Development of Embryo Suspensors for Five Genera of Crassulaceae with Special Emphasis on Plasmodesmata Distribution and Ultrastructure
Abstract
1. Introduction
2. Results
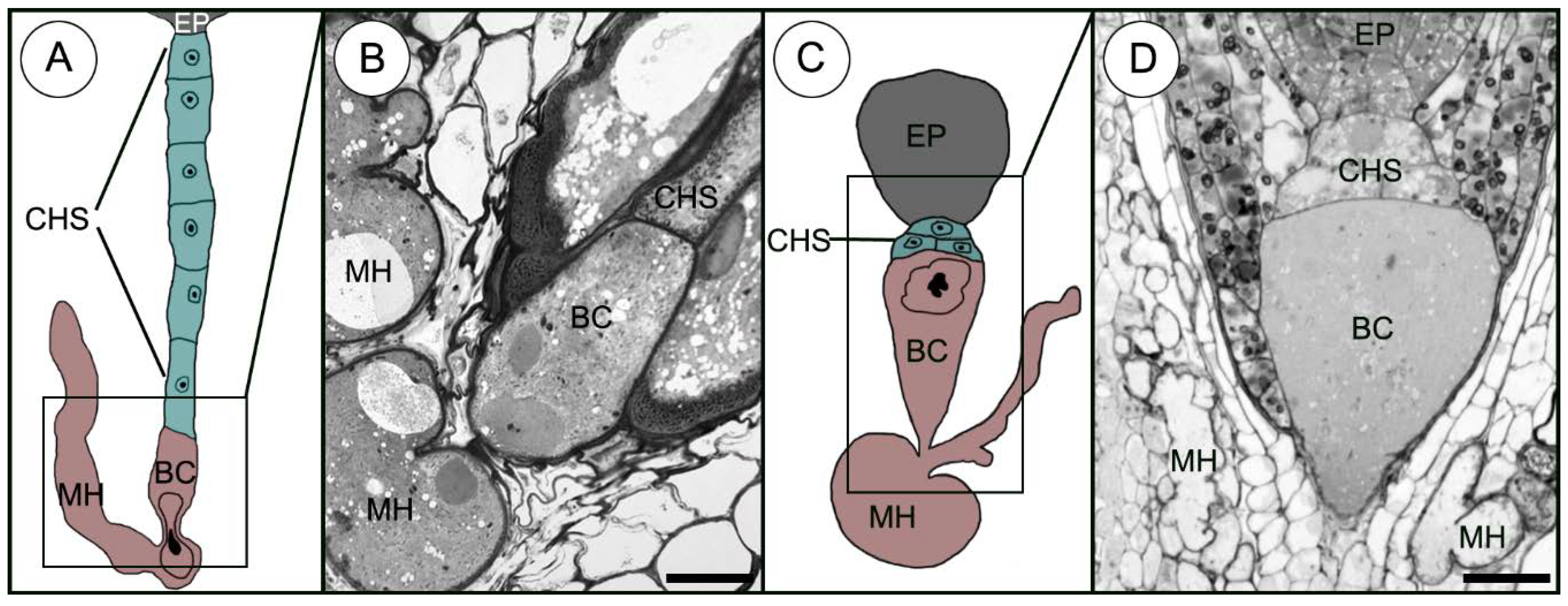
2.1. Uniseriate Suspensor Morphology
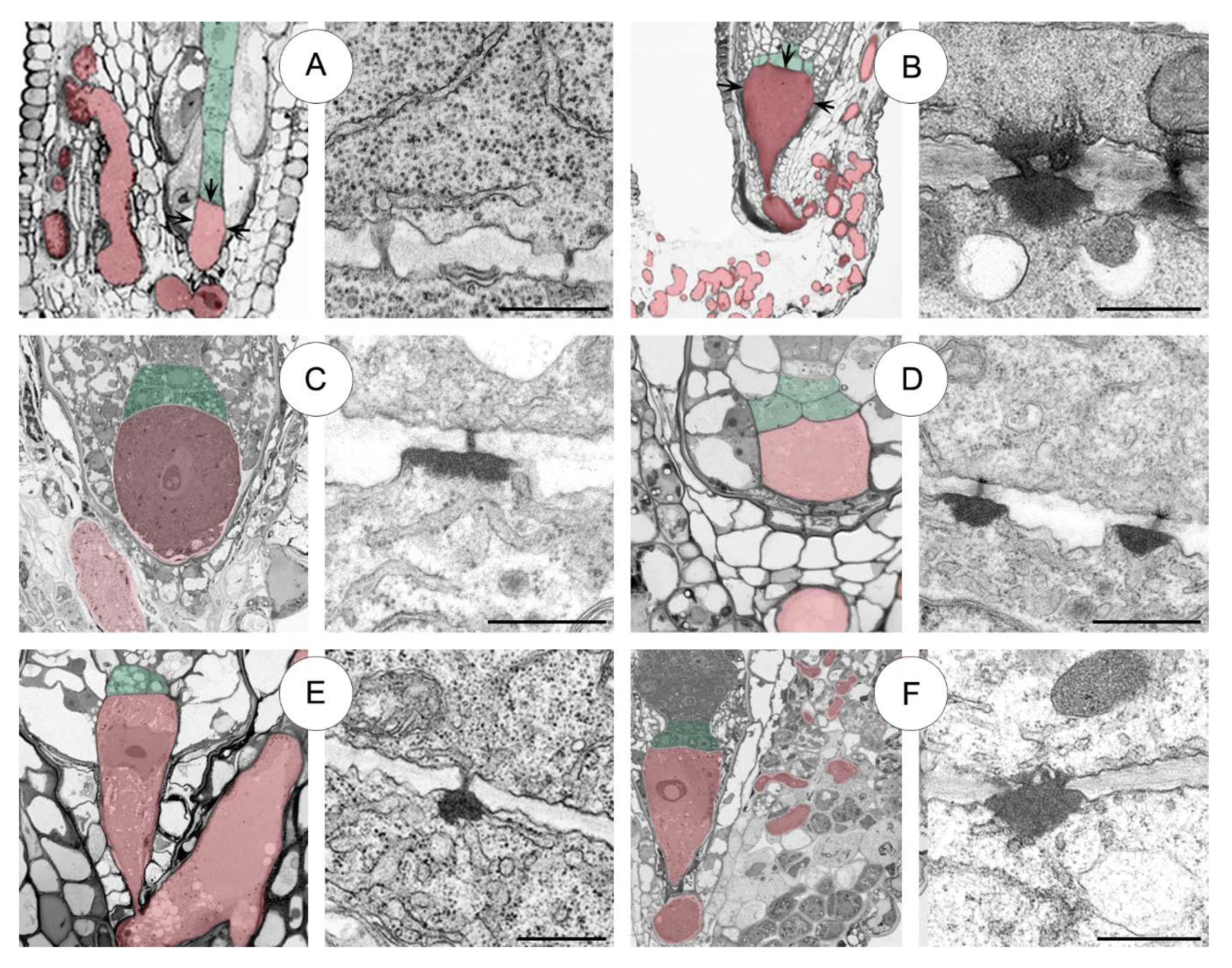
Sedum sediforme (Figure 2A)
2.2. Few-Celled Multiseriate Suspensor Morphology
2.2.1. Sedum atratum (Figure 2B)
2.2.2. Aeonium sedifolium (Figure 2C)
2.2.3. Monanthes anagensis (Figure 2D)
2.2.4. Aichryson laxum (Figure 2E)
2.2.5. Echeveria lutea (Figure 2F)
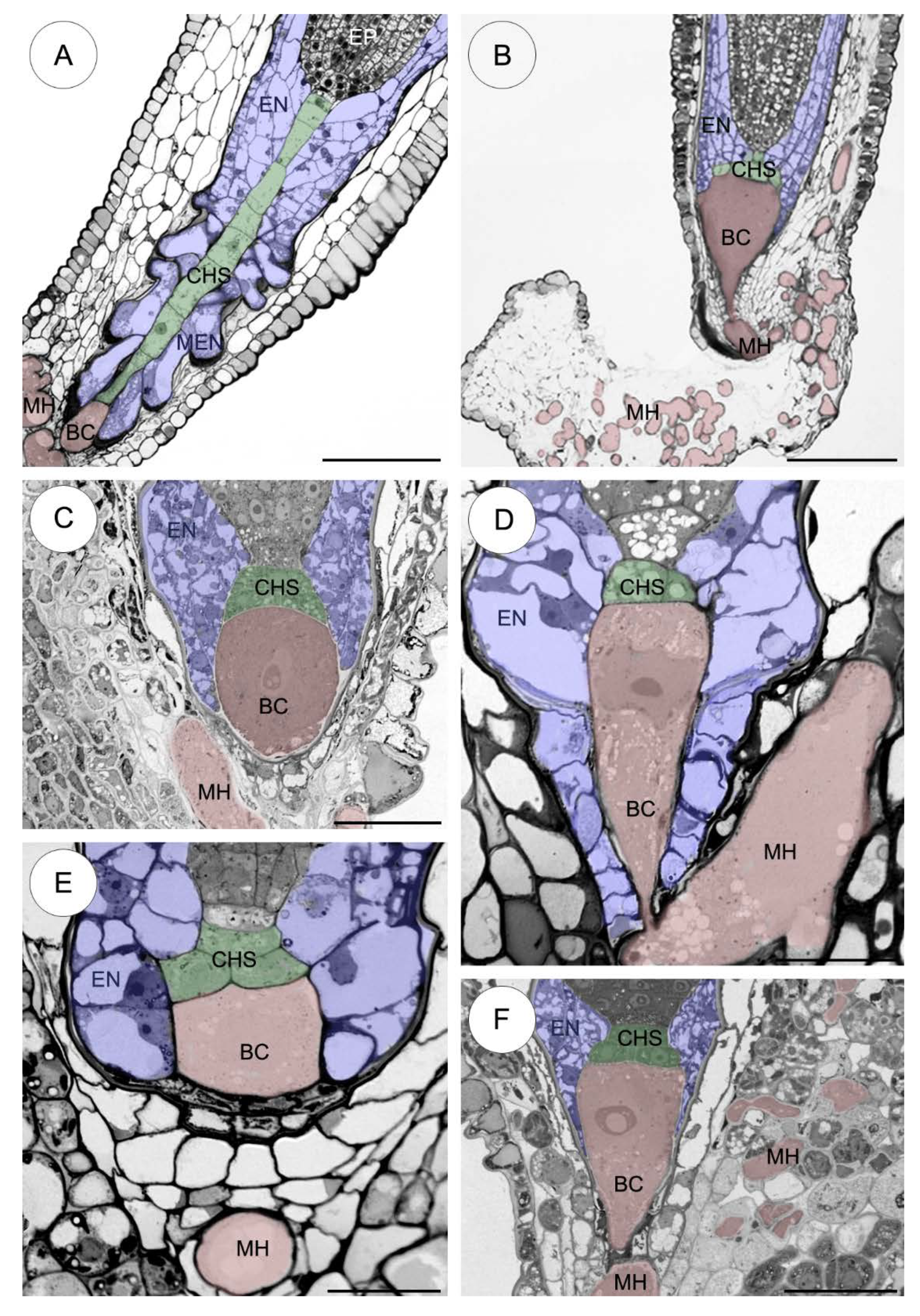
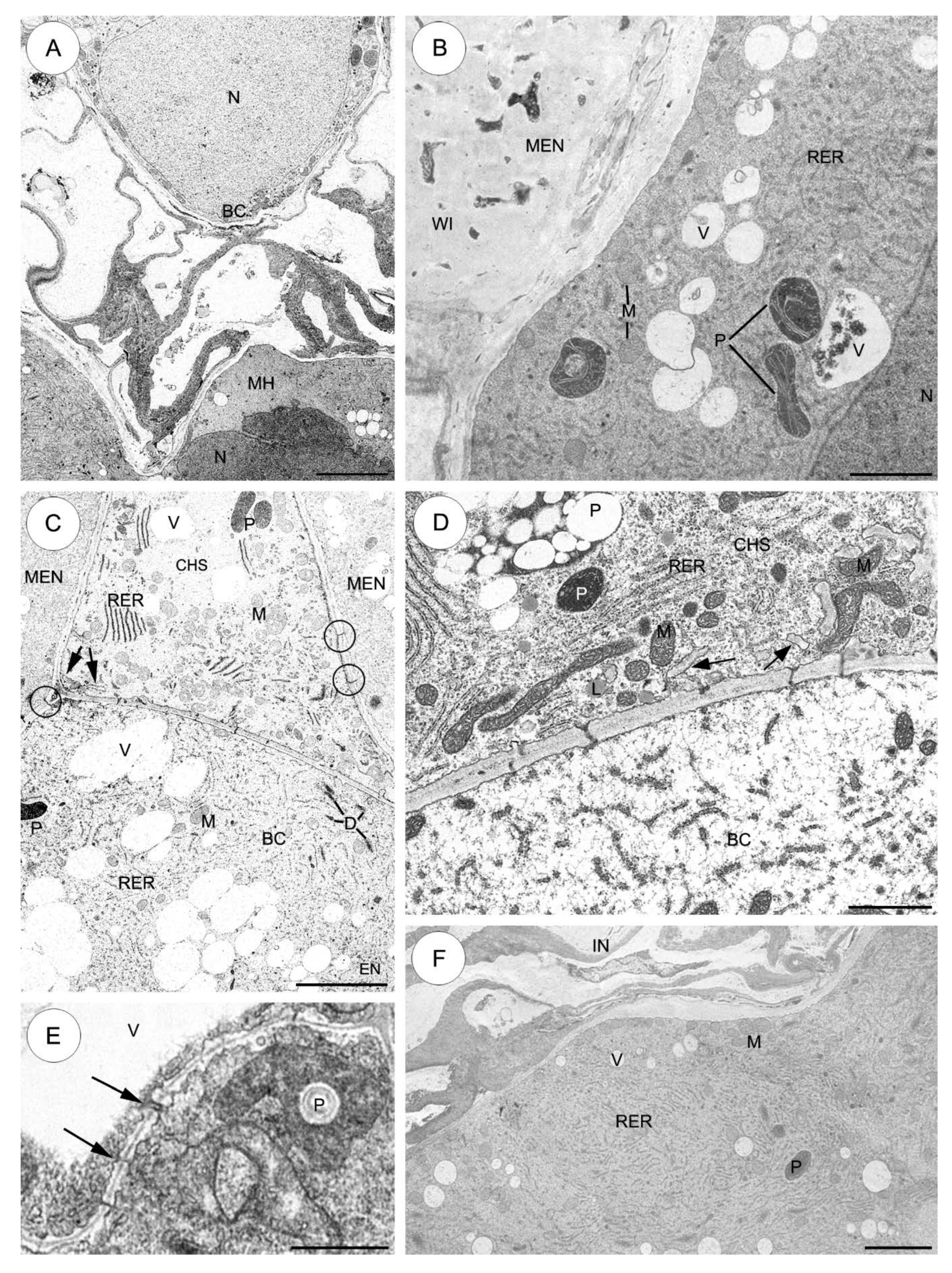
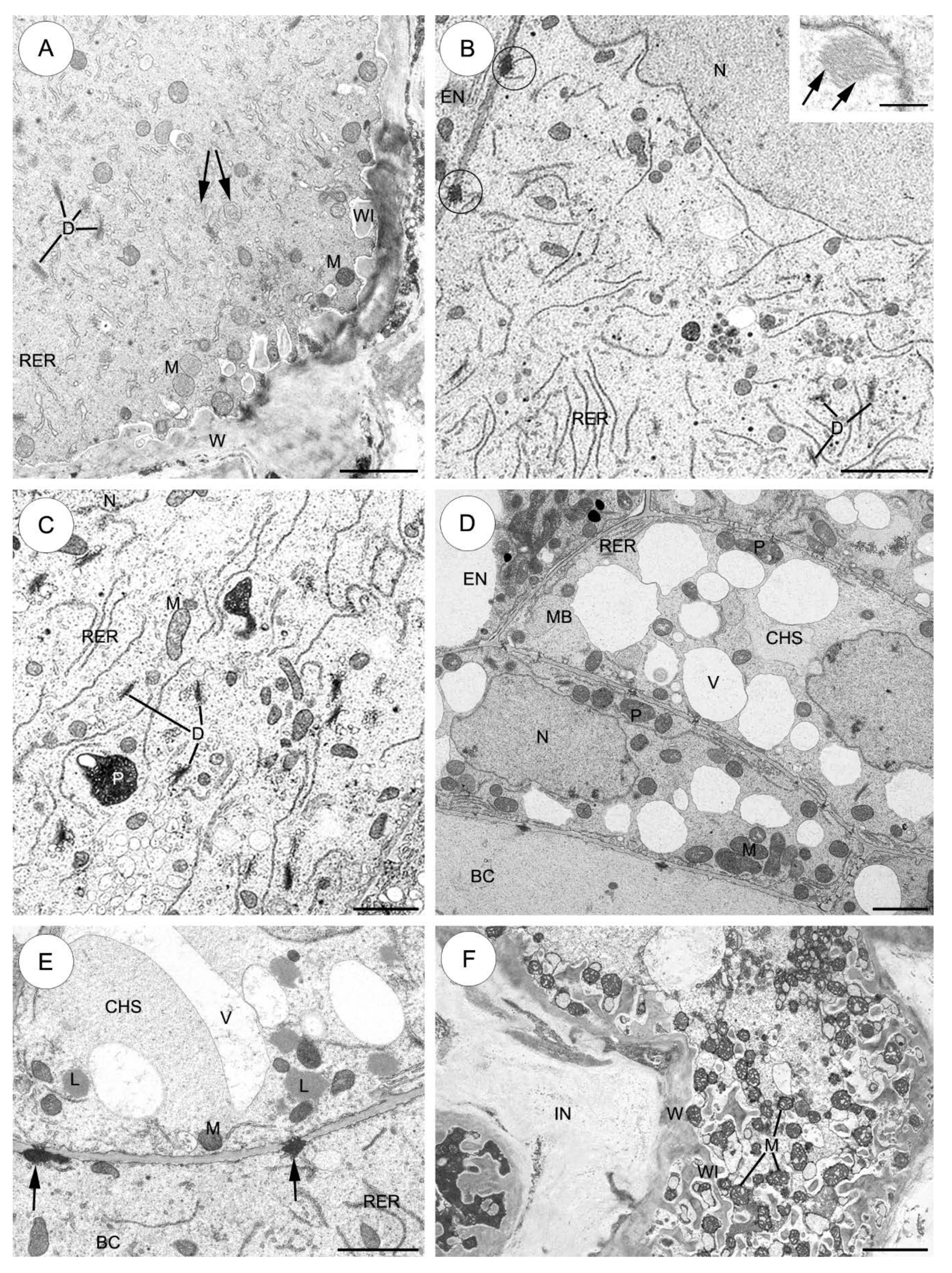
2.3. Ultrastructure of the Suspensor
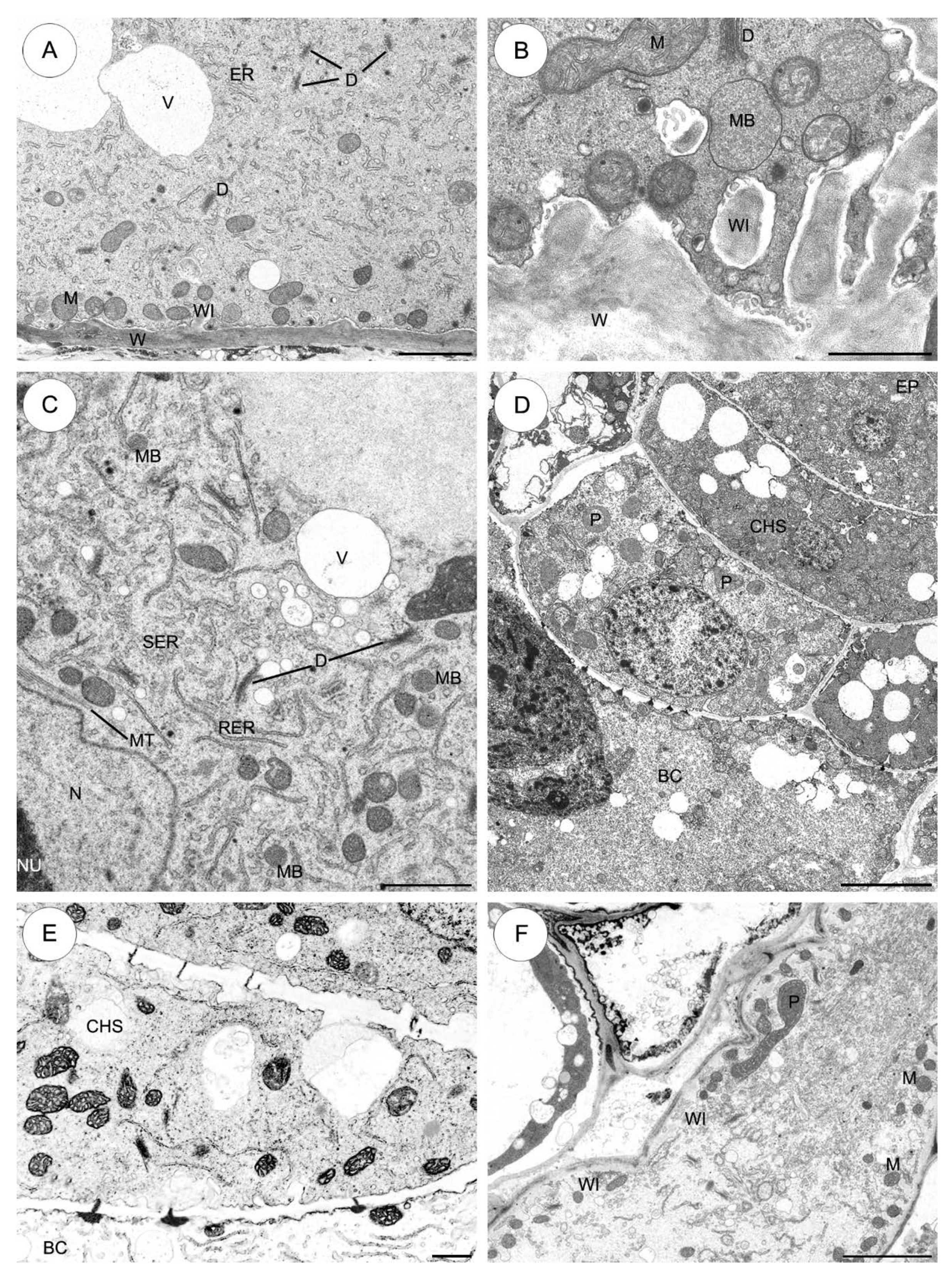
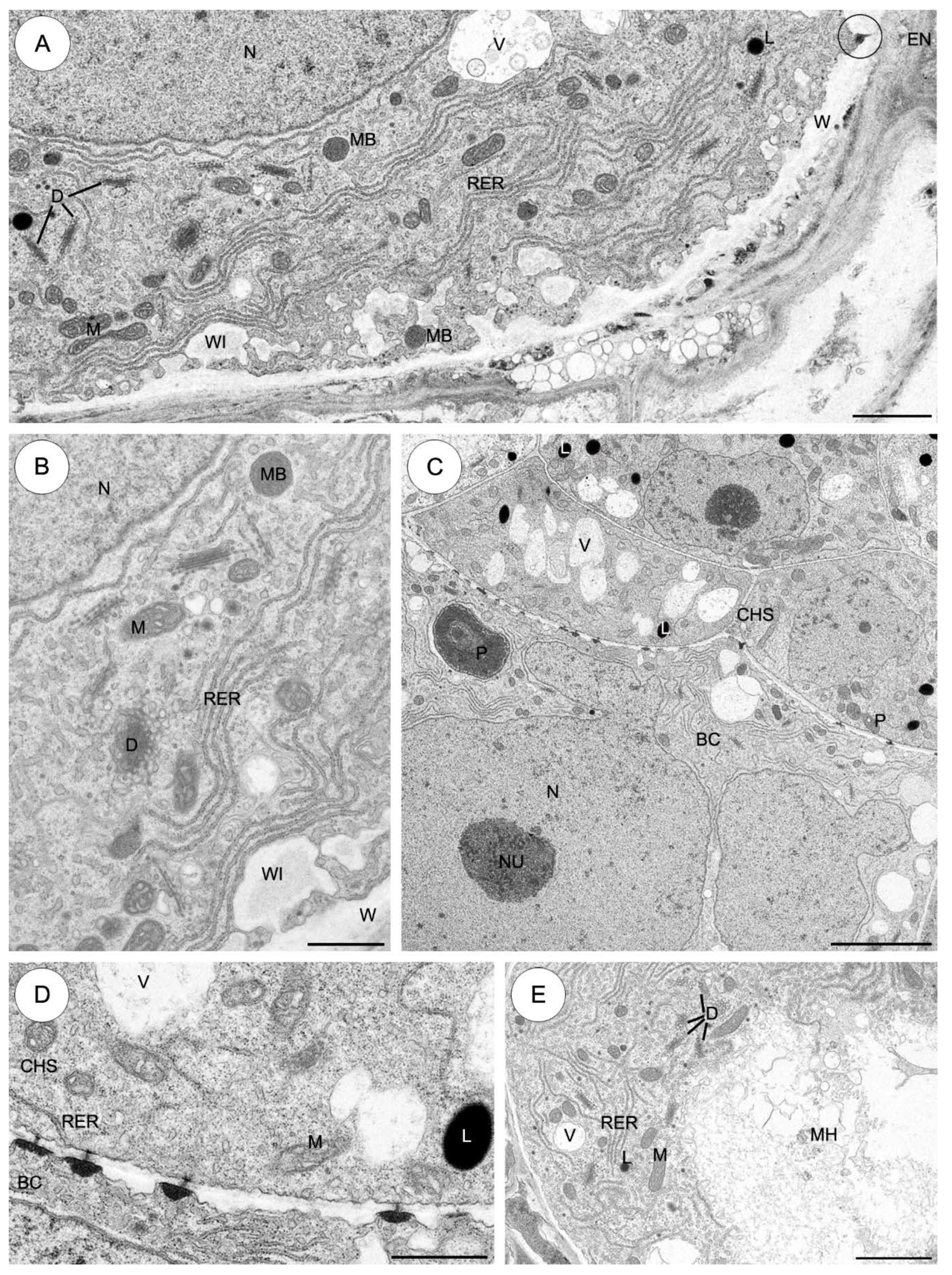
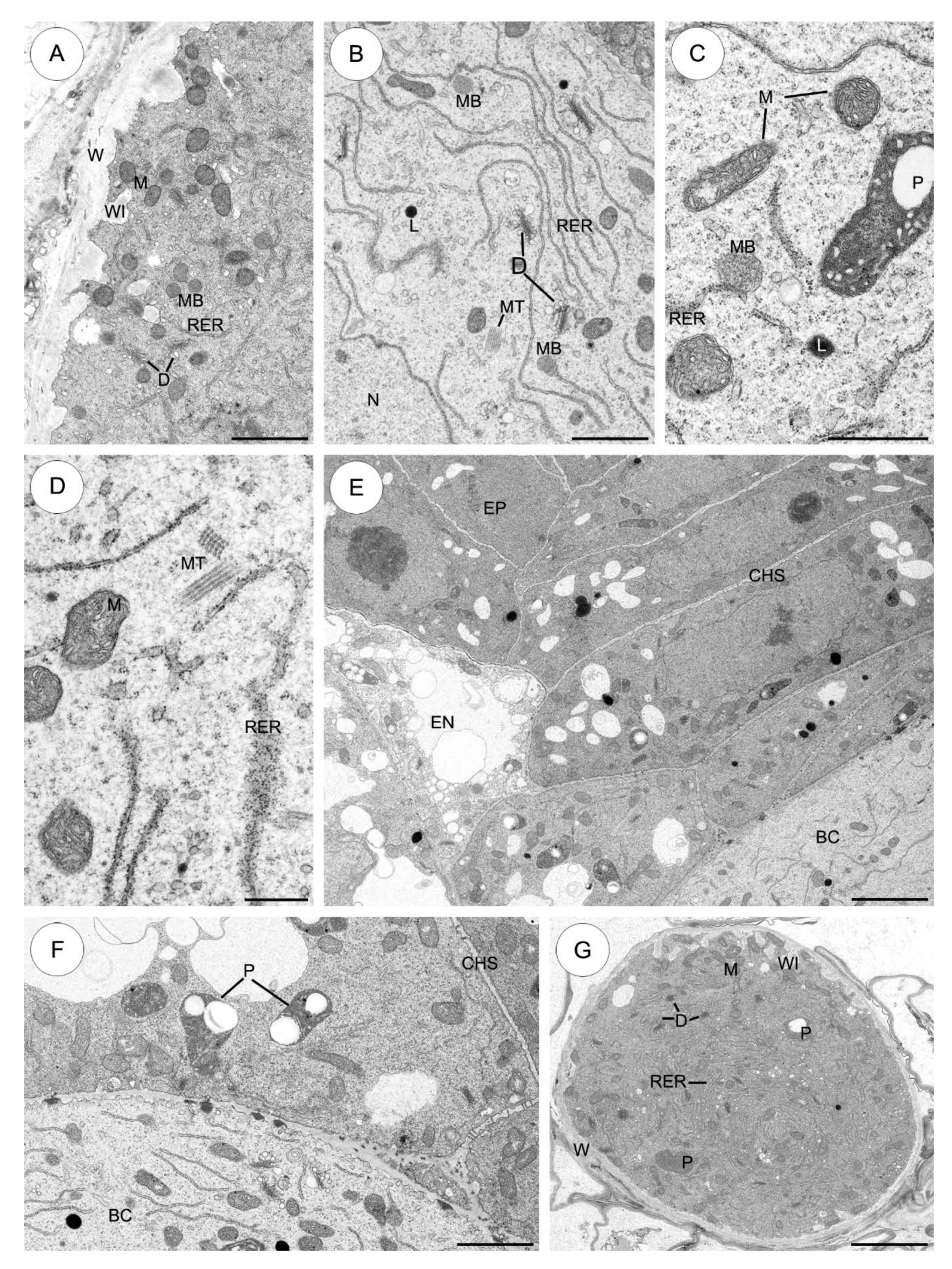
2.3.1. Sedum sediforme (Figure 3)
2.3.2. Sedum atratum (Figure 4)
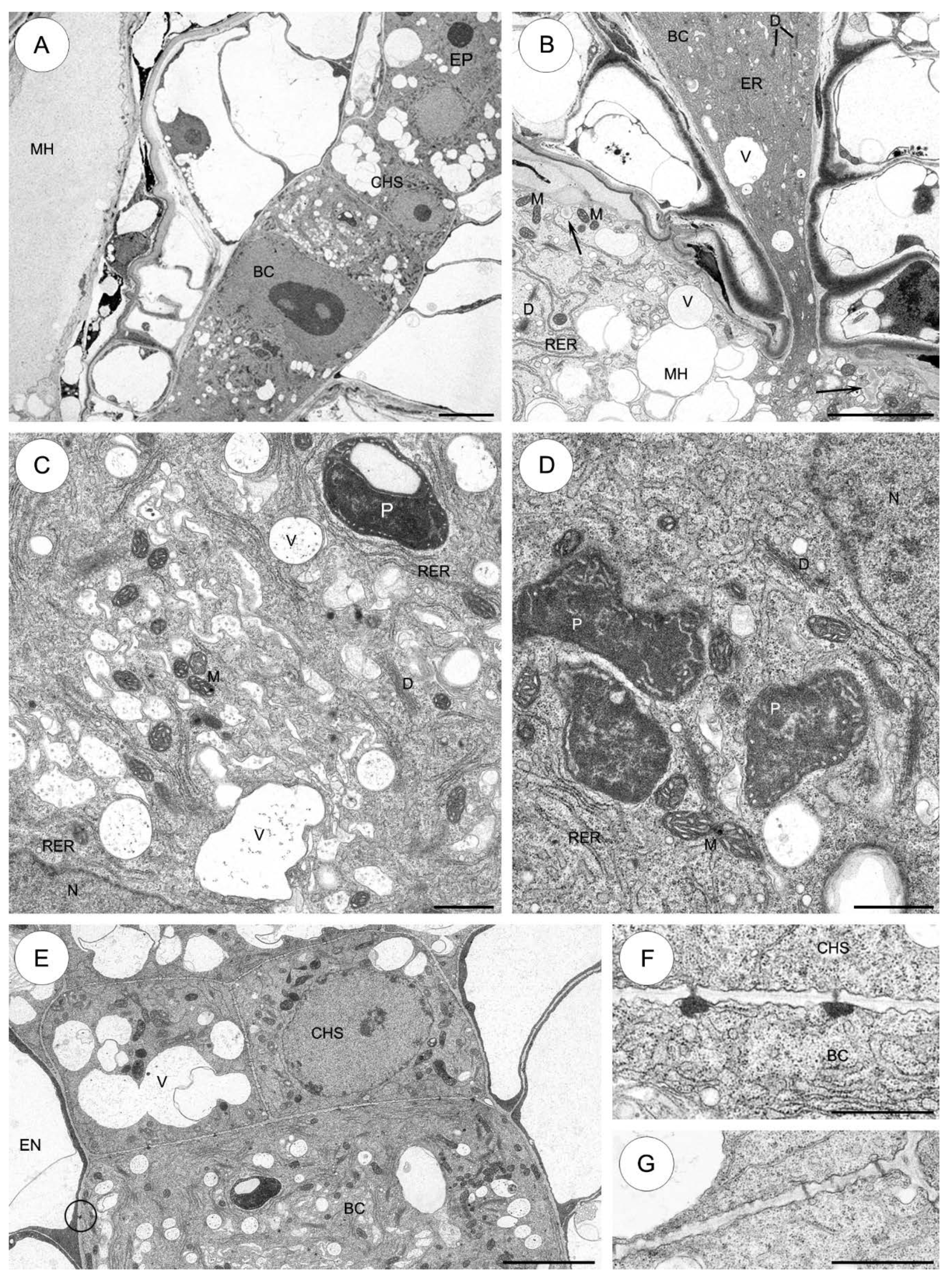
2.3.3. Aeonium sedifolium (Figure 5)
2.3.4. Monanthes anagensis (Figure 6)
2.3.5. Aichryson laxum (Figure 7)
2.3.6. Echeveria lutea (Figure 8)
3. Discussion
3.1. Suspensor Diversity
3.2. Ultrastructure of the Suspensor
3.3. Suspensor as a Connecting Structure
3.4. Suspensor Specialization
4. Materials and Methods
4.1. Plant Material
4.2. Electron Microscopy
4.3. Light Microscopy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shirley, N.J.; Aubert, M.K.; Wilkinson, L.G.; Bird, D.C.; Lora, J.; Yang, X.; Tucker, M.R. Translating auxin responses into ovules, seeds and yield: Insight from Arabidopsis and the cereals. J. Integr. Plant Biol. 2019, 61, 310–336. [Google Scholar] [CrossRef]
- Robert, H.S. Molecular communication for coordinated seed and fruit development: What can we learn from auxin and sugars? Int. J. Mol. Sci. 2019, 20, 936. [Google Scholar] [CrossRef] [PubMed]
- Bleckmann, A.; Alter, S.; Dresselhaus, T. The beginning of a seed: Regulatory mechanisms of double fertilization. Front. Plant Sci. 2014, 5, 452. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.C.; Meinke, D.W. Embryogenesis in angiosperms: Development of the suspensor. Plant Cell 1993, 5, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.W.; Vernon, D.A.; Meinke, D.W. Development of the suspensor: Differentiation, communication and programmed cell death during plant embryogenesis. In Cellular and Molecular Biology of Plant Seed Development (Advances in Cellular and Molecular Biology of Plants); Larkins, B.A., Vasil, I.K., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; Volume 4, pp. 53–72. [Google Scholar]
- Kawashima, T.; Goldberg, R.B. The suspensor: Not just suspending the embryo. Trends Plant Sci. 2010, 15, 23–30. [Google Scholar] [CrossRef]
- Raghavan, V. Double fertilization. In Embryo and Endosperm Development in Flowering Plants; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- D’Amato, F. Embryology of angiosperms. In Role of Polyploidy in Reproductive Organs and Tissues; Johri, B.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 519–566. [Google Scholar]
- Brodsky, V.Y.A.; Uryvayeva, J.V. Genome multiplication in growth and development. In Biology of Polyploid and Polytene Cells; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- Bozhkov, P.V.; Filonova, L.H.; Suarez, M.F. Programmed cell death in plant embryogenesis. Curr. Top. Dev. Biol. 2005, 67, 135–179. [Google Scholar]
- Henry, K.F.; Goldberg, R.B. Using giant scarlet runner bean embryos to uncover regulatory networks controlling suspensor gene activity. Front. Plant Sci. 2015, 6, 44. [Google Scholar] [CrossRef]
- Gunnung, B.E.S.; Pate, J.S. Transfer cells. In Dynamic Aspects of Plant Ultrastructure; Robards, A.W., Ed.; McGraw-Hill: London, UK, 1974; pp. 441–480. [Google Scholar]
- Talbot, M.J.; Offler, C.E.; McCurdy, D.W. Transfer cell wall architecture: A contribution towards understanding localized wall deposition. Protoplasma 2002, 219, 197–209. [Google Scholar] [CrossRef]
- Mauritzon, J. Studien über die Embryologie der Familien Crassulaceae und Saxifragaceae. Ph.D. Thesis, University of Lund, Lund, Sweden, 1933. [Google Scholar]
- Kozieradzka-Kiszkurno, M.; Płachno, B.J.; Bohdanowicz, J. New data about the suspensor of succulent angiosperms: Ultrastructure and cytochemical study of the embryo-suspensor of Sempervivum arachnoideum L. and Jovibarba sobolifera (Sims) Opiz. Protoplasma 2012, 249, 613–624. [Google Scholar] [CrossRef]
- Czaplejewicz, D.; Kozieradzka-Kiszkurno, M. Ultrastructural and cytochemical studies of the embryo suspensor of Sedum reflexum L. (Crassulaceae). Acta Biol. Cracov. Ser. Bot. 2013, 55, 76–89. [Google Scholar] [CrossRef]
- Eggli, U. Illustrated Handbook of Succulent Plants; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Gontcharova, S.B.; Gontcharov, A.A. Molecular phylogeny and systematics of flowering plants of the family Crassulaceae DC. Mol. Biol. 2009, 437, 94–803. [Google Scholar] [CrossRef]
- Nikulin, V.; Gontcharova, S.B.; Stephenson, R.; Gontcharov, A.A. Phylogenetic relationships between Sedum, L. and related genera (Crassulaceae) based on ITS rDNA sequence comparisons. Flora 2016, 224, 218–229. [Google Scholar] [CrossRef]
- Kozieradzka-Kiszkurno, M.; Bohdanowicz, J. Sedum acre embryogenesis: Polyploidization in the suspensor. Acta Biol. Cracov. Ser. Bot. 2003, 45, 159–163. [Google Scholar]
- Kozieradzka-Kiszkurno, M.; Bohdanowicz, J. Development and cytochemistry of the embryo suspensor in Sedum. Acta Biol. Cracov. Ser. Bot. 2006, 48, 67–72. [Google Scholar]
- Kozieradzka-Kiszkurno, M.; Świerczyńska, J.; Bohdanowicz, J. Embryogenesis in Sedum acre L.: Structural and immunocytochemical aspects of suspensor development. Protoplasma 2011, 248, 775–784. [Google Scholar] [CrossRef][Green Version]
- Kozieradzka-Kiszkurno, M.; Bohdanowicz, J. Unusual electron-dense dome associates with compound plasmodesmata in the embryo-suspensor of genus Sedum (Crassulaceae). Protoplasma 2010, 247, 117–120. [Google Scholar] [CrossRef]
- Kozieradzka-Kiszkurno, M.; Płachno, B.J.; Bohdanowicz, J. Are unusual plasmodesmata in the embryo-suspensor restricted to species from the genus Sedum among Crassulaceae? Flora 2011, 206, 684–690. [Google Scholar] [CrossRef]
- Kozieradzka-Kiszkurno, M.; Płachno, B.J. Are there symplastic connections between the endosperm and embryo in some angiosperms?—A lesson from the Crassulaceae family. Protoplasma 2012, 249, 1081–1089. [Google Scholar] [CrossRef][Green Version]
- Dute, R.R.; Peterson, C.M.; Rushing, A.E. Utrastructural changes of the egg apparatus associated with fertilization and proembryo development of soybean, Glycine max (Fabaceae). Ann. Bot. 1989, 64, 123–136. [Google Scholar] [CrossRef]
- Sangduen, N.; Kreitner, G.L.; Sorensen, E.L. Light and electron microscopy of embryo development in perennial and annual Medicago species. Can. J. Bot. 1983, 61, 837–849. [Google Scholar] [CrossRef]
- Johansson, M.; Walles, B. Functional anatomy of the ovule in broad bean, Vicia faba L. II. Ultrastructural development up to early embryogenesis. Int. J. Plant Sci. 1993, 154, 535–549. [Google Scholar] [CrossRef]
- Wróbel-Marek, J.; Kurczyńska, E.; Płachno, B.J.; Kozieradzka-Kiszkurno, M. Identification of symplasmic domains in the embryo and seed of Sedum acre L. (Crassulaceae). Planta 2017, 245, 491–505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raghavan, V. Embryogenesis in angiosperms. In A Developmental and Experimental Study; Cambridge University Press: New York, NY, USA, 1986. [Google Scholar]
- Majcher, D. Zróżnicowanie Budowy Wieszadełka Zarodkowego w Obrębie Rodzaju Sedum (Crassulaceae). Ph.D. Thesis, University of Gdańsk, Gdansk, Poland, 2017. (In Polish). [Google Scholar]
- Thiede, J.; Eggli, U. Crassulaceae. In The Familie and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 83–118. [Google Scholar]
- Mort, M.E.; Soltis, D.E.; Soltis, P.S.; Francisco-Ortega, J.; Santos-Guerra, A. Phylogenetic relationships and evolution of Crassulaceae inferred from matKsequence data. Am. J. Bot. 2001, 88, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Gunning, B.E.S.; Pate, J.S. Transfer cells: Plant cells with wall ingrowths, specialized in relation to short distance transport of solutes—Their occurrence, structure, and development. Protoplasma 1969, 68, 107–113. [Google Scholar] [CrossRef]
- Pate, J.S.; Gunning, B.E.S. Transfer cells. Annu. Rev. Plant Physiol. 1972, 23, 173–196. [Google Scholar] [CrossRef]
- Schnepf, E.; Pross, E. Differentiation and redifferentiation of a transfer cell: Development of septal nectaries of Aloe and Gasteria. Protoplasma 1976, 89, 105–115. [Google Scholar] [CrossRef]
- Newcomb, W.; Fowke, L.C. Stellaria media embryogenesis: The development and ultrastructure of the suspensor. Can. J. Bot. 1974, 52, 607–614. [Google Scholar] [CrossRef]
- Nagl, W.; Kühner, S. Early embryogenesis in Tropaeolum majus L.: Diversification of plastids. Planta 1976, 133, 15–19. [Google Scholar] [CrossRef]
- Wredle, U.; Walles, B.; Hakman, I. Chromoplasts are formed in Vicia faba suspensor cells. Int. J. Plant Sci. 2000, 161, 713–719. [Google Scholar] [CrossRef]
- Kozieradzka-Kiszkurno, M.; Płachno, B.J. Diversity of plastid morphology and structure along the micropyle-chalaza axis of different Crassulaceae. Flora 2013, 208, 128–137. [Google Scholar] [CrossRef]
- Selga, T.; Selga, M.; Gobiņš, V.; Ozoliņa, A.; Selga, T. Plastid nuclear complexes: Permanent structures in photosynthesizing tissues of vascular plants. Environ. Exp. Bot. 2010, 8, 85–92. [Google Scholar]
- Breuers, F.K.; Bräutigam, A.; Geimer, S.; Welzel, U.Y.; Stefano, G.; Renna, L.; Brandizzi, F.; Weber, A.P. Dynamic remodeling of the plastid envelope membranes—A tool for chloroplast envelope in vivo localizations. Front. Plant Sci. 2012, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Bohdanowicz, J. Alisma embryogenesis: The development and ultrastructure of the suspensor. Protoplasma 1987, 137, 71–83. [Google Scholar] [CrossRef]
- Tolbert, N.E.; Essner, E. Microbodies: Peroxisomes and glyoxysomes. J. Cell Biol. 1981, 91, 271–283. [Google Scholar] [CrossRef]
- Schulz, S.R.; Jensen, W.A. Capsella embryogenesis: The suspensor and the basal cell. Protoplasma 1969, 67, 139–163. [Google Scholar] [CrossRef]
- Yeung, E.C.; Clutter, M.E. Embryogeny of Phaseolus coccineus: The ultrastructure and development of the suspensor. Can. J. Bot. 1979, 57, 120–136. [Google Scholar] [CrossRef]
- Westafer, J.M.; Brown, R.M., Jr. Electron microscopy of the cotton fibre: New observations on cell wall formation. Cytobios 1976, 15, 111–138. [Google Scholar]
- Stadler, R.; Lauterbach, C.; Sauer, N. Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol. 2005, 139, 701–712. [Google Scholar] [CrossRef]
- Lafon-Placette, C.; Köhler, C. Embryo and endosperm, partners in seed development. Curr. Opin. Plant Biol. 2014, 17, 64–69. [Google Scholar] [CrossRef]
- Brzezicka, E.; Kozieradzka-Kiszkurno, M. Ultrastructural and cytochemical aspects of female gametophyte development in Sedum hispanicum L. (Crassulaceae). Protoplasma 2018, 255, 247–261. [Google Scholar] [CrossRef]
- Brzezicka, E.; Kozieradzka-Kiszkurno, M. Female gametophyte development in Sedum sediforme (Jacq.) Pau (Crassulaceae): An anatomical, cytochemical and ultrastructural analysis. Protoplasma 2019, 256, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Brady, T. Feulgen cytophotometric determination of the DNA content of the embryo proper and suspensor cells of Phaseolus coccineus. Cell Differ. 1973, 2, 65–75. [Google Scholar] [CrossRef]
- Kozieradzka-Kiszkurno, M.; Świerczyńska, J.; Bohdanowicz, J. Polyploidization in the suspensor of Triglochin palustre L. (Juncaginaceae). Acta Biol. Cracov. Ser. Bot. 2002, 44, 189–193. [Google Scholar]
- Mathur, J.; Hülskamp, M. Microtubules and microfilaments in cell morphogenesis in higher plants. Curr. Biol. 2002, 12, 669–676. [Google Scholar]
- Wasteneys, G.O.; Yang, Z. New views on the plant cytoskeleton. Plant Physiol. 2004, 136, 3884–3891. [Google Scholar] [CrossRef]
- Świerczyńska, J.; Bohdanowicz, B. Alisma plantago-aquatica L.: The cytoskeleton of the suspensor development. Acta Soc. Bot. Pol. 2014, 83, 159–166. [Google Scholar] [CrossRef][Green Version]
- Świerczyńska, J.; Bohdanowicz, B. Suspensor development in Gagea lutea (L.) Ker Gawl., with emphasis on the cytoskeleton. Acta Biol. Cracov. Ser. Bot. 2014, 56, 79–90. [Google Scholar] [CrossRef]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Venable, J.H.; Coggeshall, R. A simplified lead citrate stain for use in electron microscopy. J. Plant Biol. 1965, 24, 407–408. [Google Scholar] [CrossRef]
| Suspensor’s Characters | Sedum | Aeonium | Aichryson | Monanthes | Echeveria | ||
|---|---|---|---|---|---|---|---|
| S. sediforme | S. atratum | A. sedifolium | A. laxum | M. anagensis | E. lutea | ||
| Type of Suspensor | A long uniseriate | + | - | - | - | - | - |
| A few-celled multiseriate | - | + | + | + | + | + | |
| Type of Plasmodesmata | Simple | + | - | - | - | - | - |
| Unbranched/branched with an electron-dense material | - | + | + | + | + | + | |
| Symplasmic connection via PD between suspensor and embryo and between suspensor and endosperm | Present in all genera/species | ||||||
| Apoplasmic connection with the ovule tissue (by haustoria and transfer cells) | Present in all genera/species | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozieradzka-Kiszkurno, M.; Majcher, D.; Brzezicka, E.; Rojek, J.; Wróbel-Marek, J.; Kurczyńska, E. Development of Embryo Suspensors for Five Genera of Crassulaceae with Special Emphasis on Plasmodesmata Distribution and Ultrastructure. Plants 2020, 9, 320. https://doi.org/10.3390/plants9030320
Kozieradzka-Kiszkurno M, Majcher D, Brzezicka E, Rojek J, Wróbel-Marek J, Kurczyńska E. Development of Embryo Suspensors for Five Genera of Crassulaceae with Special Emphasis on Plasmodesmata Distribution and Ultrastructure. Plants. 2020; 9(3):320. https://doi.org/10.3390/plants9030320
Chicago/Turabian StyleKozieradzka-Kiszkurno, Małgorzata, Daria Majcher, Emilia Brzezicka, Joanna Rojek, Justyna Wróbel-Marek, and Ewa Kurczyńska. 2020. "Development of Embryo Suspensors for Five Genera of Crassulaceae with Special Emphasis on Plasmodesmata Distribution and Ultrastructure" Plants 9, no. 3: 320. https://doi.org/10.3390/plants9030320
APA StyleKozieradzka-Kiszkurno, M., Majcher, D., Brzezicka, E., Rojek, J., Wróbel-Marek, J., & Kurczyńska, E. (2020). Development of Embryo Suspensors for Five Genera of Crassulaceae with Special Emphasis on Plasmodesmata Distribution and Ultrastructure. Plants, 9(3), 320. https://doi.org/10.3390/plants9030320








