Investigation of Indigenous Arbuscular Mycorrhizal Performance Using a Lotus japonicus Mycorrhizal Mutant
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant, Soil Samples, and Fungal Materials
2.2. Plant Growth and AMF Inoculation
2.3. Fungal Cell Wall Staining
2.4. Percent Root Length Colonization
3. Results and Discussion
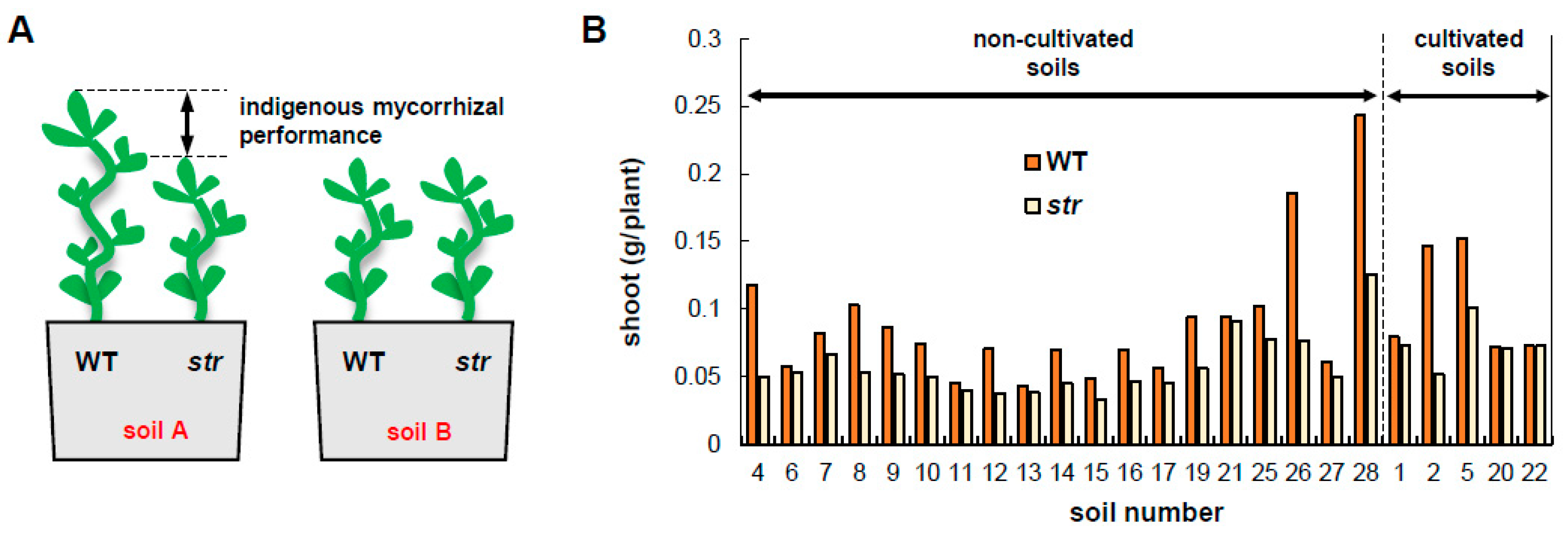
3.1. The Performance of Indigenous AMF Varies Among Soils
3.2. Indigenous Mycorrhizal Performance is not Related to the Early Colonization Potential of Indigenous AMF and the Levels of P in the Soil
3.3. Relationship between Indigenous Mycorrhizal Performance and the Effect of Exotic AMF Inoculation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.G.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, UK, 2008. [Google Scholar]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Van Tuinen, D.; Jacquot, E.; Zhao, B.; Gollotte, A.; Gianinazzi-Pearson, V. Characterization of root colonization profiles by a microcosm community of arbuscular mycorrhizal fungi using 25S rDNA-targeted nested PCR. Mol. Ecol. 1998, 7, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Metsis, M.; Daniell, T.J.; Zobel, M.; Moora, M. Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol. 2009, 184, 424–437. [Google Scholar] [CrossRef]
- Fortin, J.A.; Bécard, G.; Declerck, S.; Dalpé, Y.; St-Arnaud, M.; Coughlan, A.P.; Piché, Y. Arbuscular mycorrhiza on root-organ cultures. Can. J. Bot. 2002, 80, 1–20. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Chaudhary, V.B.; Abbott, L.K. Fungal inoculants in the field: Is the reward greater than the risk? Funct. Ecol. 2018, 32, 126–135. [Google Scholar] [CrossRef]
- Lehnert, H.; Serfling, A.; Enders, M.; Friedt, W.; Ordon, F. Genetics of mycorrhizal symbiosis in winter wheat (Triticum aestivum). New Phytol. 2017, 215, 779–791. [Google Scholar] [CrossRef]
- De Vita, P.; Avio, L.; Sbrana, C.; Laidò, G.; Marone, D.; Mastrangelo, A.M.; Cattivelli, L.; Giovannetti, M. Genetic markers associated to arbuscular mycorrhizal colonization in durum wheat. Sci. Rep. 2018, 8, 10612. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef]
- Srivastava, P.; Saxena, B.; Giri, B. Arbuscular mycorrhizal fungi: Green approach/technology for sustainable agriculture and environment. In Mycorrhiza – Nutrient Uptake, Biocontrol, Ecorestoration; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 355–386. [Google Scholar]
- Tawaraya, K.; Hirose, R.; Wagatsuma, T. Inoculation of arbuscular mycorrhizal fungi can substantially reduce phosphate fertilizer application to Allium fistulosum L. and achieve marketable yield under field condition. Biol. Fert. Soils 2012, 48, 839–843. [Google Scholar] [CrossRef]
- Elliott, A.J.; Daniell, T.J.; Cameron, D.D.; Field, K.J. A commercial arbuscular mycorrhizal inoculum increases root colonization across wheat cultivars but does not increase assimilation of mycorrhiza-acquired nutrients. Plants People Planet 2019. [Google Scholar] [CrossRef]
- Arihara, J.; Karasawa, T. Effect of previous crops on arbuscular mycorrhizal formation and growth of succeeding maize. Soil Sci. Plant Nutr. 2000, 46, 43–51. [Google Scholar] [CrossRef]
- Karasawa, T. Arbuscular mycorrhizal associations and interactions in temperate cropping systems. Res. Bull. Natl. Agric. Res. Cent. 2004, 179, 1–71. [Google Scholar]
- Karasawa, T.; Takebe, M. Temporal or spatial arrangements of cover crops to promote arbuscular mycorrhizal colonization and P uptake of upland crops grown after nonmycorrhizal crops. Plant Soil 2012, 353, 355–366. [Google Scholar] [CrossRef]
- Oka, N.; Karasawa, T.; Okazaki, K.; Takebe, M. Maintenance of soybean yield with reduced phosphorus application by previous cropping with mycorrhizal plants. Soil Sci. Plant Nutr. 2010, 56, 824–830. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef]
- Walder, F.; van der Heijden, M.G. Regulation of resource exchange in the arbuscular mycorrhizal symbiosis. Nat. Plants 2015, 1, 15159. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Renaut, S.; Daoud, R.; Masse, J.; Vialle, A.; Hijri, M. Inoculation with Rhizophagus irregularis does not alter arbuscular mycorrhizal fungal community structure within the roots of corn, wheat, and soybean crops. Microorganisms 2020, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Mansour, I.; Roy, J.; van der Heijden, M.; et al. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 2019, 222, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Lekberg, Y.; Koide, R.T. Integrating physiological, community, and evolutionary perspectives on the arbuscular mycorrhizal symbiosis. Can. J. Bot. 2014, 251, 241–251. [Google Scholar] [CrossRef]
- Kobae, Y. Dynamic phosphate uptake in arbuscular mycorrhizal roots under field conditions. Front. Environ. Sci. 2019, 6, 159. [Google Scholar] [CrossRef]
- Schreiner, R.P. Effects of native and non-native arbuscular mycorrhizal fungi on growth and nutrient uptake of ‘Pinot noir’ (Vitis vinifera L.) in two soils with contrasting levels of phosphorus. Appl. Soil Ecol. 2007, 36, 205–215. [Google Scholar] [CrossRef]
- Faye, A.; Dalpé, Y.; Ndung’u-Magiroi, K.; Jefwa, J.; Ndoye, I.; Diouf, M.; Lesueur, D. Evaluation of commercial arbuscular mycorrhizal inoculants. Can. J. Plant Sci. 2013, 93, 1201–1208. [Google Scholar] [CrossRef]
- Rodriguez, A.; Sanders, I.R. The role of community and population ecology in applying mycorrhizal fungi for improved food security. ISME J. 2015, 9, 1053–1061. [Google Scholar] [CrossRef]
- Miller, M.H.; McGonigle, T.P.; Addy, H.D. Functional ecology of vesicular arbuscular mycorrhizas as influenced by phosphate fertilization and tillage in an agricultural ecosystem. Crit. Rev. Biotechnol. 1995, 15, 241–255. [Google Scholar] [CrossRef]
- Faggioli, V.S.; Cabello, M.N.; Grilli, G.; Vasar, M.; Covacevich, F.; Öpik, M. Root colonizing and soil borne communities of arbuscular mycorrhizal fungi differ among soybean fields with contrasting historical land use. Agric. Ecosyst. Environ. 2019, 269, 174–182. [Google Scholar] [CrossRef]
- Abbott, L.K.; Robson, A.D. The role of vesicular arbuscular mycorrhizal fungi in agriculture and the selection of fungi for inoculation. Aust. J. Agric. Res. 1982, 33, 389–408. [Google Scholar] [CrossRef]
- Kojima, T.; Saito, K.; Oba, H.; Yoshida, Y.; Terasawa, J.; Umehara, Y.; Suganuma, N.; Kawaguchi, M.; Ohtomo, R. Isolation and phenotypic characterization of Lotus japonicus mutants specifically defective in arbuscular mycorrhizal formation. Plant Cell Physiol. 2014, 55, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Brands, M.; Wewer, V.; Dörmann, P.; Harrison, M.J. Arbuscular mycorrhiza-specific enzymes FatM and RAM 2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol. 2017, 214, 1631–1645. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Keymer, A.; Pimprikar, P.; Wewer, V.; Huber, C.; Brands, M.; Bucerius, S.L.; Delaux, P.M.; Klingl, V.; Röpenack-Lahaye, E.V.; Wang, T.L.; et al. Lipid transfer from plants to arbuscular mycorrhiza fungi. elife 2017, 6, e29107. [Google Scholar] [CrossRef] [PubMed]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; Van Erp, H.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.D.; Eastmond, P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Wewer, V.; Brands, M.; Dörmann, P. Fatty acid synthesis and lipid metabolism in the obligate biotrophic fungus Rhizophagus irregularis during mycorrhization of Lotus japonicus. Plant J. 2014, 79, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Maeda, T.; Yamaguchi, K.; Kameoka, H.; Tanaka, S.; Ezawa, T.; Shigenobu, S.; Kawaguchi, M. The genome of Rhizophagus clarus HR1 reveals a common genetic basis for auxotrophy among arbuscular mycorrhizal fungi. BMC Genom. 2018, 19, 465. [Google Scholar] [CrossRef]
- Zhang, Q.; Blaylock, L.A.; Harrison, M.J. Two Medicago truncatula half-ABC transporters are essential for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Cell 2010, 22, 1483–1497. [Google Scholar] [CrossRef]
- Gutjahr, C.; Radovanovic, D.; Geoffroy, J.; Zhang, Q.; Siegler, H.; Chiapello, M.; Casieri, L.; An, K.; An, G.; Guiderdoni, E.; et al. The half-size ABC transporters STR1 and STR2 are indispensable for mycorrhizal arbuscule formation in rice. Plant J. 2012, 69, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Obara, H.; Ohkura, T.; Takata, Y.; Kohyama, K.; Maejima, Y.; Hamazaki, T. Comprehensive soil classification system of Japan first approximation. Bull. Natl. Inst. Agro-Environ. Sci. 2011, 29, 1–73. [Google Scholar] [CrossRef]
- Cox, G.; Sanders, F. Ultrastructure of the host–fungus interface in a vesicular-arbuscular mycorrhiza. New Phytol. 1974, 73, 901–912. [Google Scholar] [CrossRef]
- Kobae, Y.; Ohtomo, R. An improved method for bright-field imaging of arbuscular mycorrhizal fungi in plant roots. Soil Sci. Plant Nutr. 2016, 62, 27–30. [Google Scholar] [CrossRef]
- Kobae, Y. The Infection Unit: An overlooked conceptual unit for arbuscular mycorrhizal function. In Root Biology-Growth, Physiology, and Functions; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Graham, J.H.; Abbott, L.K. Wheat responses to aggressive and non-aggressive arbuscular mycorrhizal fungi. Plant Soil 2000, 220, 207–218. [Google Scholar] [CrossRef]
- Grace, E.J.; Cotsaftis, O.; Tester, M.; Smith, F.A.; Smith, S.E. Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytol. 2009, 181, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Smith, F.A.; Dickson, S.; Holloway, R.E.; Smith, S.E. Plant growth depressions in arbuscular mycorrhizal symbiosis: Not just caused by carbon drain? New Phytol. 2008, 178, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Niwa, R.; Koyama, T.; Sato, T.; Adachi, K.; Tawaraya, K.; Sato, S.; Hirakawa, H.; Yoshida, Y.; Ezawa, T. Dissection of niche competition between introduced and indigenous arbuscular mycorrhizal fungi with respect to soybean yield responses. Sci. Rep. 2018, 8, 7419. [Google Scholar] [CrossRef]
- Verbruggen, E.; Kiers, E.T. Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evol. Appl. 2010, 3, 547–560. [Google Scholar] [CrossRef]
- Burleigh, S.H.; Cavagnaro, T.; Jakobsen, I. Functional diversity of arbuscular mycorrhizas extends to the expression of plant genes involved in P nutrition. J. Exp. Bot. 2002, 53, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725. [Google Scholar] [CrossRef]
- Breuillin-Sessoms, F.; Floss, D.S.; Gomez, S.K.; Pumplin, N.; Ding, Y.; Levesque-Tremblay, V.; Noar, R.D.; Daniels, D.A.; Bravo, A.; Eaglesham, J.B.; et al. Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter4 mutants is dependent on the ammonium transporter 2 family protein AMT2;3. Plant Cell 2015, 27, 1352–1366. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teranishi, T.; Kobae, Y. Investigation of Indigenous Arbuscular Mycorrhizal Performance Using a Lotus japonicus Mycorrhizal Mutant. Plants 2020, 9, 658. https://doi.org/10.3390/plants9050658
Teranishi T, Kobae Y. Investigation of Indigenous Arbuscular Mycorrhizal Performance Using a Lotus japonicus Mycorrhizal Mutant. Plants. 2020; 9(5):658. https://doi.org/10.3390/plants9050658
Chicago/Turabian StyleTeranishi, Taisuke, and Yoshihro Kobae. 2020. "Investigation of Indigenous Arbuscular Mycorrhizal Performance Using a Lotus japonicus Mycorrhizal Mutant" Plants 9, no. 5: 658. https://doi.org/10.3390/plants9050658
APA StyleTeranishi, T., & Kobae, Y. (2020). Investigation of Indigenous Arbuscular Mycorrhizal Performance Using a Lotus japonicus Mycorrhizal Mutant. Plants, 9(5), 658. https://doi.org/10.3390/plants9050658




