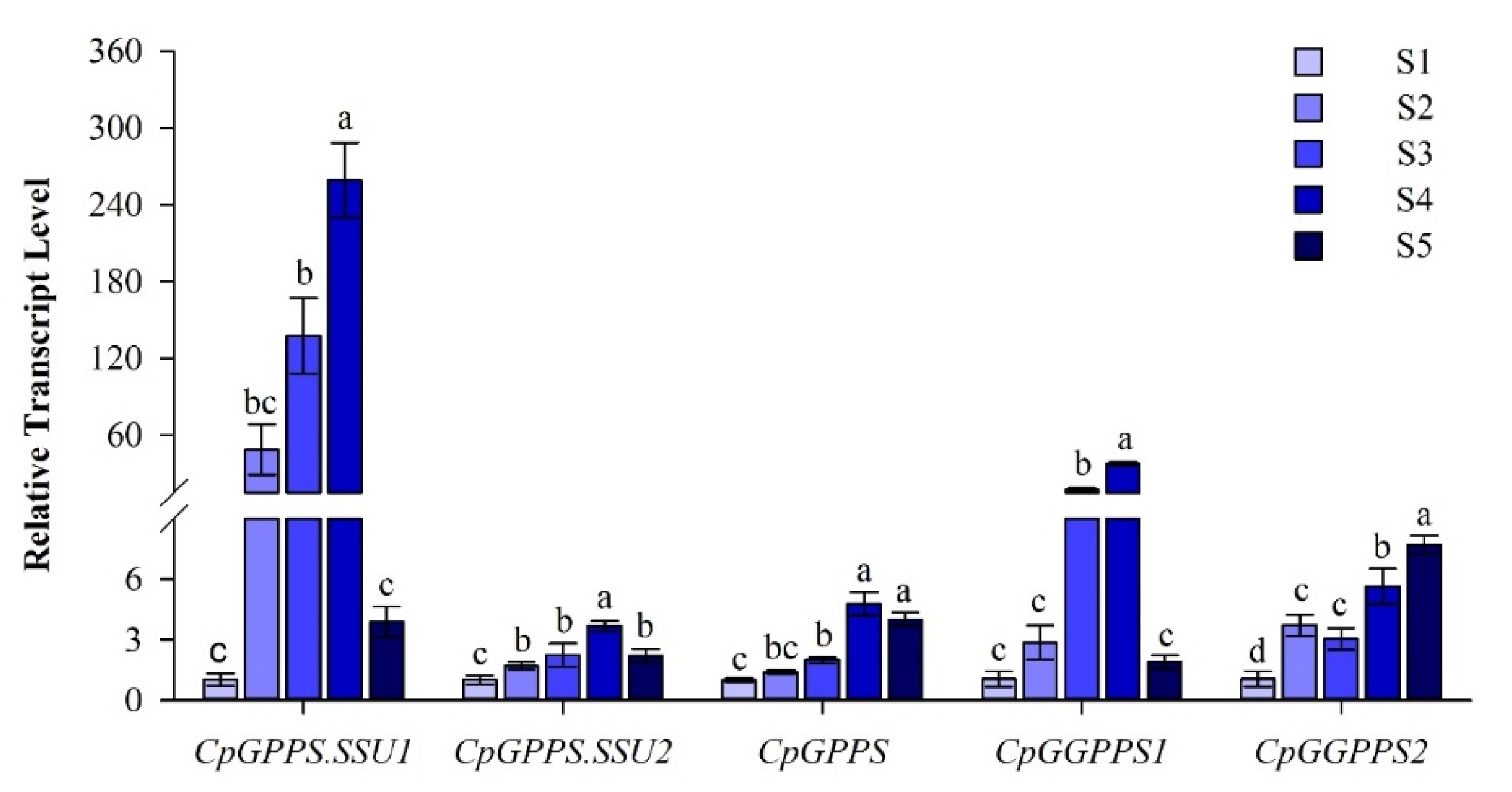
2.3. Spatiotemporal Expression Pattern of CpGPPS/GGPPS Genes
2.4. Subcellular Localization of CpGPPS/GGPPS Genes
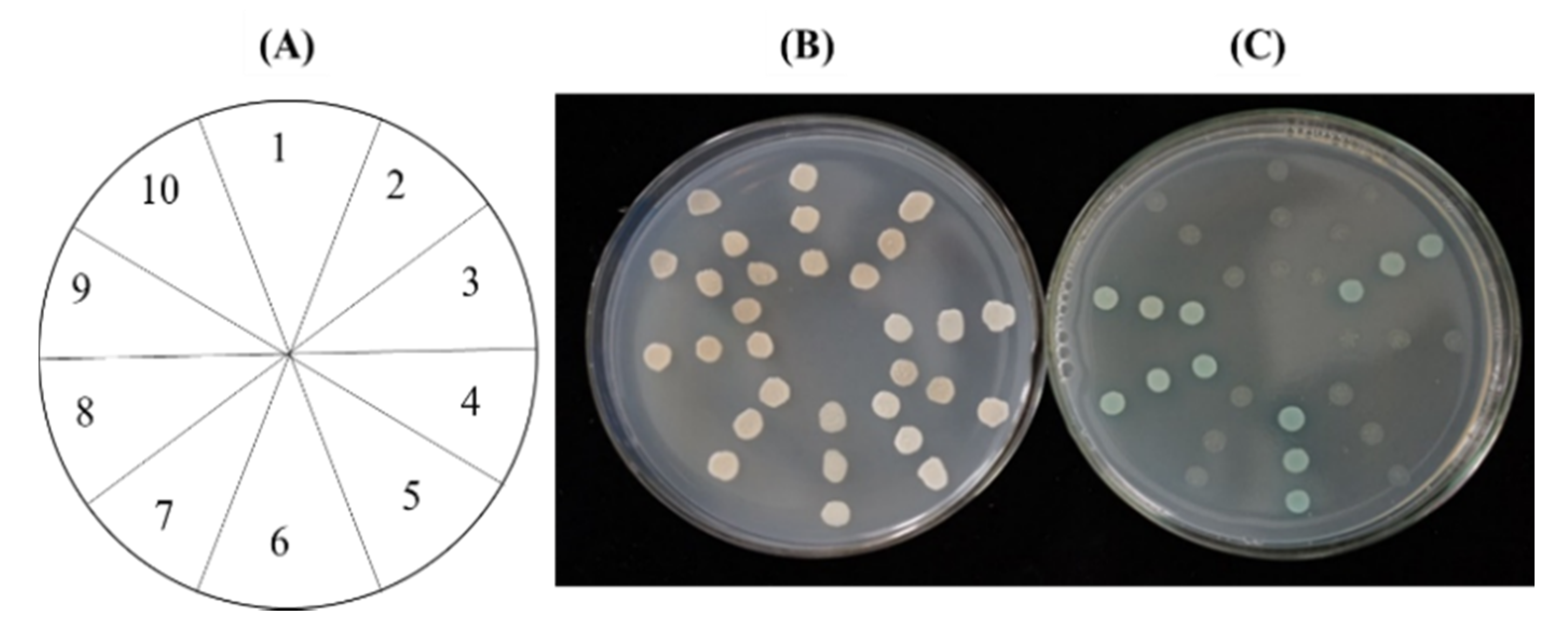
2.5. CpGPPS.SSU1 and CpGPPS.SSU2 Interaction with GGPPS
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sampling
4.2. Database Mining and Sequencing of CpGPPS cDNAs
4.3. Bioinformatics Analysis
4.4. RNA Extraction and qRT-PCR
4.5. Subcellular Localization
4.6. Yeast 2-Hybrid Assay (Y2H)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pichersky, E.; Dudareva, N. Scent engineering: Toward the goal of controlling how flowers smell. Trends Biotechnol. 2007, 25, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar]
- McGarvey, D.J.; Croteau, R. Terpenoid metabolism. Plant Cell 1995, 7, 1015–1026. [Google Scholar] [PubMed]
- Vranova, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Van Schie, C.C.; Ament, K.; Schmidt, A.; Lange, T.; Haring, M.A.; Schuurink, R.C. Geranyl diphosphate synthase is required for biosynthesis of gibberellins. Plant J. 2007, 52, 752–762. [Google Scholar] [CrossRef]
- Schmidt, A.; Gershenzon, J. Cloning and characterization of two different types of geranyl diphosphate synthases from Norway spruce (Picea abies). Phytochemistry 2008, 69, 49–57. [Google Scholar] [CrossRef]
- Tholl, D.; Kish, C.M.; Orlova, I.; Sherman, D.; Gershenzon, J.; Pichersky, E.; Dudareva, N. Formation of monoterpenes in Antirrhinum majus and Clarkia breweri flowers involves heterodimeric geranyl diphosphate synthases. Plant Cell 2004, 16, 977–992. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef]
- Lackus, N.D.; Petersen, N.P.; Nagel, R.; Schmidt, A.; Irmisch, S.; Gershenzon, J.; Köllner, T.G. Identification and Characterization of trans-Isopentenyl Diphosphate Synthases Involved in Herbivory-Induced Volatile Terpene Formation in Populus trichocarpa. Molecules 2019, 24, 2408. [Google Scholar] [CrossRef]
- Rai, A.; Smita, S.S.; Singh, A.K.; Shanker, K.; Nagegowda, D.A. Heteromeric and homomeric geranyl diphosphate synthases from Catharanthus roseus and their role in monoterpene indole alkaloid biosynthesis. Mol. Plant 2013, 6, 1531–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dixon, R.A. Heterodimeric geranyl (geranyl) diphosphate synthase from hop (Humulus lupulus) and the evolution of monoterpene biosynthesis. Proc. Natl. Acad. Sci. USA 2009, 106, 9914–9919. [Google Scholar] [CrossRef] [PubMed]
- Orlova, I.; Nagegowda, D.A.; Kish, C.M.; Gutensohn, M.; Maeda, H.; Varbanova, M.; Fridman, E.; Yamaguchi, S.; Hanada, A.; Kamiya, Y.; et al. The Small Subunit of Snapdragon Geranyl Diphosphate Synthase Modifies the Chain Length Specificity of Tobacco Geranylgeranyl Diphosphate Synthase in Planta. Plant Cell 2009, 21, 4002–4017. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.Y.; Jeng, M.F.; Tsai, W.C.; Chuang, Y.C.; Li, C.Y.; Wu, T.S.; Kuoh, C.S.; Chen, W.H.; Chen, H.H. A novel homodimeric geranyl diphosphate synthase from the orchid Phalaenopsis bellina lacking a DD (X) 2–4D motif. Plant J. 2008, 55, 719–733. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, D.; Wang, G. Heteromeric geranyl (geranyl) diphosphate synthase is involved in monoterpene biosynthesis in Arabidopsis flowers. Mol. Plant 2015, 8, 1434–1437. [Google Scholar] [CrossRef]
- Kulkarni, R.; Pandit, S.; Chidley, H.; Nagel, R.; Schmidt, A.; Gershenzon, J.; Pujari, K.; Giri, A.; Gupta, V. Characterization of three novel isoprenyl diphosphate synthases from the terpenoid rich mango fruit. Plant Physiol. Biochem. 2013, 71, 121–131. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.-Q.; Cao, T.-J.; Zhuang, Z.; Wang, R.; Lu, S. Heteromeric geranylgeranyl diphosphate synthase contributes to carotenoid biosynthesis in ripening fruits of red pepper (Capsicum annuum var. conoides). J. Agric. Food Chem. 2018, 66, 11691–11700. [Google Scholar] [CrossRef]
- Zhao, K.-G.; Zhou, M.-Q.; Chen, L.-Q.; Zhang, D.; Robert, G.W. Genetic diversity and discrimination of Chimonanthus praecox (L.) Link germplasm using ISSR and RAPD markers. HortScience 2007, 42, 1144–1148. [Google Scholar] [CrossRef]
- Deng, C.; Song, G.; Hu, Y. Rapid determination of volatile compounds emitted from Chimonanthus praecox flowers by HS-SPME-GC-MS. Z. Naturforsch. C J. Biosci. 2004, 59, 636–640. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, K.; Chen, L. Molecular cloning and expression of Chimonanthus praecox farnesyl pyrophosphate synthase gene and its possible involvement in the biosynthesis of floral volatile sesquiterpenoids. Plant Physiol. Biochem. 2010, 48, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, H.; Peng, C.; Chen, Z.; Long, Z. Cloning of SAMT gene cDNA from Chimonanthus praecox and its expression in Escherichia coli. Agric. Sci. Technol. Hunan 2012, 13, 82–87. [Google Scholar]
- Tian, J.P.; Ma, Z.Y.; Zhao, K.G.; Zhang, J.; Xiang, L.; Chen, L.Q. Transcriptomic and proteomic approaches to explore the differences in monoterpene and benzenoid biosynthesis between scented and unscented genotypes of wintersweet. Physiol. Plant. 2019, 166, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Vassão, D.G.; Gang, D.R.; Koeduka, T.; Jackson, B.; Pichersky, E.; Davin, L.B.; Lewis, N.G. Chavicol formation in sweet basil (Ocimum basilicum): Cleavage of an esterified C9 hydroxyl group with NAD (P) H-dependent reduction. Org. Biomol. Chem. 2006, 4, 2733–2744. [Google Scholar] [CrossRef]
- Dudareva, N.; Murfitt, L.M.; Mann, C.J.; Gorenstein, N.; Kolosova, N.; Kish, C.M.; Bonham, C.; Wood, K. Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell 2000, 12, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.; Coman, D.; Herren, E.; Ruiz-Sola, M.A.; Rodríguez-Concepción, M.; Gruissem, W.; Vranová, E. Characterization of the GGPP synthase gene family in Arabidopsis thaliana. Plant Mol. Biol. 2013, 82, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, B.A.; Poulter, C.D. Chain elongation in the isoprenoid biosynthetic pathway. Curr. Opin. Chem. Biol. 1997, 1, 570–578. [Google Scholar] [CrossRef]
- Azuma, H.; Toyota, M.; Asakawa, Y. Floral scent chemistry and stamen movement of Chimonanthus praecox (L.) Link (Calycanthaceae). Acta Phytotax. Geobot. 2005, 56, 197–201. [Google Scholar]
- Kim, Y.-J.; Lee, O.R.; Oh, J.Y.; Jang, M.-G.; Yang, D.-C. Functional analysis of 3-hydroxy-3-methylglutaryl coenzyme a reductase encoding genes in triterpene saponin-producing ginseng. Plant Physiol. 2014, 165, 373–387. [Google Scholar] [CrossRef]
- Xue, L.; He, Z.; Bi, X.; Xu, W.; Wei, T.; Wu, S.; Hu, S. Transcriptomic profiling reveals MEP pathway contributing to ginsenoside biosynthesis in Panax ginseng. BMC Genom. 2019, 20, 383. [Google Scholar] [CrossRef]
- Liu, D.; Sui, S.; Ma, J.; Li, Z.; Guo, Y.; Luo, D.; Yang, J.; Li, M. Transcriptomic analysis of flower development in wintersweet (Chimonanthus praecox). PLoS ONE 2014, 9, e86976. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Liu, D.; Ma, J.; Li, J.; Li, M.; Sui, S. Floral Scent Emission from Nectaries in the Adaxial Side of the Innermost and Middle Petals in Chimonanthus praecox. Int. J. Mol. Sci. 2018, 19, 3278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Y.; Gao, M.; Yin, H.; Wu, L.; Wang, Y. Overexpression of geranyl diphosphate synthase small subunit 1 (LcGPPS. SSU1) enhances the monoterpene content and biomass. Ind. Crops Prod. 2020, 143, 111926. [Google Scholar] [CrossRef]
- Adal, A.M.; Mahmoud, S.S. Short-chain isoprenyl diphosphate synthases of lavender (Lavandula). Plant Mol. Biol. 2020, 102, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019. [Google Scholar] [CrossRef]



| Gene/cDNA Name | ORF Length (aa) | Theoretical pI Value | Molecular Weight (kDa) | In Silico Subcellular Localization Prediction | Conserved Motif |
|---|---|---|---|---|---|
| CpGPPS.SSU1/c81471 | 306 | 7.07 | 33.00 | Chloroplast | CxxxC, CxxxC |
| CpGPPS.SSU2/c64849 | 341 | 5.75 | 37.60 | Chloroplast | CxxC, DDX(2–4)D, CxxxC |
| CpGPPS/c90672 | 428 | 5.85 | 46.84 | Mitochondria | DDX(2–4)D, DDxxD |
| CpGGPPS1/c80517 | 377 | 6.47 | 40.85 | Chloroplast | CxxxC, DDX(2–4)D, DDxxD |
| CpGGPPS2/c51170 | 384 | 5.97 | 41.39 | Chloroplast | CxxxC, DDX(2–4)D, DDxxD |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamran, H.M.; Hussain, S.B.; Junzhong, S.; Xiang, L.; Chen, L.-Q. Identification and Molecular Characterization of Geranyl Diphosphate Synthase (GPPS) Genes in Wintersweet Flower. Plants 2020, 9, 666. https://doi.org/10.3390/plants9050666
Kamran HM, Hussain SB, Junzhong S, Xiang L, Chen L-Q. Identification and Molecular Characterization of Geranyl Diphosphate Synthase (GPPS) Genes in Wintersweet Flower. Plants. 2020; 9(5):666. https://doi.org/10.3390/plants9050666
Chicago/Turabian StyleKamran, Hafiz Muhammad, Syed Bilal Hussain, Shang Junzhong, Lin Xiang, and Long-Qing Chen. 2020. "Identification and Molecular Characterization of Geranyl Diphosphate Synthase (GPPS) Genes in Wintersweet Flower" Plants 9, no. 5: 666. https://doi.org/10.3390/plants9050666
APA StyleKamran, H. M., Hussain, S. B., Junzhong, S., Xiang, L., & Chen, L.-Q. (2020). Identification and Molecular Characterization of Geranyl Diphosphate Synthase (GPPS) Genes in Wintersweet Flower. Plants, 9(5), 666. https://doi.org/10.3390/plants9050666





