The Role of Root Exudates of Barley Colonized by Pseudomonas fluorescens in Enhancing Root Colonization by Fusarium culmorum
Abstract
1. Introduction
2. Results
2.1. The Effect of Experimental Conditions on the Amount of F. culmorum and P. fluorescens in the Roots and Root Rot Incidence in Barley Plants
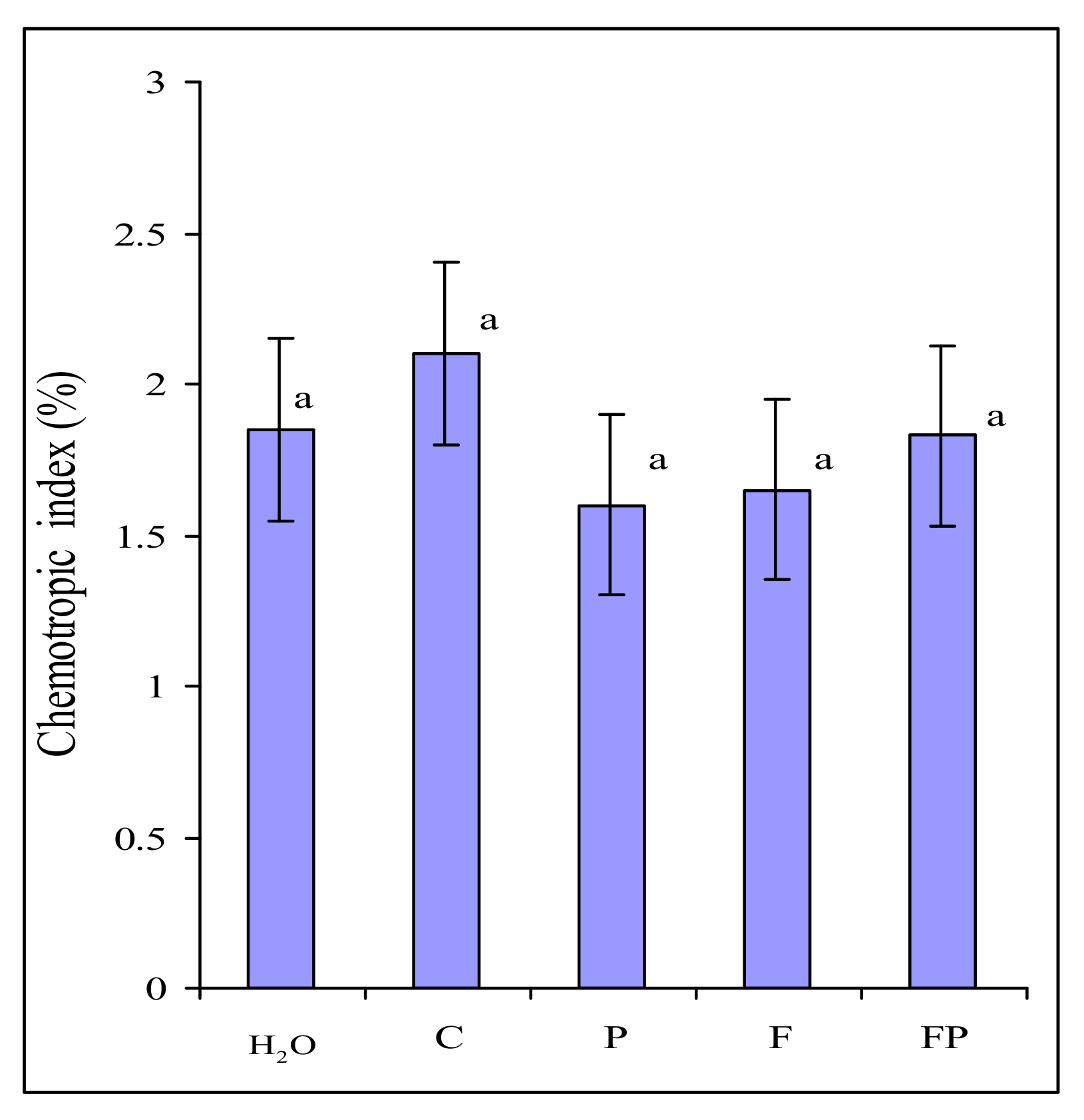
2.2. Testing the Chemotropic Response in F. culmorum Towards Barley Root Exudates
2.3. Influence of Root Exudates on the Growth of F. culmorum
2.4. The Effect of P. fluorescens on the Growth of F. culmorum
2.5. Quantitative Composition of Sugars, Organic Acids and Amino Acids in Root Exudates of Control Barley, Barley Colonized by P. fluorescens and Jointly by F. culmorum + P. fluorescens
3. Discussion
4. Materials and methods
4.1. Study Objects
4.2. Experimental Conditions
4.3. Testing the Chemotropism of F. culmorum Towards Barley Exudates
4.4. Assessment of the influence of barley root exudates on the growth of F. culmorum
4.5. Assessing the Effect of P. fluorescens on the Growth of F. culmorum
4.6. Chromatographic Analysis of Low-Molecular-Weight Components of Root Exudates
4.7. Statistical Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDA | Czapek-Dox agar |
| CDB | Czapek-Dox broth |
| UPLC | ultra performance liquid chromatography |
| ACA | aromatic carboxylic acids |
| DW | Dry Weight |
References
- Cook, R.J.; Baker, K.F. The Nature and Practice of Biological Control of Plant Pathogens, 2nd ed.; APS Press: St. Paul, MN, USA, 1983; p. 539. [Google Scholar]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef]
- Beccari, G.; Covarelli, L.; Nicholson, P. Infection processes and soft wheat response to root rot and crown rot caused by Fusarium culmorum. Plant Pathol. 2011, 60, 671–684. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Steed, A.; Nicholson, P. Colonization of soft wheat following infection of the stem base by Fusarium culmorum and translocation of deoxynivalenol to the head. Plant Pathol. 2012, 61, 1121–1129. [Google Scholar] [CrossRef]
- Becher, R.; Miedaner, T.; Wirsel, S.G.R. Biology, diversity, and management of FHB-causing Fusarium species in small-grain cereals. In The Mycota XI, Agricultural Applications, 2nd ed.; Kempken, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 11, pp. 199–241. [Google Scholar]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Mesterhazy, A. Role of deoxynivalenol in aggressiveness of Fusarium graminearum and F. culmorum and in resistance to Fusarium head blight. Eur. J. Plant Pathol. 2002, 108, 675–684. [Google Scholar] [CrossRef]
- Wagacha, J.M.; Muthomi, J.W. Fusarium culmorum: Infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop. Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- Winter, M.; Koopmann, B.; Döll, K.; Karlovsky, P.; Kropf, U.; Schlüter, K.; von Tiedemann, A. Mechanisms regulating grain contamination with trichothecenes translocated from the stem base of wheat (Triticum aestivum) infected with Fusarium culmorum. Phytopathology 2013, 103, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Rocha, O.; Ansari, K.; Doohan, F.M. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit. Contam. 2005, 22, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Bensassi, F.; Gallerne, C.; El Dein, O.S.; Lemaire, C.; Hajlaoui, M.R.; Bacha, H. Involvement of mitochondria-mediated apoptosis in deoxynivalenol cytotoxicity. Food Chem. Toxicol. 2012, 50, 1680–1689. [Google Scholar] [CrossRef]
- Weller, D.M.; Landa, B.B.; Mavrodi, O.V.; Schroeder, L.K.; De la Fuente, L.; Blouin Bankhead, S.; Allende Molar, R.; Bonsall, R.F.; Mavrodi, D.V.; Thomashow, L.S. Role of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biol. 2007, 9, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; de Bruijn, I.; de Kock, M.J.D. Cyclic lipopeptide production by plant-associated Pseudomonas species: Diversity, activity, biosynthesis and regulation. Mol. Plant Microb. Interact. 2006, 19, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Clement, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo-and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Strunnikova, O.K.; Shakhnazarova, V.Y.; Vishnevskaya, N.A.; Chebotar, V.K.; Tikhonovich, I.A. Development and relations of Fusarium culmorum and Pseudomonas fluorescens in soil. Microbiology 2007, 76, 596–602. [Google Scholar] [CrossRef]
- Strunnikova, O.K.; Shakhnazarova, V.Y.; Vishnevskaya, N.A.; Chebotar, V.K.; Tikhonovich, I.A. Interactions between Fusarium culmorum and Pseudomonas fluorescens in the rhizosphere and rhizoplane of barley. Mycol. Phytopathol. 2008, 42, 70–77. [Google Scholar]
- Strunnikova, O.K.; Vishnevskaya, N.A.; Tikhonovich, I.A. Colonization of barley roots by Fusarium culmorum and influence of Pseudomonas fluorescens on the process. Microbiology 2010, 79, 865–870. [Google Scholar] [CrossRef]
- Strunnikova, O.K.; Vishnevskaya, N.A.; Ruchiy, A.S.; Shakhnazarova, V.Y.; Vorobyov, N.I.; Chebotar, V.K. The influence of soils with different textures on development, colonization capacity and interactions between Fusarium culmorum and Pseudomonas fluorescens in soil and on barley roots. Plant Soil 2015, 389, 131–144. [Google Scholar] [CrossRef]
- De Weert, S.; Vermeiren, H.; Mulders, I.H.M.; Kuiper, I.; Hendrickx, N.; Bloemberg, G.V.; Vanderleyden, J.; De Mot, R.; Lugtenberg, B.J.J. Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol. Plant Microb. Interact. 2002, 15, 1173–1180. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.J.; Pare, P.W.; Bais, H.P. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef]
- Ling, N.; Raza, W.; Ma, J.; Huang, Q.; Shen, Q. Identification and role of organic acids in watermelon root exudates for recruiting Paenibacillus polymyxa SQR-21 in the rhizosphere. Eur. J. Soil Biol. 2011, 47, 374–379. [Google Scholar] [CrossRef]
- Neal, A.L.; Ahmad, S.; Gordon-Weeks, R.; Ton, J. Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS ONE 2012, 4, e35498. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yang, C.; Mei, X.; Shen, S.; Raza, W.; Shen, Q.; Xu, Y. The effect of organic acids from tomato root exudates on rhizosphere colonization of Bacillus amyloliquefaciens T-5. Appl. Soil Ecol. 2013, 64, 15–22. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, D.; Liu, Y.; Li, S.; Shen, Q.; Zhang, R. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 2013, 374, 689–700. [Google Scholar] [CrossRef]
- Turrà, D.; El Ghalid, M.; Rossi, F.; Di Pietro, A. Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature 2015, 527, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Vitale, S.; Turrà, D.; Reverberi, M.; Fanelli, C.; Vinale, F.; Marra, R.; Ruocco, M.; Pascale, A.; d’Errico, G.; et al. Root exudates of stressed plants stimulate and attract Trichoderma soil fungi. MPMI 2018, 31, 982–994. [Google Scholar] [CrossRef]
- Shaposhnikov, A.I.; Vishnevskaya, N.A.; Shakhnazarova, V.Y.; Belimov, A.A.; Strunnikova, O.K. The role of barley root exudates as nutrition source in the interactions between Fusarium culmorum and Pseudomonas fluorescens. Mycol. Phytopathol. 2019, 53, 301–308. [Google Scholar]
- Akhter, A.; Hage-Ahmed, K.; Soja, G.; Steinkellner, S. Potential of Fusarium wilt-inducing chlamydospores in vitro behaviour in root exudates and physiology of tomato in biochar and compost amended soil. Plant Soil 2016, 406, 425–440. [Google Scholar] [CrossRef]
- Kamilova, F.; Lamers, G.; Lugtenberg, B. Biocontrol strain Pseudomonas fluorescens WCS365 inhibits germination of Fusarium oxysporum spores in tomato root exudates as well as subsequent formation of new spores. Environ. Microbiol. 2008, 10, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, S.; Mammerler, R.; Vierheilig, H. Microconidia germination of the tomato pathogen Fusarium oxysporum in the presence of root exudates. J. Plant Interact. 2005, 1, 23–30. [Google Scholar] [CrossRef]
- Steinkellner, S.; Mammerler, R.; Vierheilig, H. Germination of Fusarium oxysporum in root exudates from tomato plants challenged with different Fusarium oxysporum strains. Eur. J. Plant Pathol. 2008, 122, 395–401. [Google Scholar] [CrossRef]
- Li, X.G.; Wei, Q.; Liu, B.; Alam, M.S.; Wang, X.X.; Shen, W.; Han, Z.M. Root exudates of transgenic cotton and their effects on Fusarium oxysporum. Front. Biosci. 2013, 18, 725–733. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaposhnikov, A.I.; Shakhnazarova, V.Y.; Vishnevskaya, N.A.; Borodina, E.V.; Strunnikova, O.K. Aromatic carboxylic acids in root exudates of barley and their influence on growth of Fusarium culmorum and Pseudomonas fluorescens. Appl. Biochem. Microbiol. 2020, 56, 1–9. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Ann. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Makarova, N.; Lugtenberg, B. Effects of the tomato pathogen Fusarium oxysporum f. sp radicis-lycopersici and of the biocontrol bacterium Pseudomonas fluorescens WCS365 on the composition of organic acids and sugars in tomato root exudate. Mol. Plant-Microb. Interact. 2006, 19, 1121–1126. [Google Scholar] [CrossRef]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Azarova, T.; Makarova, N.; Lugtenberg, B. Organic acids, sugars, and Ltryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol. Plant-Microb. Interact. 2006, 19, 250–256. [Google Scholar] [CrossRef]
- Strunnikova, O.K.; Feoktistova, A.S.; Vishnevskaya, N.A.; Chebotar, V.K. Role competition between Pseudomonas fluorescens 2137GUS and Fusarium culmorum for colonization of barley roots. Mycol. Phytopathol. 2011, 45, 362–369. [Google Scholar]
- Jones, D.L.; Darrah, P.R. Re-sorption of organic-compounds by roots of Zea mays L. and its consequences in the rhizosphere. 2. Experimental and model evidence for simultaneous exudation and re-sorption of soluble C compounds. Plant Soil 1993, 153, 47–59. [Google Scholar] [CrossRef]
- Jones, D.L.; Darrah, P.R. Amino-acid influx at the soil-root interface of Zea mays L. and its implications in the rhizosphere. Plant Soil 1994, 163, 1–12. [Google Scholar] [CrossRef]
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon flow in the rhizosphere: Carbon trading at the soil–root interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Phillips, D.A.; Fox, T.C.; King, M.D.; Bhuvaneswari, T.V.; Teuber, L.R. Microbial products trigger amino acid exudation from plant roots. Plant Physiol. 2004, 136, 2887–2894. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar]


| Conditions of the Experiment | Vermiculite Inoculation | Diseased Plants (%) | Amount of the Fungus after the Growth of Barley | Amount of the Bacterium after the Growth of Barley | ||||
|---|---|---|---|---|---|---|---|---|
| in the Vermiculite CFU/g of Root (103) | In Water | In the Vermiculite CFU/g of Root (105) | In Water | |||||
| CFU/g of Root (103) | CFU/mL (102) | CFU/g of Root (105) | CFU/mL (103) | |||||
| Experiment 1: The growth of barley in the vermiculite ‒ 67 h, the exudation in water ‒ 24 h. The age of barley at the end of the experiment - 91 h | None | 0 | ||||||
| F. culmorum | 16 | 1.2 ± 0.3 a | ||||||
| P. fluorescens | 0 | 95.7 ± 5 a | ||||||
| F. culmorum + P. fluorescens | 8 | 29 ± 10 b | 48.7 ± 14 b | |||||
| Experiment 2: The growth of barley in the vermiculite ‒ 36 h, the exudation in water ‒ 96 h. The age of barley at the end of the experiment -132 h | None | 0 | ||||||
| F. culmorum | 16 | 19.9 ± 3 a | 11.7 ± 3.6 c | 0.5 ± 0.05 a | ||||
| P. fluorescens | 0 | 221.9 ± 63 a | 83.3 ± 25 c | 15 ± 2 a | ||||
| F. culmorum + P. fluorescens | 10 | 158.9 ± 35 b | 40.3 ± 6 d | 3,3 ± 1 b | 90,7 ± 27 c | 46.7 ± 11 b | 12,3 ± 1,8 a | |
| Components of Barley Root Exudates | Initial Amount (µg/mL) | Amount (µg/mL) Consumed by | |
|---|---|---|---|
| F. culmorum | P. fluorescens | ||
| Sugars: | |||
| glucose | 357.6 ± 68 | 328.8 ± 59 a | 240 ± 23.5 b |
| fructose | 34.7 ± 7.3 | 16.8 ± 3.8 a | 24.5 ± 4.7 a |
| other | 147.1 ± 20.7 | 1.1 ± 0.2 a | 1 ± 0.1 a |
| Organic acids: | |||
| malic | 11.7 ± 2.1 | 6 ± 1.3 a | 9.2 ± 2 a |
| lactic | 9 ± 1.6 | 8 ± 1.9 a | 7.7 ± 1.4 a |
| other | 14.8 ± 2.8 | 0.1 ± 0.01a | 11 ± 3.3 b |
| Amino acids: | |||
| proline | 1.75 ± 0.22 | 0.9 ± 0.2 a | 0.4 ± 0.06 b |
| phenylalanine | 1.2 ± 0.2 | 0.8 ± 0.1 a | 1.1 ± 0.2 b |
| tryptophan | 0.85 ± 0.2 | 0.75 ± 0.08 a | 0.8 ± 0.09 b |
| histidine | 0.55 ± 0.13 | 0.33 ± 0.09 a | 0.47 ± 0.14 a |
| tyrosine | 0.45 ± 0.11 | 0.32 ± 0.1 a | 0.4 ± 0.11 a |
| valine | 0.57 ± 0.12 | 0.39 ± 0.1 a | 0.5 ± 0.1 a |
| lysine | 0.35 ± 0.1 | 0.15 ± 0.05 a | 0.32 ± 0.07 b |
| leucine | 0.69 ± 0.17 | 0.35 ± 0.09 a | 0.67 ± 0.12 b |
| isoleucine | 0.42 ± 0.13 | 0.19 ± 0.04 a | 0.4 ± 0.08 b |
| other | 2.08 ± 0.25 | 0.35 ± 0.1 a | 1.47 ± 0.2 b |
| Sugars | Amount of Sugars (μg/ g DW) in the Absence and Presence of Microbes | |||||
|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | |||||
| None | P. fluorescens | F. culmorum + P. fluorescens | None | P. fluorescens | F. culmorum + P. fluorescens | |
| Melibiose | 62 ± 14 a | 29.5 ± 15 b | 22 ± 9 b | ND | ND | ND |
| Maltose | 2968 ± 590 a | 3500 ± 350 a | 492 ± 190 b | 736 ± 244 a | 658 ± 151 a | 286 ± 83 b |
| Sucrose | 21 ± 10 a | 60 ± 30 ab | 9.5 ± 5 ac | 62 ± 29 a | 2 ± 1.1 b | 2.7 ± 1.2 b |
| Glucose | 1800 ± 485 a | 4500 ± 845 b | 1346 ± 497 a | 122.5 ± 27 a | 490 ± 152 b | 67 ± 31 a |
| Fructose | 140 ± 30 a | 210 ± 100 a | 133 ± 40 a | 132 ± 37 a | 32 ± 23 b | 21 ± 6 b |
| Arabinose | 729 ± 195 a | 359 ± 39 b | 104 ± 28 c | 141 ± 43 a | 167 ± 79 a | 89 ± 41 a |
| Xylose | 51 ± 9 a | 161 ± 27 b | 84 ± 28 a | 31 ± 9 a | 81 ± 19 b | 13 ± 7 c |
| Ribose | 199 ± 49 a | 127 ± 14 b | 150 ± 48 ab | 144 ± 58 a | 133 ± 72 a | 87 ± 37 a |
| Organic Acids | Amount of Organic Acids (μg/ g DW) in the Absence and Presence of Microbes | |||||
|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | |||||
| None | P. fluorescens | F. culmorum + P. fluorescens | None | P. fluorescens | F. culmorum + P. fluorescens | |
| Pyroglutamic | 12 ± 2 a | 4 ± 2 b | 0.7 ± 0.3 c | 18 ± 1 | ND | ND |
| Propionic | 523 ± 46 a | 2100 ± 298 b | 1980 ± 280 b | 74 ± 36 a | ND | 188 ± 86 a |
| Fumaric | 11 ± 5 a | 1.4 ± 0.5 b | 2.5 ± 0.6 b | 0.7 ± 0.3 a | 0.3 ± 0.13 a | ND |
| Acetic | 1673 ± 344 a | 912 ± 45 b | 919 ± 108 b | 1654 ± 334 a | 7865 ± 1320 b | 2184 ± 832 a |
| Lactic | 284 ± 76 a | 579 ± 128 b | 340 ± 78 a | 196 ± 10 a | 45 ± 8 b | 37 ± 14 b |
| Succinic | 935 ± 229 a | 60 ± 19 b | 132 ± 52 c | 1363 ± 341 a | 1173 ± 296 a | 295 ± 91 b |
| t-Aconitic | 15 ± 3 a | 10 ± 1 b | 6 ± 0.8 c | 210 ± 7 a | 49 ± 18 b | 2.6 ± 1.3 c |
| Malic | 1400 ± 298 a | 290 ± 82 b | 312 ± 97 b | 198 ± 21 a | 120 ± 6 b | ND |
| Pyruvic | 169 ± 33 a | 81 ± 15 b | 52 ± 11 c | 68 ± 4.5 a | 79 ± 13 a | 33 ± 6 b |
| Citric | 25 ± 3 a | 6 ± 0.8 b | 6 ± 0.8 b | 309 ± 93 a | 166 ± 48 b | 2.2 ± 0.5 c |
| Oxalic | 65 ± 14 a | 38 ± 8 ab | 25 ± 7 b | ND | ND | ND |
| Amino acids | Amount of amino acids (μg/ g DW) in the absence and presence of microbes | |||||
|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | |||||
| None | P. fluorescens | F. culmorum+ P. fluorescens | None | P. fluorescens | F. culmorum + P. fluorescens | |
| Phenylalanine | 36 ± 1.8 a | 21 ± 2.6 b | 4 ± 0.5 c | 2.8 ± 0.2 a | 17 ± 1.3 b | 5.4 ± 0.1 c |
| Leucine | 42 ± 4.5 a | 17 ± 1.8 b | 10 ± 2 c | 7.5 ± 0.2 a | 45 ± 2 b | 6 ± 0.2 a |
| Isoleucine | 27 ± 1 a | 10 ± 1.3 b | 7 ± 0.8 b | 1.9 ± 0.1 a | 34 ± 3 b | 1.9 ± 0.1 a |
| Lysine | 25 ± 5 a | 12 ± 1.6 b | 9 ± 1.1 b | 8 ± 0.2 a | 50 ± 2.5 b | 4 ± 0.1 c |
| Ornitine | 3 ± 0.9 a | 0.8 ± 0.2 b | 0.6 ± 0.1 b | 0.25 ± 0.1 a | 0.5 ± 0.15 a | 0.6 ± 0.15 a |
| Methionine | 4.3 ± 0.9 a | 1.7 ± 0.6 b | 0.7 ± 0.2 c | 0.9 ± 0.1 a | 4.5 ± 0.38 b | 1 ± 0.1 a |
| Valine | 38 ± 6 a | 14 ± 2.6 b | 7 ± 1.3 c | 8 ± 0.3 a | 70 ± 3.5 b | 3 ± 0.2 c |
| Tyrosine | 23 ± 1.5 a | 10 ± 1.1 b | 3.2 ± 0.5 c | 2.5 ± 0.2 a | 23 ± 1.1 b | 1.2 ± 0.1 c |
| Cysteine | 3 ± 0.3 a | 6.5 ± 0.9 b | 1.2 ± 0.5 a | 4 ± 0.2.3 a | 0.5 ± 0.1 b | 4 ± 1.9 a |
| γ-Aminobutyric acid | 75 ± 8 a | 7.2 ± 1.4 b | 7.6 ± 1.5 b | 290 ± 45 a | 130 ± 21 b | 2 ± 0.1 c |
| Proline | 129 ± 18 a | 43 ± 9 b | 16.5 ± 3.5 c | 44 ± 7 a | 8.5 ± 0.4 b | 6 ± 0.2 c |
| Alanine | 41 ± 11 a | 29 ± 7 a | 28 ± 5 a | 20 ± 1 a | 23 ± 1 a | 7.2 ± 0.4 b |
| Threonine | 7.2 ± 0.8 a | 3.5 ± 0.6 b | 3.5 ± 0.5 b | 0.8 ± 0.2 a | 2.4 ± 0.8 a | 0.8 ± 0.2 a |
| Arginine | 14 ± 2.8 a | 7.3 ± 0.6 b | 0.4 ± 0.1 c | 3 ± 0.3 a | 8 ± 0.5 b | 8.5 ± 0.4 b |
| Histidine | 6 ± 0.9 a | 5.4 ± 0.9 a | 3.9 ± 0.6 a | 19 ± 0.8 a | 24.5 ± 1 b | 3.6 ± 0.4 c |
| Glycine | 20 ± 4.5 a | 16 ± 3.8 a | 9.5 ± 2 c | 1.5 ± 0.1 a | 21 ± 2 b | 3 ± 0.3 c |
| Glutamic acid | 130 ± 24 a | 64 ± 7 b | 82 ± 12 c | 4 ± 0.25 a | 12 ± 1.1 b | 5 ± 0.4 a |
| Serine | 45 ± 11 a | 19 ± 4 b | 12 ± 3 b | 22 ± 1.5 a | 84 ± 3 b | 3 ± 0.2 c |
| Aspartic acid | 66 ± 11 a | 15 ± 3 b | 39 ± 9 c | 5 ± 0.2 a | 39 ± 2.5 b | 3 ± 0.1 c |
| Tryptophane | 20 ± 6 a | 19.5 ± 5 a | 2.4 ± 0.7 b | 0.7 ± 0.1 a | 9 ± 0.4 b | 3 ± 0.2 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnevskaya, N.; Shakhnazarova, V.; Shaposhnikov, A.; Strunnikova, O. The Role of Root Exudates of Barley Colonized by Pseudomonas fluorescens in Enhancing Root Colonization by Fusarium culmorum. Plants 2020, 9, 366. https://doi.org/10.3390/plants9030366
Vishnevskaya N, Shakhnazarova V, Shaposhnikov A, Strunnikova O. The Role of Root Exudates of Barley Colonized by Pseudomonas fluorescens in Enhancing Root Colonization by Fusarium culmorum. Plants. 2020; 9(3):366. https://doi.org/10.3390/plants9030366
Chicago/Turabian StyleVishnevskaya, Nadezhda, Vlada Shakhnazarova, Alexander Shaposhnikov, and Olga Strunnikova. 2020. "The Role of Root Exudates of Barley Colonized by Pseudomonas fluorescens in Enhancing Root Colonization by Fusarium culmorum" Plants 9, no. 3: 366. https://doi.org/10.3390/plants9030366
APA StyleVishnevskaya, N., Shakhnazarova, V., Shaposhnikov, A., & Strunnikova, O. (2020). The Role of Root Exudates of Barley Colonized by Pseudomonas fluorescens in Enhancing Root Colonization by Fusarium culmorum. Plants, 9(3), 366. https://doi.org/10.3390/plants9030366




