RETRACTED: Neuroprotective Effects of Dried Tubers of Aconitum napellus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection of Plant Material
2.3. Detoxification and Extraction
2.4. HPLC Analysis
2.5. Preparation of Standard Solutions
2.6. Animals
Induction of Diabetes
2.7. Parameters
2.7.1. Blood Sugar Level and Body Mass
2.7.2. Oral Glucose Tolerance Test (OGTT)
2.8. Behavioral Studies
2.8.1. Tail Immersion Test for the Assessment Hyperalgesia
2.8.2. Allodynia by Cold Water
2.8.3. Motor Co-Ordination Test
2.8.4. Locomotor Activity
2.9. Thio Barbituric Acid Reactive Substances (TBARS)
2.10. Reduced Glutathione-GSH
2.11. Catalase
2.12. Superoxide Dismutase Assay
2.13. Histopathology
2.14. Development of Cell Culture and Treatment Protocol
2.15. MTT Assay to Evaluate the Antiproliferative Activity of A. napellus Extracts on Neuroblastoma Cells
2.16. Statistical Analysis
3. Results
3.1. Yield of Plant Extract
3.2. HPLC Analysis
3.3. Effect of the Chloroform Extract of Goat Milk Treated A. napellus on Body Mass:
3.4. Effect of the Extract of Goat Milk Treated A. napellus on Blood Sugar Level
3.5. Effect of the Extract of Goat Milk Treated A. napellus on Oral Glucose Tolerance:
3.6. Effect of the Extract of Goat Milk Treated A. napellus on Thermal Hyperalgesia:
3.7. Effect of the Chloroform Extract of Goat Milk Treated A. napellus on Cold Allodynia
3.8. Effect of the Chloroform Extract of Goat Milk Treated A. napellus on Motor Co-Ordination:
3.9. Effect of Chloroform Extract of Goat Milk Treated A. napellus on Locomotors Activity
3.10. Effect of the Chloroform Extract of Goat Milk Treated A. napellus on Oxidative Biomarkers
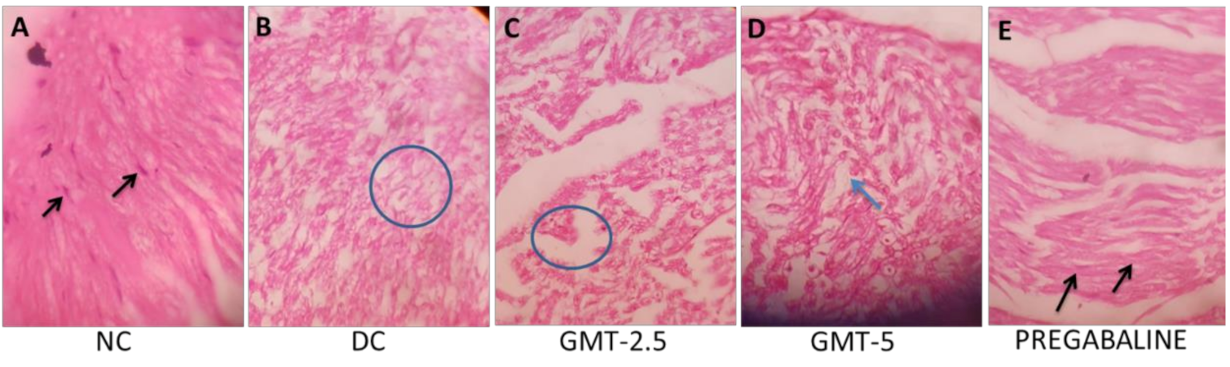
3.11. Histopathology of the Sciatic Nerve
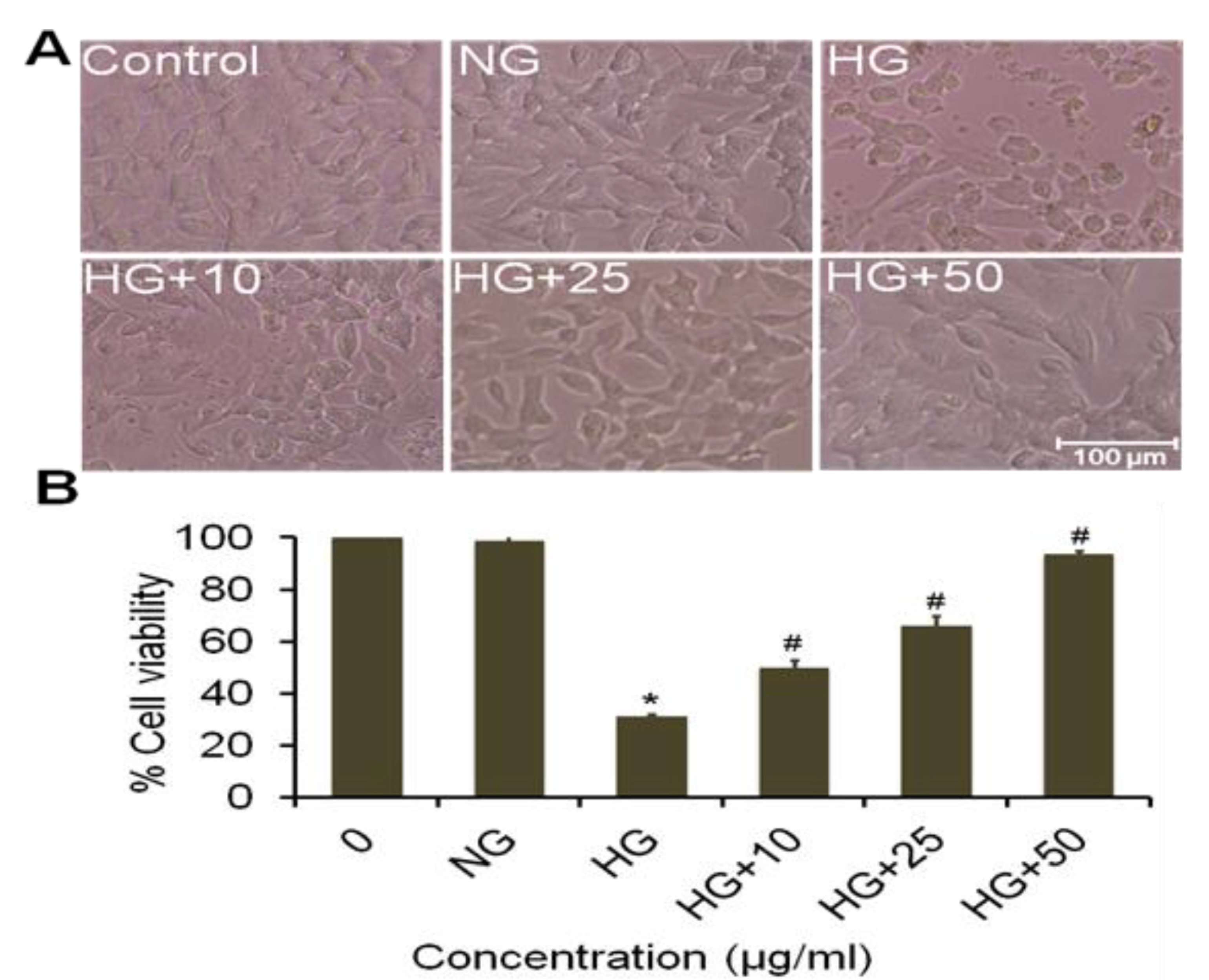
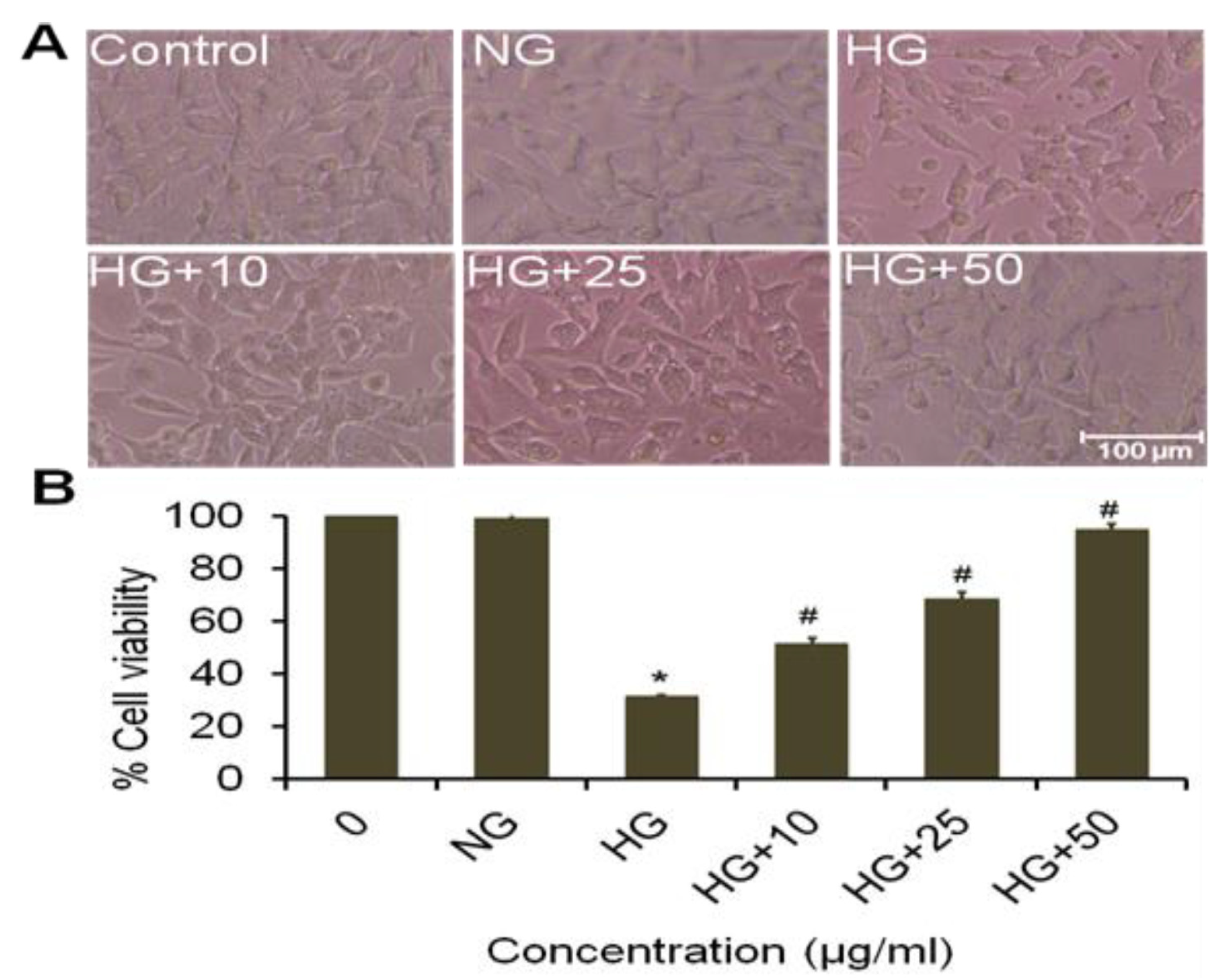
3.12. Effect of A. napellus Extract on Morphological Variation and Cell Viability on Neuroblastoma Cells
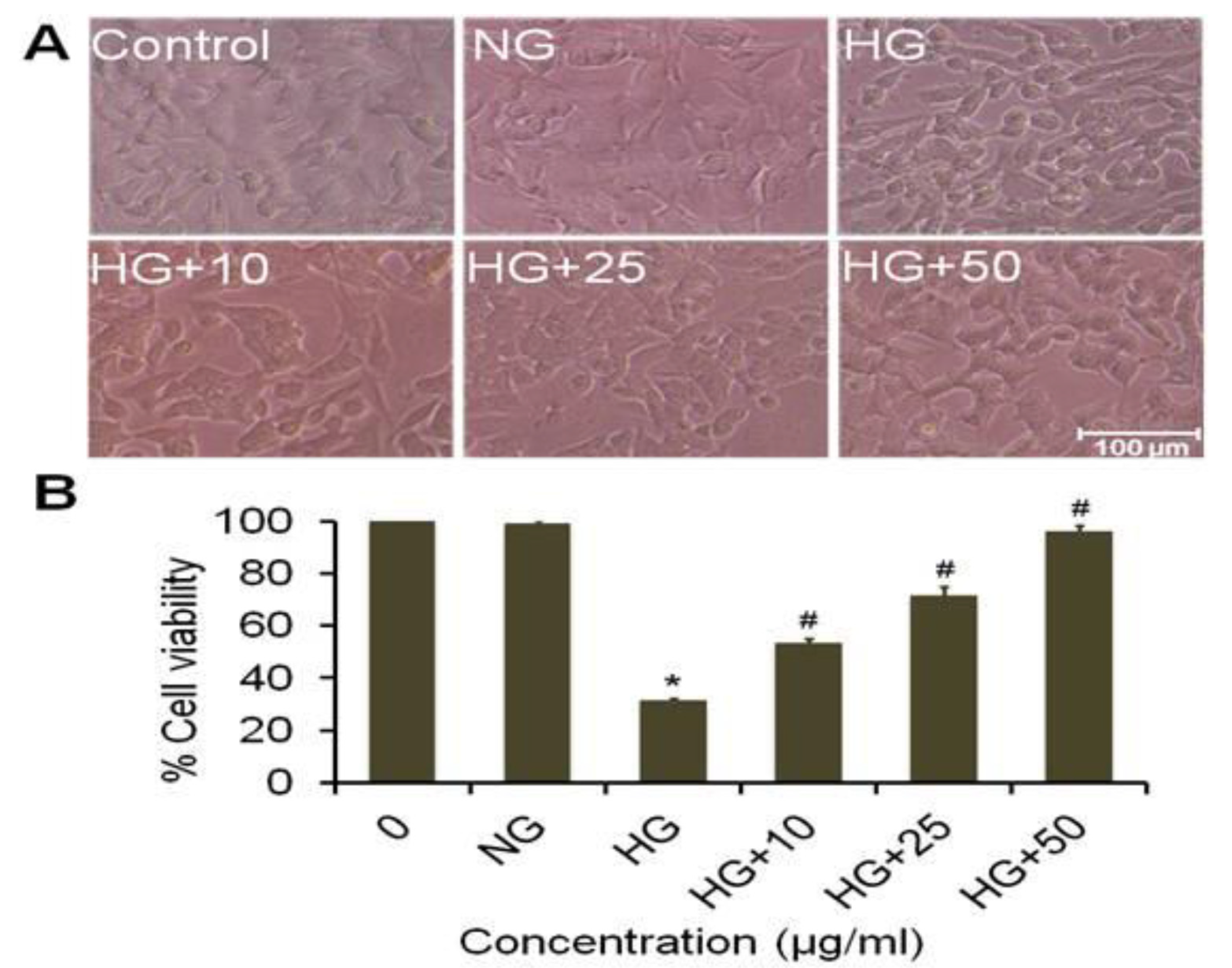
3.13. Effect of Cow Milk Treated Extract of A. napellus (CMT) on Morphological Variation and Cell Viability on Neuroblastoma Cells
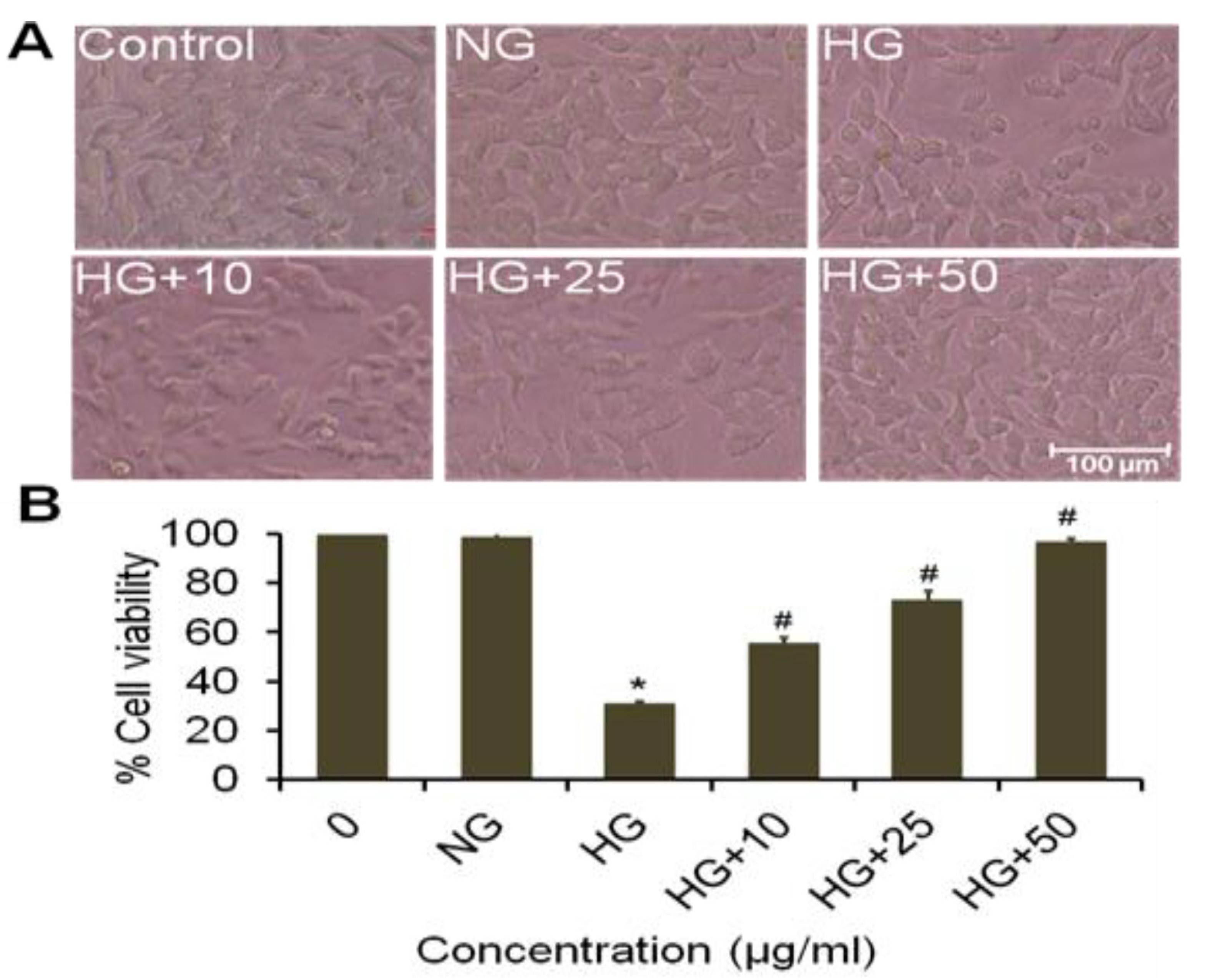
3.14. Effect of Goat Milk Treated Extract of A. napellus (GMT) on Morphological Variation on Neuroblastoma Cells
3.15. Effect of Water Treated Extract of A. napellus (WT) on Cell Viability and Morphological Variation in Neuroblastoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tabana, Y.M.; Al-Suede, F.S.; Ahamed, M.B.; Dahham, S.S.; Hassan, L.E.; Khalilpour, S.; Taleb-Agha, M.; Sandai, D.; Majid, A.S.; Majid, A.M. Cat’s whiskers (Orthosiphon stamineus) tea modulates arthritis pathogenesis via the angiogenesis and inflammatory cascade. BMC Complement. Altern. Med. 2016, 16, 480. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, A.; Siddiqui, H.; Dixit, R.; Badruddeen; Ahsan, F. Current Pharmacotherapeutics approaches to treat diabetic neuropathy. J. Chem. Pharm. Res. 2016, 8, 449–458. [Google Scholar]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C., Jr.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Czerwinska, M.E.; Gasinska, E.; Lesniak, A.; Krawczyk, P.; Kiss, A.K.; Naruszewicz, M.; Bujalska-Zadrozny, M. Inhibitory effect of Ligustrum vulgare leaf extract on the development of neuropathic pain in a streptozotocin-induced rat model of diabetes. Phytomedicine 2018, 49, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu 2012, 33, 10–19. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Zhou, S.-F.; Gao, S.-H.; Yu, Z.-L.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ma, D.-L.; Han, Y.-F.; Fong, W.-F.; et al. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evid. Based Complement. Altern. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef]
- Singhuber, J.; Zhu, M.; Prinz, S.; Kopp, B. Aconitum in traditional Chinese medicine: A valuable drug or an unpredictable risk? J. Ethnopharmacol. 2009, 126, 18–30. [Google Scholar] [CrossRef]
- Chang, L.K.; Whitaker, D.C. The impact of herbal medicines on dermatologic surgery. Derm. Surg. 2001, 27, 759–763. [Google Scholar]
- Venkataraghavan, S.; Sundareesan, T. A short note on contraceptive in Ayurveda. J. Sci. Res. Pl. Med. 1981, 2, 39. [Google Scholar]
- Kelly, S. Aconite poisoning. Med. J. Aust. 1990, 153, 499. [Google Scholar] [CrossRef]
- Prajapati, N.D.; Kumar, U. Agro’s Dictionary of Medicinal Plants; Agrobios: Jodhpur, India, 2003; Available online: https://content.kopykitab.com/ebooks/2014/04/3140/sample/sample_3140.pdf (accessed on 30 November 2019).
- Shoaib, A.; Badruddeen; Siddiqui, H.H.; Dixit, R.K.; Akhtar, J. Aconitum Napellus: Detoxification and Acute Toxicity Investigation Followed by Sub-Acute Toxicity and Bioavailability Assessment of Highest and Lowest LD50 Extract. J. Biol. Act. Prod. Nat. 2019, 9, 108–119. [Google Scholar] [CrossRef]
- Wang, R.H.; Guo, W.S.; Liu, Y.J. The effect of Modified Mahuangfuzixixin treating arthralgia on 50 Cases. China Mod. Med. 2011, 18, 95–96. [Google Scholar]
- Barkana, Y.; Belkin, M. Neuroprotection in ophthalmology: A review. Brain Res. Bull. 2004, 62, 447–453. [Google Scholar] [CrossRef]
- Zhang, F.; Hong, S.; Stone, V.; Smith, P.J. Expression of cannabinoid CB1 receptors in models of diabetic neuropathy. J. Pharmacol. Exp. Ther. 2007, 323, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Shindo, H.; Thomas, T.P.; Larkin, D.D.; Karihaloo, A.K.; Inada, H.; Onaya, T.; Stevens, M.J.; Greene, D.A. Modulation of basal nitric oxide-dependent cyclic-GMP production by ambient glucose, myo-inositol, and protein kinase C in SH-SY5Y human neuroblastoma cells. J. Clin. Investig. 1996, 97, 736–745. [Google Scholar] [CrossRef]
- Shoaib, A.; Dixit, R.K.; Badruddeen; Rahman, M.A.; Bagga, P.; Kaleem, S.; Siddiqui, S.; Arshad, M.; Siddiqui, H.H. Cure of human diabetic neuropathy by HPLC validated bark extract of Onosma echioides L. root. Nat. Prod. Res. 2018, 33, 1–5. [Google Scholar] [CrossRef]
- Rastogi, S. A review of aconite (Vatsanabha) usage in Ayurvedic formulations: Traditional views and their references. Spatula DD 2011, 1, 233–244. [Google Scholar] [CrossRef]
- Jaiswal, Y.; Liang, Z.; Yong, P.; Chen, H.; Zhao, Z. A comparative study on the traditional Indian Shodhana and Chinese processing methods for aconite roots by characterization and determination of the major components. Chem. Cent. J. 2013, 7, 169. [Google Scholar] [CrossRef]
- Shoaib, A.; Salem-Bekhit, M.M.; Siddiqui, H.H.; Dixit, R.K.; Bayomi, M.; Khalid, M.; Badruddeen; Shakeel, F. Antidiabetic activity of standardized dried tubers extract of Aconitum napellus in streptozotocin-induced diabetic rats. 3 Biotech 2020, 10, 56. [Google Scholar] [CrossRef]
- Dubey, N.; Dubey, N.; Mehta, R. Development and Validation of Selective High-Performance Liquid Chromatographic Method Using Photodiode Array Detection for Estimation of Aconitine in Polyherbal Ayurvedic Taila Preparations. Chromatogr. Res. Int. 2012, 2012. [Google Scholar] [CrossRef]
- Matthews, J.; Altman, D.G.; Campbell, M.; Royston, P. Analysis of serial measurements in medical research. Bmj 1990, 300, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.; Asmawi, M.Z.; Sadikun, A. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008, 55, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulkarni, S.K.; Agrewala, J.N.; Chopra, K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur. J. Pharmacol. 2006, 536, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Mangaiarkkarasi, A.; Rameshkannan, S.; Ali, R.M. Effect of gabapentin and pregabalin in rat model of taxol induced neuropathic pain. J. Clin. Diagn. Res. JCDR 2015, 9, FF11. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.J.; Morton, J.; Dunnett, S.B. Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 2001, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shortall, S.E.; Spicer, C.H.; Ebling, F.J.; Green, A.R.; Fone, K.C.; King, M.V. Contribution of serotonin and dopamine to changes in core body temperature and locomotor activity in rats following repeated administration of mephedrone. Addict. Biol. 2016, 21, 1127–1139. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahmad, E.; Gupta, M.; Rawat, V.; Shivnath, N.; Banerjee, M.; Khan, M.; Arshad, M. Cissus quadrangularis Linn exerts dose-dependent biphasic effects: Osteogenic and anti-proliferative, through modulating ROS, cell cycle and Runx2 gene expression in primary rat osteoblasts. Cell Prolif. 2015, 48, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Singleton, J.R. Diabetic neuropathy. Contin. Lifelong Learn. Neurol. 2012, 18, 60–84. [Google Scholar] [CrossRef] [PubMed]
- Shyaula, S.L. Phytochemicals, traditional uses and processing of Aconitum species in Nepal. Nepal J. Sci. Technol. 2011, 12, 171–178. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, R.; Kishore, K. Onosma L.: A review of phytochemistry and ethnopharmacology. Pharmacogn. Rev. 2013, 7, 140. [Google Scholar] [CrossRef]
- Han, J.; Tan, P.; Li, Z.; Wu, Y.; Li, C.; Wang, Y.; Wang, B.; Zhao, S.; Liu, Y. Fuzi attenuates diabetic neuropathy in rats and protects schwann cells from apoptosis induced by high glucose. PLoS ONE 2014, 9, e86539. [Google Scholar] [CrossRef]
- Ahmad, H.; Ahmad, S.; Shah, S.A.A.; Khan, H.U.; Khan, F.A.; Ali, M.; Latif, A.; Shaheen, F.; Ahmad, M. Selective dual cholinesterase inhibitors from Aconitum leave. J. Asian Nat. Prod. Res. 2018, 20, 172–181. [Google Scholar] [CrossRef]
- Hikmah, N.; Shita, A.D.P.; Maulana, H. Diabetic Blood Glucose Level Profile with Stratified Dose Streptozotocin (SD-STZ) and Multi Low Dose Streptozotocin (MLD-STZ) Induction Methods. J. Trop. Life Sci. 2015, 5, 30–34. [Google Scholar]
- Lee, H.-W.; Park, Y.-S.; Choi, J.-W.; Yi, S.-Y.; Shin, W.-S. Antidiabetic effects of chitosan oligosaccharides in neonatal streptozotocin-induced noninsulin-dependent diabetes mellitus in rats. Biol. Pharm. Bull. 2003, 26, 1100–1103. [Google Scholar] [CrossRef]
- Nasiry, D.; Ahmadvand, H.; Amiri, F.T.; Akbari, E. Protective effects of methanolic extract of Juglans regia L. leaf on streptozotocin-induced diabetic peripheral neuropathy in rats. BMC Complement. Altern. Med. 2017, 17, 476. [Google Scholar] [CrossRef]
- Saini, A.K.; Hs, A.K.; Sharma, S.S. Preventive and curative effect of edaravone on nerve functions and oxidative stress in experimental diabetic neuropathy. Eur. J. Pharmacol. 2007, 568, 164–172. [Google Scholar] [CrossRef]
- Field, M.J.; McCleary, S.; Hughes, J.; Singh, L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain 1999, 80, 391–398. [Google Scholar] [CrossRef]
- Azmi, S.; ElHadd, K.T.; Nelson, A.; Chapman, A.; Bowling, F.L.; Perumbalath, A.; Lim, J.; Marshall, A.; Malik, R.A.; Alam, U. Pregabalin in the Management of Painful Diabetic Neuropathy: A Narrative Review. Diabetes 2019, 10, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Muthuraman, A.; Singh, N. Attenuating effect of Acorus calamus extract in chronic constriction injury induced neuropathic pain in rats: An evidence of anti-oxidative, anti-inflammatory, neuroprotective and calcium inhibitory effects. BMC Complement. Altern. Med. 2011, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.; Rathi, S.; Vats, V. Amelioration of experimental diabetic neuropathy and gastropathy in rats following oral administration of plant (Eugenia jambolana, Mucurna pruriens and Tinospora cordifolia) extracts. Indian J. Exp. Biol. 2002, 40, 273–276. [Google Scholar]
- Solanki, N.D.; Bhavsar, S.K. An evaluation of the protective role of Ficus racemosa Linn. In streptozotocin-induced diabetic neuropathy with neurodegeneration. Indian J. Pharm. 2015, 47, 610–615. [Google Scholar] [CrossRef]
- Sugimoto, K.; Yasujima, M.; Yagihashi, S. Role of advanced glycation end products in diabetic neuropathy. Curr. Pharm. Des. 2008, 14, 953–961. [Google Scholar] [CrossRef]
- Tolkovsky, A. Apoptosis in diabetic neuropathy. Int. Rev. Neurobiol. 2002, 50, 145–159. [Google Scholar]
- Sekido, H.; Suzuki, T.; Jomori, T.; Takeuchi, M.; Yabe-Nishimura, C.; Yagihashi, S. Reduced cell replication and induction of apoptosis by advanced glycation end products in rat Schwann cells. Biochem. Biophys. Res. Commun. 2004, 320, 241–248. [Google Scholar] [CrossRef]
| Group(s) | Change in Body Mass (g) | |||
|---|---|---|---|---|
| At week 0 (g) | At week 2 (g) | % Body Mass Variation | ||
| % Gain | % Loss | |||
| NC | 232.76 ± 19.24 | 317.95 ± 20.81 | 30.64 ± 2.61 | - |
| DC | 162.91±13.29 | 142.06 ± 14.10 | 13.42 ± 2.26 * | |
| GMT-2.5 | 238.28 ± 17.69 | 260.10 ± 16.27 | 10.23 ± 3.40 # | - |
| GMT-5 | 203.48 ± 7.53 | 264.75 ± 6.54 | 30.04 ± 6.71 # | - |
| Pregabalin | 176.11 ± 14.27 | 201.54 ± 19.65 | 20.03 ± 21.74 # | - |
| Group(s) | Blood Glucose Concentration (mg/dL) | Oral Glucose Tolerance (h.mmol/L) | |
|---|---|---|---|
| Initial Plasma Glucose | Final Plasma Glucose | Area Under Curve | |
| NC | 82.40 ± 3.47 | 85.60 ± 2.94 | 12.94 ± 0.75 |
| DC | 455.40 ± 58.87 | 504.60 ± 41.09 * | 63.83 ± 4.31 * |
| GMT-2.5 | 514.00 ± 67.62 | 204.80 ± 14.02 # | 30.85 ± 1.72 # |
| GMT-5 | 533.80 ± 59.01 | 184.00 ± 24.65 # | 27.01 ± 2.67 # |
| Pregabalin | 552.20 ± 56.75 | 372.80 ± 9.73 ns | 51.33 ± 1.61 ns |
| Group(s) | Change in Thermal Hyperalgesia (in sec) by Tail Immersion (Warm Water) Test on Different Weeks | |
|---|---|---|
| 0 week | 2 week | |
| NC | 9.38 ± 0.45 | 10.70 ± 0.48 |
| DC | 5.94 ± 0.36 | 3.62 ± 0.20 * |
| GMT-2.5 | 5.33 ± 0.25 | 7.62 ± 0.46 # |
| GMT-5 | 5.25 ± 0.32 | 8.97± 0.50 # |
| Pregabalin | 7.34 ± 0.66 | 11.75 ± 0.07 # |
| Groups(s) | Change in Cold Allodynia (sec) on Different Weeks | |
|---|---|---|
| 0 week | 2 week | |
| NC | 13.00 ± 0.30 | 13.08 ± 0.45 |
| DC | 6.46 ± 0.17 | 3.23 ± 0.42 * |
| GMT-2.5 | 6.96 ± 0.41 | 12.08 ± 0.37 # |
| GMT-5 | 7.66 ± 0.50 | 12.43 ± 0.43 # |
| Pregabalin | 7.06 ± 0.25 | 13.81 ± 0.24 # |
| Group(s) | Motor Coordination (sec) | Locomotor Activity ( Count in 5 min) | ||
|---|---|---|---|---|
| 0 week | 2 week | 0 week | 2 week | |
| NC | 32.65 ± 1.57 | 33.10 ± 0.94 | 140.80 ± 1.24 | 143.80 ± 4.20 |
| DC | 10.82 ± 0.44 | 10.94 ± 0.49* | 65.00 ± 3.39 | 36.00 ± 2.77 * |
| GMT-2.5 | 10.90 ± 0.38 | 16.56 ± 0.66 # | 66.60 ± 4.09 | 128.8 ± 8.49 # |
| GMT-5 | 11.00 ± 0.83 | 21.68 ± 0.66 # | 64.40 ± 4.35 | 133.6 ± 6.13 # |
| Pregabalin | 11.38 ± 0.41 | 28.20 ± 0.87 # | 63.80 ± 5.73 | 140 ± 5.32 # |
| Group(s) | Oxidative Parameters | |||
|---|---|---|---|---|
| TBARS (m mole/mg protein) | SOD (units/mg of protein) | CATALASE (units/mg of protein) | GSH (m mole/mg protein) | |
| CONTROL | 1.12 ± 0.05 | 1.83 ± 0.05 | 2.78 ± 0.05 | 3.26 ± 0.16 |
| DC | 2.85 ± 0.22 * | 0.64 ± 0.08 * | 0.56 ± 0.02 * | 1.564 ± 0.10 * |
| GMT-2.5 | 1.63 ± 0.11 # | 1.44 ± 0.07 # | 1.19 ± 0.02 # | 1.934 ± 0.06 # |
| GMT-5 | 1.22 ± 0.09 # | 1.72 ± 0.04 # | 2.18 ± 0.05 # | 2.194 ± 0.14 # |
| Pregabalin | 2.09 ± 0.07 # | 1.95 ± 0.01 # | 2.71 ± 0.03 # | 2.468 ± 0.05 # |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoaib, A.; Siddiqui, H.H.; Dixit, R.K.; Siddiqui, S.; Deen, B.; Khan, A.; Alrokayan, S.H.; Khan, H.A.; Ahmad, P. RETRACTED: Neuroprotective Effects of Dried Tubers of Aconitum napellus. Plants 2020, 9, 356. https://doi.org/10.3390/plants9030356
Shoaib A, Siddiqui HH, Dixit RK, Siddiqui S, Deen B, Khan A, Alrokayan SH, Khan HA, Ahmad P. RETRACTED: Neuroprotective Effects of Dried Tubers of Aconitum napellus. Plants. 2020; 9(3):356. https://doi.org/10.3390/plants9030356
Chicago/Turabian StyleShoaib, Ambreen, Hefazat Hussain Siddiqui, Rakesh Kumar Dixit, Sahabjada Siddiqui, Badrud Deen, Andleeb Khan, Salman H. Alrokayan, Haseeb A. Khan, and Parvaiz Ahmad. 2020. "RETRACTED: Neuroprotective Effects of Dried Tubers of Aconitum napellus" Plants 9, no. 3: 356. https://doi.org/10.3390/plants9030356
APA StyleShoaib, A., Siddiqui, H. H., Dixit, R. K., Siddiqui, S., Deen, B., Khan, A., Alrokayan, S. H., Khan, H. A., & Ahmad, P. (2020). RETRACTED: Neuroprotective Effects of Dried Tubers of Aconitum napellus. Plants, 9(3), 356. https://doi.org/10.3390/plants9030356







