Effect of Leaf Litter from Cistus ladanifer L. on the Germination and Growth of Accompanying Shrubland Species
Abstract
:1. Introduction
2. Results
2.1. Quantification of Compounds with Allelopathic Activity
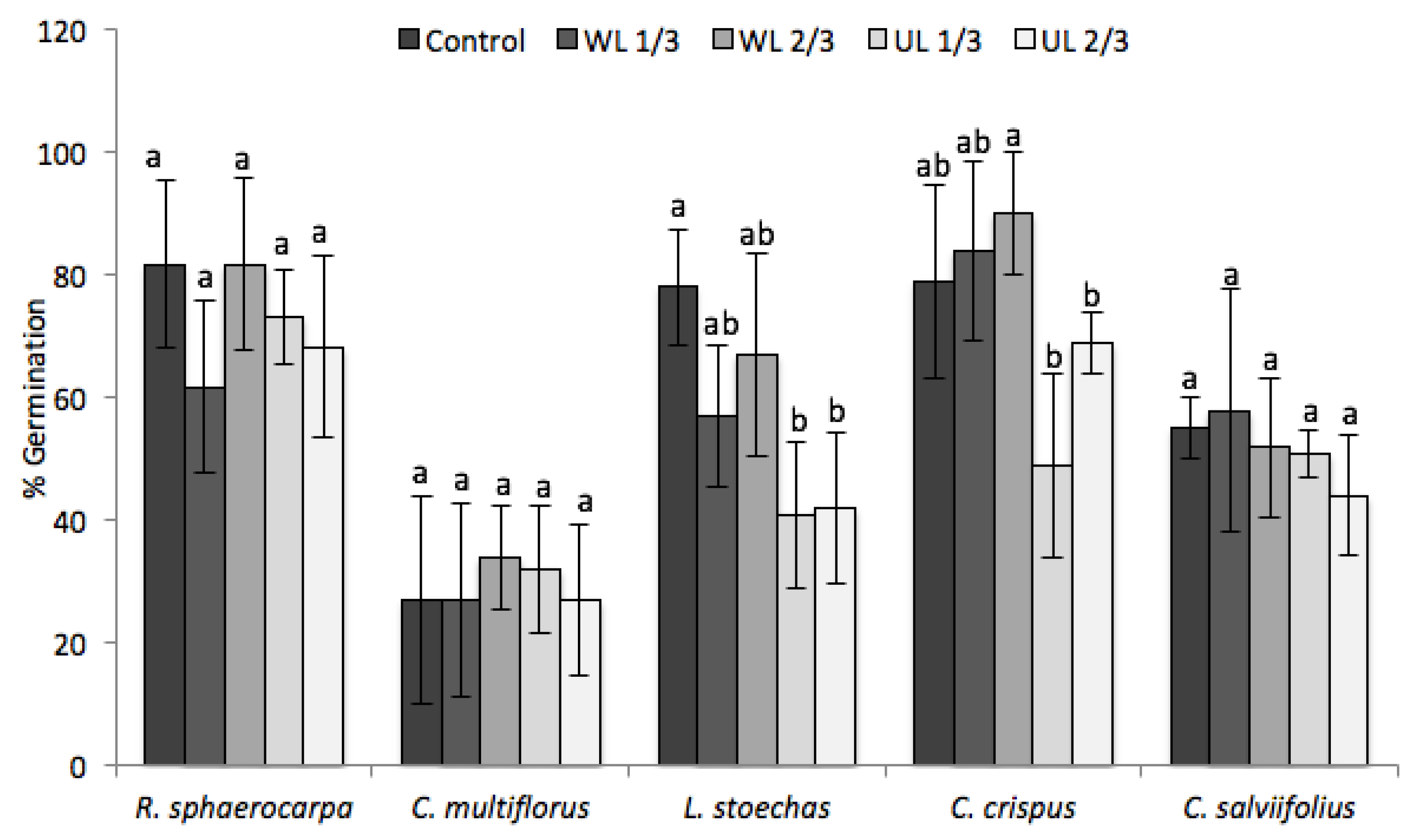
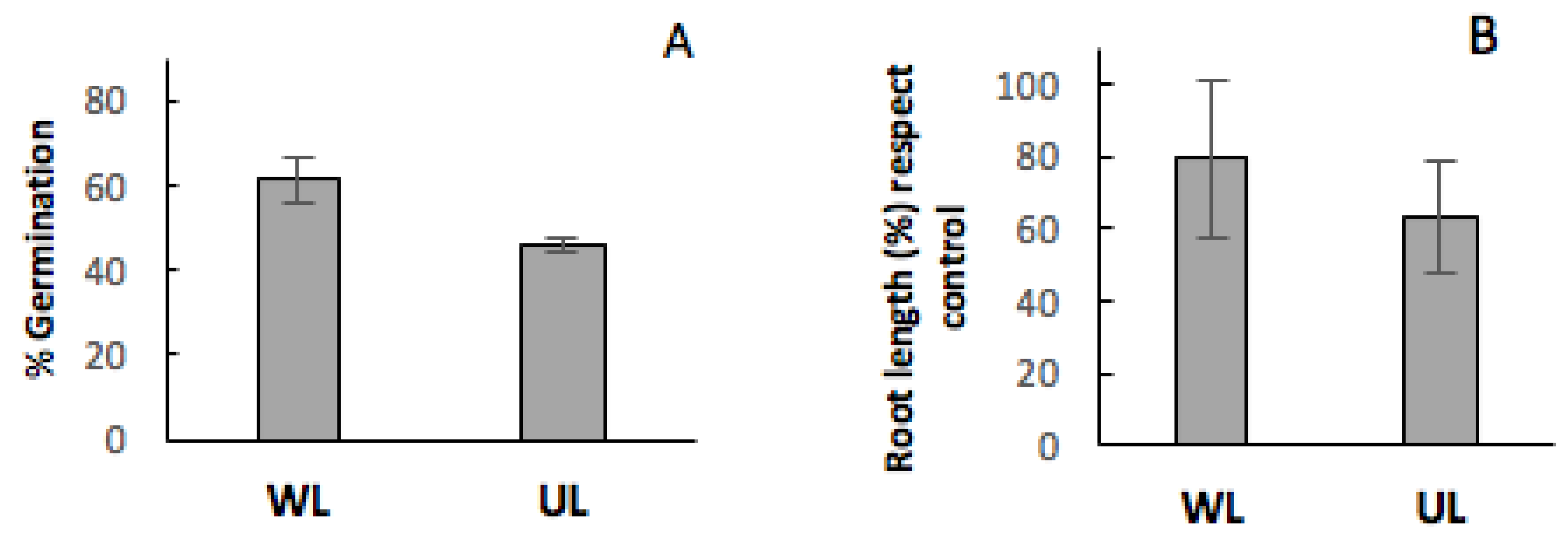
2.2. Effect of Leaf Litter on Germination
2.3. Effect of Leaf Litter at the Stage of Seedling Growth
3. Discussion
4. Materials and Methods
4.1. Collection of Leaf Litter and Preparation of Substrates
- Vermiculite (control): substrate composed only of vermiculite (2 dm3).
- Untreated Leaf Litter ⅓ (UL 1/3): 1.33 dm3 vermiculite and 0.66 dm3 untreated leaf litter.
- Untreated Leaf Litter ⅔ (UL 2/3): 0.66 dm3 vermiculite and 1.33 dm3 untreated leaf litter.
- Washed Leaf litter ⅓ (WL 1/3): 1.33 dm3 vermiculite and 0.66 dm3 washed leaf litter.
- Washed Leaf litter ⅔ (WL 2/3): 0.66 dm3 vermiculite and 1.33 dm3 washed leaf litter.
4.2. Target Species
4.3. Experiment Design
- -
- Germination percentage (GP): The final germination percentage was calculated using the following formula:GP = number of germinated seeds/number of seeded seeds.
- -
- Germination rate (GR): The germination rate is an arithmetic mean that indicates the days required for germination [71]. It was calculated using the formula cited by [72]:where GR is the germination rate; N1, N2, …, Nn represent the number of days from the beginning of the germination test; and G1, G2, …, Gn represent the number of germinated seeds at day n.
- -
- The time to reach 50% germination (T50). It was calculated according to the following formula [73]:where N is the final number of emergence and ni, nj cumulative number of seeds germinated by adjacent counts at times ti and tj, respectively when ni < N/2 < nj.
- -
- Root and stem length: Immediately after removing the seedlings from the culture media, the length of the roots and above-ground part of each individual was measured, with millimetric precision.
4.4. Quantification of Allelopathic Compounds
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- IPPC. Climate Change 2007: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Bonanomi, G.; Incerti, G.; Antignani, V.; Capodilupo, M.; Mazzoleni, S. Decomposition and nutrient dynamics in mixed litter of Mediterranean species. Plant Soil 2010, 331, 481–496. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I. Deuterium labelling of roots provides evidence of deep water access and hydraulic lift by Pinus nigra in a Mediterranean forest of NE Spain. Environ. Exp. Bot. 2003, 49, 201–208. [Google Scholar] [CrossRef]
- Cardillo, E.; Bernal, C. Morphological response and growth of cork oak (Quercus suber L.) seedlings at different shade levels. For. Ecol. Manag. 2006, 222, 296–301. [Google Scholar] [CrossRef]
- Gutiérrez, J.R.; Squeo, F.A. Importancia de los arbustos en los ecosistemas semiáridos de Chile. Ecosistemas 2004, 13, 36–45. [Google Scholar]
- Montané, F.; Romanyà, J.; Rovira, P.; Casals, P. Aboveground litter quality changes may drive soil organic carbon increase after shrub encroachment into mountain grasslands. Plant Soil 2010, 337, 151–165. [Google Scholar] [CrossRef]
- Alías, J.C.; García, M.; Sosa, T.; Valares, C.; Chaves, N.; Alías, J.C. Carbon storage in the different compartments of two systems of shrubs of the southwestern Iberian Peninsula. Agrofor. Syst. 2015, 89, 575–585. [Google Scholar] [CrossRef]
- Rebecca, A.; Montgomery, P.B.; Reich, B.J.P. Untangling positive and negative biotic interactions: Views from above and below ground in a forest ecosystem. Ecology 2010, 91, 3641–3655. [Google Scholar]
- Tálamo, A.; Barchuk, A.; Cardozo, S.; Trucco, C.; Marás, G.; Trigo, C. Direct versus indirect facilitation (herbivore mediated) among woody plants in a semiarid Chaco forest: A spatial association approach. Austral Ecol. 2015, 40, 573–580. [Google Scholar] [CrossRef]
- Costa, A.; Villa, S.; Alonso, P.; García-Rodríguez, J.; Martín, F.; Martínez-Ruiz, C.; Fernández-Santos, B. Can native shrubs facilitate the early establishment of contrasted co-occurring oaks in Mediterranean grazed areas? J. Veg. Sci. 2017, 28, 1047–1056. [Google Scholar] [CrossRef]
- Van Zonneveld, M.; Gutiérrez, J.R.; Holmgren, M. Shrub facilitation increases plant diversity along an arid scrubland-temperate rain forest boundary in South America. J. Veg. Sci. 2012, 23, 541–551. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Gómez, J.M.; Zamora, R.; Boettinger, J.L. Canopy vs. soil effects of shrubs facilitating tree seedlings in Mediterranean montane ecosystems. J. Veg. Sci. 2005, 16, 191–198. [Google Scholar] [CrossRef]
- Nuñez, J.J. Respuestas Ecofisiológicas y Demográficas de Quercis Ilex l. a Alteraciones del Balance Facilitación/Competencia del Matorral en un Ambiente Semiárido. Ph.D. Thesis, Universidad of Extremadura, Extremadura, Spain, 2013. [Google Scholar]
- Rolo, V.; Moreno, G. Interspecific competition induces asymmetrical rooting profile adjustments in shrub-encroached open oak woodlands. Trees 2012, 26, 997–1006. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Al Harun, A.Y.; Johnson, J.; Robinson, R. The contribution of volatilization and exudation to the allelopathic phytotoxicity of invasive Chrysanthemoides monilifera subsp. monilifera (boneseed). Boil. Invasions 2015, 17, 3609–3624. [Google Scholar] [CrossRef]
- Al Harun, A.Y.; Johnson, J.; Uddin, N.; Robinson, R. The effects of temperature on decomposition and allelopathic phytotoxicity of boneseed litter. J. Environ. Sci. 2015, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reigosa, M.J.; Ocaña, A.C. Studies on the allelopathic potential of Acacia dealbata Link.: Allelopathic potential produced during laboratory decomposition of plant residues incorporated into soil. J. Alleloch. Interac. 2017, 3, 23–33. [Google Scholar]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and Exotic Plant Invasion: From Molecules and Genes to Species Interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H. Roles of Allelopathy in Plant Biodiversity and Sustainable Agriculture. Crit. Rev. Plant Sci. 1999, 18, 609–636. [Google Scholar] [CrossRef]
- Fernandez, C.; Lelong, B.; Vila, B.; Mévy, J.-P.; Robles, C.; Greff, S.; Dupouyet, S.; Bousquet-Mélou, A. Potential allelopathic effect of Pinus halepensis in the secondary succession: An experimental approach. Chemoecology 2006, 16, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Quintela-Sabaris, C.; Vendramin, G.G.; Castro-Fernández, D.; Fraga, M.I. Chloroplast DNA phylogeography of the shrub Cistus ladanifer L. (Cistaceae) in the highly diverse Western Mediterranean region. Plant Boil. 2011, 13, 391–400. [Google Scholar] [CrossRef]
- Lousa, M.; Espírito-Santo, M.D.; Rosa, M.L.; Luz, J.P. Estevais do Centro e Sul de Portugal. Alguns tipos. Stvdia Botánica 2009, 8, 67–77. [Google Scholar]
- Díaz-González, T.E.; López-Pacheco, M.J.; Pérez-Morales, C.; Llamas-García, F.; Penas-Merino, A. La clase Cisto-Lavanduletea en la provincia de León. Acta Bot. Malcit. 1989, 14, 226–230. [Google Scholar]
- Tárrega, R.; Luis-Calabuig, E.; Valbuena, L. Eleven years of recovery dynamic after experimental burning and cutting in two Cistus communities. Acta Oecologica 2001, 22, 277–283. [Google Scholar] [CrossRef]
- Mendes, P.; Meireles, C.; Vila-Viçosa, C.M.; Musarella, C.M.; Pinto-Gomes, C. Best management practices to face degraded territories occupied by Cistus ladanifer shrublands—Portugal case study. Plant Biosyst. 2015, 149, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Calvo, L.; Tárrega, R.; Luis, E.; Valbuena, L.; Marcos, E. Recovery after Experimental Cutting and Burning in Three Shrub Communities with Different Dominant Species. Plant Ecol. 2005, 180, 175–185. [Google Scholar] [CrossRef]
- Castro, H.; Freitas, H. Above-ground biomass and productivity in the Montado: From herbaceous to shrub dominated communities. J. Arid. Environ. 2009, 73, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Escudero, J.C.; Núñez-Olivera, E.; Martínez-Abaigar, J.; Garcia-Novo, F. A comparative study of Cistus ladanifer shrublands in Extremadura (CW Spain) on the basis of woody species composition and cover. Vegetatio 1995, 117, 123–132. [Google Scholar] [CrossRef]
- Chaves, N.; Alías, J.C.; Sosa, T. Phytotoxicity of Cistus ladanifer L.: Role of allelopathy. Allelopath. J. 2016, 38, 113–131. [Google Scholar]
- Chaves, N.; Escudero, J.C. Allelopathic effect of Cistus ladanifer on seed germination. Funct. Ecol. 1997, 11, 432–440. [Google Scholar] [CrossRef]
- Dias, A.S.; Dias, L.S.; Pereira, I.P. Activity of water extracts of Cistus ladanifer and Lavandula stoechas in soil on germination and early growth of wheat and Phalaris minor. Allelopath. J. 2004, 14, 59–64. [Google Scholar]
- Alías, J.C. Influence of Climatic Factors on the Synthesis and Activity of Phytotoxic Compounds Secretred by Cistus ladanifer L. Ph.D. Thesis, Universidad of Extremadura, Extremadura, Spain, 2006. [Google Scholar]
- Chaves, N.; Sosa, T.; Escudero, J.C. Plant growth inhibiting flavonoids in exudate of Cistus ladanifer and in associated soils. J. Chem. Ecol. 2001, 27, 623–631. [Google Scholar] [CrossRef]
- Herranz, J.M.; Ferrandis, P.; Copete, M.A.; Duro, E.M.; Zalacain, A. Effect of allelopathic compounds produced by Cistus ladanifer on germination of 20 Mediterranean taxa. Plant Ecol. 2005, 184, 259–272. [Google Scholar] [CrossRef]
- Scognamiglio, M.; D’Abrosca, B.; Esposito, A.; Pacifico, S.; Monaco, P.; Fiorentino, A. Plant growth inhibitors: Allelopathic role or phytotoxic effects? Focus on Mediterranean biomes. Phytochem. Rev. 2013, 12, 803–830. [Google Scholar] [CrossRef]
- Sosa, T.; Valares, C.; Alías, J.C.; Lobón, N.C.; Alías, J.C. Persistence of flavonoids in Cistus ladanifer soils. Plant Soil 2010, 337, 51–63. [Google Scholar] [CrossRef]
- Katsuichiro, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar]
- Chaves, N.; Ríos, J.J.; Gutierrez, C.; Escudero, J.C.; Olías, J.M. Analysis of secreted flavonoids of Cistus ladanifer L. by high-performance liquid chromatography–particle beam mass spectrometry. J. Chromatogr. A 1998, 799, 111–115. [Google Scholar] [CrossRef]
- Alías, J.C.; Sosa, T.; Valares, C.; Escudero, J.C.; Chaves, N. Seasonal Variation of Cistus ladanifer L. Diterpenes. Plants 2012, 1, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, N.; Sosa, T.; Valares, C.; Alías, J.C. Routes of incorporation of phytotoxic compounds of Cistus ladanifer L. into soil. Allelopath. J. 2015, 36, 25–36. [Google Scholar]
- Dorrepaal, E.; Cornelissen, J.H.C.; Aerts, R. Changing leaf litter feedbacks on plant production across contrasting sub-arctic peatland species and growth forms. Oecologia 2006, 151, 251–261. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Barile, E.; Capodilupo, M.; Antignani, V.; Mingo, A.; Lanzotti, V.; Scala, F.; Mazzoleni, S. Phytotoxicity, not nitrogen immobilization, explains plant litter inhibitory effects: Evidence from solid-state 13C NMR spectroscopy. New Phytol. 2011, 191, 1018–1030. [Google Scholar] [CrossRef] [Green Version]
- Samedani, B.; Juraimi, A.S.; Rafii, M.Y.; Anuar, A.R.; Awadz, S.A.S.; Anwar, P. Allelopathic Effects of Litter Axonopus compressus against Two Weedy Species and Its Persistence in Soil. Sci. World J. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Iglesias, B.; Olmo, M.; Gallardo, A.; Villar, R. Short-term effects of litter from 21 woody species on plant growth and root development. Plant Soil 2014, 381, 177–191. [Google Scholar] [CrossRef]
- López-Pintor, A.; Sal, A.G.; Benayas, J.M.R. Shrubs as a source of spatial heterogeneity—the case of Retama sphaerocarpa in Mediterranean pastures of central Spain. Acta Oecologica 2006, 29, 247–255. [Google Scholar] [CrossRef]
- Chaves, N.; Escudero, J.C.; Gutiérrez-Merino, C. Role of Ecological Variables in the Seasonal Variation of Flavonoid Content of Cistus ladanifer Exudate. J. Chem. Ecol. 1997, 23, 579–603. [Google Scholar] [CrossRef]
- Maqbool, N.; Farooq, M.; Cheema, Z.A.; Siddique, K.H.M. Allelopathy and Abiotic Stress Interaction in Crop Plants. In Allelopathy; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; pp. 451–468. [Google Scholar]
- Masa, C.V.; Díaz, T.S.; Alías, J.C.; Lobón, N.C. Quantitative Variation of Flavonoids and Diterpenes in Leaves and Stems of Cistus ladanifer L. at Different Ages. Molecules 2016, 21, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Chemical composition and herbicidal activity of the essential oil from a Cistus ladanifer L. population from Spain. Nat. Prod. Res. 2012, 26, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Zackrisson, O.; Nilsson, M.C. Allelopathic effects by Empetrum hermaphroditum on seed germination of two boreal tree species. Canad. J. For. Res. 2011, 22, 1310–1319. [Google Scholar] [CrossRef]
- Skulman, B.W.; Mattice, J.D.; Cain, M.D.; Gbur, E.E. Evidence for allelopathic interference of Japanese honeysuckle (Lonicera japonica) to loblolly and shortleaf pine regeneration. Weed Sci. 2004, 52, 433–439. [Google Scholar] [CrossRef]
- Green, S.; Kirkham, M.; Clothier, B. Root uptake and transpiration: From measurements and models to sustainable irrigation. Agric. Water Manag. 2006, 86, 165–176. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J. Exp. Bot. 2001, 52, 419–426. [Google Scholar] [CrossRef]
- Peer, W.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef]
- Alias, J.C.; Sosa, T.; Escudero, J.C.; Chaves, N. Autotoxicity Against Germination and Seedling Emergence in Cistus ladanifer L. Plant Soil 2006, 282, 327–332. [Google Scholar] [CrossRef]
- Lobón, N.C.; De La Cruz, I.F.; Alías, J.C. Autotoxicity of Diterpenes Present in Leaves of Cistus ladanifer L. Plants 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chon, S.U.; Jennings, J.A.; Nelson, C.J. Alfalfa (Medicago sativa L.) autotoxicity: Current Status. Allelopath J. 2006, 18, 1–24. [Google Scholar]
- Ghimire, B.K.; Ghimire, B.; Yu, C.Y.; Chung, I.-M. Allelopathic and Autotoxic Effects of Medicago sativa-Derived Allelochemicals. Plants 2019, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. The use of shrubs as nurse plants: A new technique for reforestation in Mediterranean mountains. Restor. Ecol. 2002, 10, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Koorem, K.; Price, J.N.; Moora, M. Species-Specific Effects of Woody Litter on Seedling Emergence and Growth of Herbaceous Plants. PLoS ONE 2011, 6, e26505. [Google Scholar] [CrossRef] [Green Version]
- Chaves, N.; Sosa, T.; Alías, J.C.; Escudero, J.C. Germination inhibition of herbs in Cistus ladanifer L. soil: Possible involvemente of allelochemicals. Allelopath J. 2003, 11, 31–42. [Google Scholar]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Castellanos, D.; Simonet, A.M.; Molinillo, J.M. Structure-Activity Relationship (sar) studies of benzoxazinones, their degradation products, and analogues. Phytotoxicity on problematic weeds Avena fatua L. and Lolium rigidum Gaud. J. Agric. Food Chem. 2006, 54, 1040–1048. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Alías, J.C.; Escudero, J.C. Identification and effects of interaction phytotoxic compounds from exudate of Cistus ladanifer leaves. J. Chem. Ecol. 2001, 27, 611–621. [Google Scholar] [CrossRef]
- Ruiz, R.E.; Isla, L.H.; Sánchez, L.R.B.; Aro, M.H.; García, S.T.; González, Y.R.; Mill, M.E. Synergic effect of tannins and flavonoids in Terminalia catappa L. on the mycelial growth of Rhizoctonia solani Kühn and Sclerotium rolfsii Sacc. Fitosanidad 2012, 16, 27–32. [Google Scholar]
- Cleavitt, N.L.; Fahey, T.J.; Battles, J.J. Regeneration ecology of sugar maple (Acer saccharum): Seedling survival in relation to nutrition, site factors, and damage by insects and pathogens. Can. J. For. Res. 2011, 41, 235–244. [Google Scholar] [CrossRef]
- Delgado, J.A.; Serrano, J.M.; López, F.; Acosta, F.J. Heat shock, mass-dependent germination, and seed yield as related components of fitness in Cistus ladanifer. Environ. Exp. Bot. 2001, 46, 11–20. [Google Scholar] [CrossRef]
- Scuderi, D.; Gregorio, R.D.; Toscano, S.; Cassaniti, C.; Romano, D.M. Germination behaviour of four mediterranean Cistus L. species in relation to high temperature. Ecol. Quest. 2010, 12, 175–186. [Google Scholar] [CrossRef]
- Ruiz de la Torre, J. Manual de la Flora para la Restauración de Áreas Críticas y Diversificación en Masas Forestales; Consejería de Medio Ambiente de la Junta de Andalucía: Sevilla, Spain, 1996. [Google Scholar]
- Nonogaki, H. Seed dormancy and germination—emerging mechanisms and new hypotheses. Front. Plant Sci. 2014, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pece, M.G.; De Benítez, C.G.; Acosta, M.; Bruno, C.; Saavedra, S.; Buvenas, O. Germinación de Tipuana tipu (Benth.) O. Kuntze (tipa blanca) en condiciones de laboratorio. Quebracho 2010, 18, 5–15. [Google Scholar]
- Nakagawa. Teste de Vigor Baseados no Desempenho das Plântulas. In Vigor de Sementes: Conceitos e Testes; Abrates: Londrinas, Brasil, 1999. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Hafeez, K.; Ahmad, N. Thermal hardening: A new seed vigour enhancement tool in rice. J. Integ. Plant Biol. 2005, 47, 187–193. [Google Scholar] [CrossRef]



| Compound | UL (μg/g dw) | WL (μg/g dw) | Quantity Removed (%) |
|---|---|---|---|
| Ap | 6.0 ± 0.4 | 1.2 ± 0.1 | 80.6 ± 2.7 |
| Ap-4 | 57.8 ± 3.2 | 25.6 ± 0.1 | 55.6 ± 2.6 |
| Ap-7 | 385.5 ± 10.4 | 44.1 ± 1.4 | 88.5 ± 0.2 |
| K-3 | 85.8 ± 17.1 | 1.5 ± 0.1 | 98.2 ± 0.4 |
| K-3,4 | 86.1 ± 10.6 | 12.7 ± 0.2 | 85.1 ± 2.0 |
| K-3,7 | 2564.7 ± 114.7 | 126.6 ± 15.7 | 91.5 ± 0.6 |
| D1 | 25.8 ± 0.8 | 2.5 ± 0.1 | 90.1 ± 0.7 |
| D2 | 2.2 ± 0.1 | 0.3 ± 0.0 | 88.1 ± 1.0 |
| D3 | 3.9 ± 0.9 | 0.4 ± 0.0 | 88.8 ± 2.8 |
| Treatment | R. sphaerocarpa | C. multiflorus | L. stoechas | C. crispus | C. salviifolius | |
|---|---|---|---|---|---|---|
| C | 9.8 ± 1.8 a | 17.4 ± 3.8 a | 15.6 ± 1.4 a | 27.4 ± 1.9 a | 32.2 ± 2.5 a | |
| WL ⅓ | 9.8 ± 0.5 a | 21.7 ± 4.0 a | 27.1 ± 5.4 b | 31.5 ± 2.2 ba | 29.6 ± 2.8 a | |
| GR | WL ⅔ | 11.6 ± 2.7 a | 20.2 ± 2.3 a | 24.9 ± 2.6 b | 26.9 ± 1.9 a | 33.0 ± 3.4 a |
| UL ⅓ | 9.7 ± 1.4 a | 19.5 ± 2.4 a | 31.2 ± 1.3 b | 45.3 ± 14.0 b | 32.85 ± 0.4 a | |
| UL ⅔ | 13.8 ± 4.7 b | 17.1 ± 4.0 a | 28.5 ± 3.4 b | 39.7 ± 8.8 b | 35.8 ± 3.4 a | |
| C | 8.33 ±0.7 a | 16.8 ± 3.0 a | 13.1 ± 1.5 a | 23.9 ± 1.0 a | 31.7 ± 5.9 a | |
| WL ⅓ | 8.50 ± 0.4 a | 21.6 ± 3.7 a | 22.5 ± 10.4 ab | 23.3 ± 2.9 a | 28.8 ± 3.8 a | |
| T50 | WL ⅔ | 9.18 ± 1.3 a | 19.3 ± 3.7 a | 24.0 ± 2.2 b | 23.8 ± 0.7 a | 32.7 ± 3.7 a |
| UL ⅓ | 8.15 ± 0.3 a | 19.3 ± 8.2 a | 27.1 ± 0.9 b | 31.6 ± 3.6 b | 31.7 ± 0.9 a | |
| UL ⅔ | 10.2 ± 4.3 a | 16.9 ± 4.8 a | 29.4 ± 3.9 b | 28.2 ± 3.2 b | 35.6 ± 4.0 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego, J.C.A.; Caro, J.G.; Campos, V.H.; Lobón, N.C. Effect of Leaf Litter from Cistus ladanifer L. on the Germination and Growth of Accompanying Shrubland Species. Plants 2020, 9, 593. https://doi.org/10.3390/plants9050593
Gallego JCA, Caro JG, Campos VH, Lobón NC. Effect of Leaf Litter from Cistus ladanifer L. on the Germination and Growth of Accompanying Shrubland Species. Plants. 2020; 9(5):593. https://doi.org/10.3390/plants9050593
Chicago/Turabian StyleGallego, Juan Carlos Alías, Jonás González Caro, Virginia Hinojal Campos, and Natividad Chaves Lobón. 2020. "Effect of Leaf Litter from Cistus ladanifer L. on the Germination and Growth of Accompanying Shrubland Species" Plants 9, no. 5: 593. https://doi.org/10.3390/plants9050593
APA StyleGallego, J. C. A., Caro, J. G., Campos, V. H., & Lobón, N. C. (2020). Effect of Leaf Litter from Cistus ladanifer L. on the Germination and Growth of Accompanying Shrubland Species. Plants, 9(5), 593. https://doi.org/10.3390/plants9050593





