Epipactis tremolsii Seed Diversity in Two Close but Extremely Different Populations: Just a Case of Intraspecific Variability?
Abstract
1. Introduction
2. Results
3. Discussion
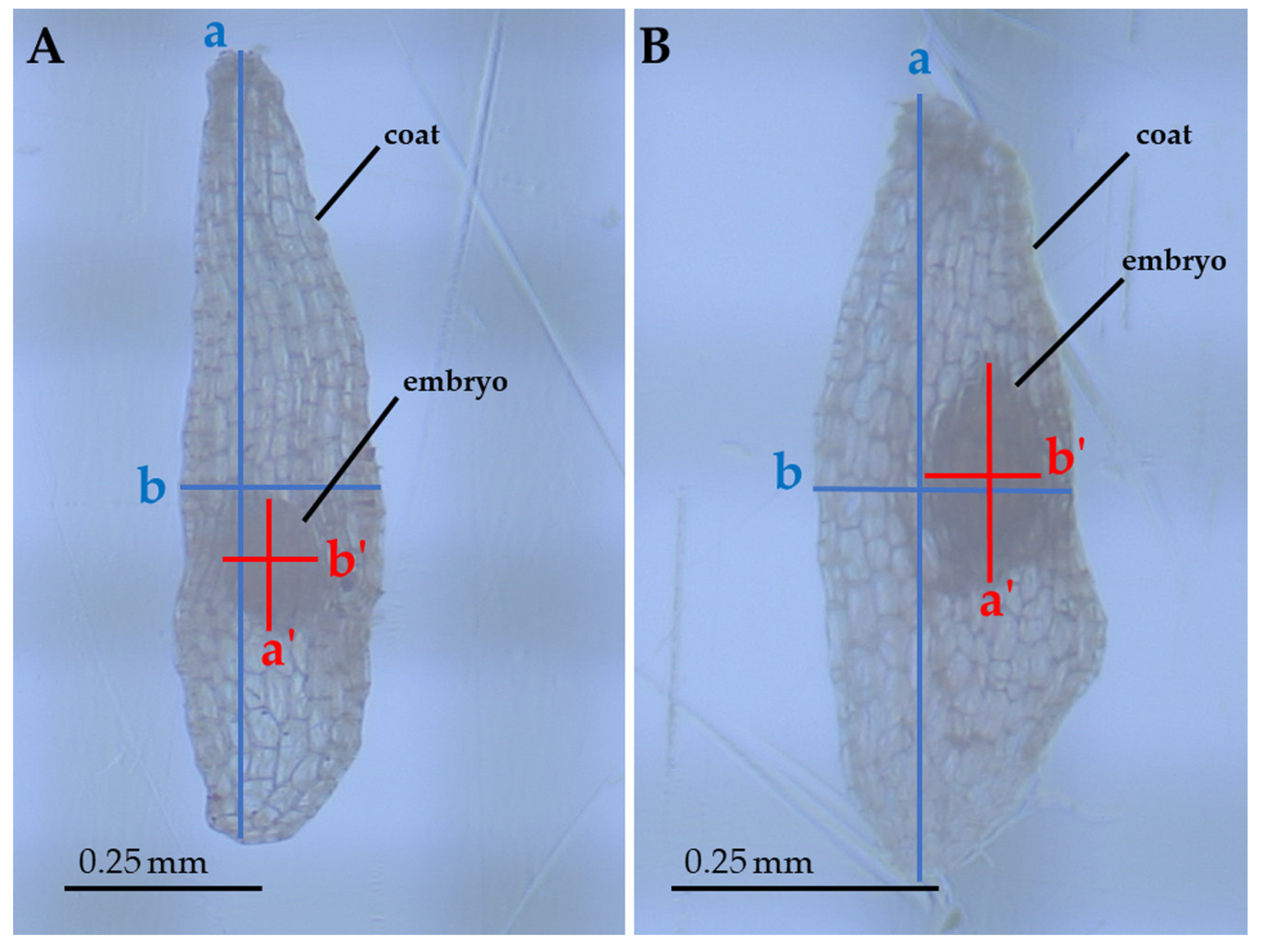
4. Materials and Methods
4.1. Species Description
4.2. Data Collection
4.3. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lussu, M.; De Agostini, A.; Cogoni, A.; Marignani, M.; Cortis, P. Does size really matter? A comparative study on floral traits in orchids with two different pollination strategies. Plant Biol. 2019, 21, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Gögler, J.; Stökl, J.; Cortis, P.; Beyrle, H.; Lumaga, M.R.B.; Cozzolino, S.; Ayasse, M. Increased divergence in floral morphology strongly reduces gene flow in sympatric sexually deceptive orchids with the same pollinator. Evol. Ecol. 2015, 29, 703–717. [Google Scholar] [CrossRef]
- Arditti, J.; Elliott, J.; Kitching, I.J.; Wasserthal, L.T. ‘Good Heavens what insect can suck it’- Charles Darwin, Angraecum sesquipedale and Xanthopan morganii praedicta. Bot. J. Linn. Soc. 2012, 169, 403–432. [Google Scholar] [CrossRef]
- Schiestl, F.P. On the success of a swindle: Pollination by deception in orchids. Naturwissenschaften 2005, 92, 255–264. [Google Scholar] [CrossRef]
- Pellegrino, G.; Bellusci, F.; Musacchio, A. The effects of inflorescence size and flower position on female reproductive success in three deceptive orchids. Bot. Stud. 2010, 51, 351–356. [Google Scholar]
- Arditti, J.; Ghani, A.K.A. Tansley Review No. 110. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef]
- Barsberg, S.; Lee, Y.-I.; Rasmussen, H.N. Development of C-lignin with G/S-lignin and lipids in orchid seed coats—An unexpected diversity exposed by ATR-FT-IR spectroscopy. Seed Sci. Res. 2018, 28, 41–51. [Google Scholar] [CrossRef]
- Şeker Şenay, S.; Şenel, G. Comparative seed micromorphology and morphometry of some orchid species (Orchidaceae) belong to the related Anacamptis, Orchis and Neotinea genera. Biologia 2017, 72, 14–23. [Google Scholar] [CrossRef]
- Diantina, S.; McGill, C.R.; Millner, J.; Nadarajan, J.; Pritchard, H.W.; McCormick, A.C. Comparative Seed Morphology of Tropical and Temperate Orchid Species with Different Growth Habits. Plants 2020, 9, 161. [Google Scholar] [CrossRef]
- Miura, C.; Saisho, M.; Yagame, T.; Yamato, M.; Kaminaka, H. Bletilla striata (Orchidaceae) Seed Coat Restricts the Invasion of Fungal Hyphae at the Initial Stage of Fungal Colonization. Plants 2019, 8, 280. [Google Scholar] [CrossRef]
- Fan, X.-L.; Chomicki, G.; Hao, K.; Liu, Q.; Xiong, Y.-Z.; Renner, S.S.; Gao, J.-Y.; Huang, S.-Q. Transitions between the Terrestrial and Epiphytic Habit Drove the Evolution of Seed-Aerodynamic Traits in Orchids. Am. Nat. 2019, 195, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.D.; Clark, J.Y.; Culham, A. The Cinderella discipline: Morphometrics and their use in botanical classification. Bot. J. Linn. Soc. 2020, 194, 385–396. [Google Scholar] [CrossRef]
- Arditti, J.; Michaud, J.D.; Healey, P.L. Morphometry of Orchid Seeds. II. Native California and Related Species of Calypso, Cephalanthera, Corallorhiza and Epipactis. Am. J. Bot. 1980, 67, 347–360. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Broeck, A.V.; Brys, R.; De Kort, H. Immigrant and extrinsic hybrid seed inviability contribute to reproductive isolation between forest and dune ecotypes of Epipactis helleborine (Orchidaceae). Oikos 2017, 127, 73–84. [Google Scholar] [CrossRef]
- Jacquemyn, H.; De Kort, H.; Broeck, A.V.; Brys, R. Low genetic divergence and variation in coastal dune populations of the widespread terrestrial orchid Epipactis helleborine. Bot. J. Linn. Soc. 2020, 193, 419–430. [Google Scholar] [CrossRef]
- Rewicz, A.; Kołodziejek, J.; Jakubska-Busse, A. The role of anthropogenic habitats as substitutes for natural habitats: A case study on Epipactis helleborine (L.) Crantz (Orchidaceae, Neottieae).Variations in size and nutrient composition of seeds. Turk. J. Bot. 2016, 40, 258–268. [Google Scholar] [CrossRef]
- Jakubska-Busse, A.; Żołubak, E.; Górniak, M.; Łobas, Z.; Tsiftsis, S.; Steiu, C. A Revision of the Taxonomy and Identification of Epipactis greuteri (Orchidaceae, Neottieae). Plants 2020, 9, 783. [Google Scholar] [CrossRef]
- Delforge, P. Epipactis. In Orchidées d’Europe, d’Afrique du Nord et du Påroche Orient, 4th ed.; Delachaux et Niestlé: Paris, France, 2016; pp. 45–46. [Google Scholar]
- De Agostini, A.; Caltagirone, C.; Caredda, A.; Cicatelli, A.; Cogoni, A.; Farci, D.; Guarino, F.; Garau, A.; Labra, M.; Lussu, M.; et al. Heavy metal tolerance of orchid populations growing on abandoned mine tailings: A case study in Sardinia Island (Italy). Ecotoxicol. Environ. Saf. 2020, 189, 110018. [Google Scholar] [CrossRef]
- Jurkiewicz, A.; Turnau, K.; Mesjasz-Przybyłowicz, J.; Przybyłowicz, W.; Godzik, B. Heavy metal localisation in mycorrhizas ofEpipactis atrorubens (Hoffm.) Besser (Orchidaceae) from zinc mine tailings. Protoplasma 2001, 218, 117–124. [Google Scholar] [CrossRef]
- Richards, A.J.; Swan, G.A. Epipactis leptochila (Godfery) Godfery and E. phyllanthes G. E. Srn. occurring in South Northumberland on lead and zinc soils. Watsonia 1976, 11, 1–5. [Google Scholar]
- Shefferson, R.P.; Kull, T.; Tali, K. Mycorrhizal interactions of orchids colonizing Estonian mine tailings hills. Am. J. Bot. 2008, 95, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Szarek-Łukaszewska, G. Vegetation of reclaimed and spontaneously vegetated Zn-Pb mine wastes in Southern Poland. Pol. J. Environ. 2009, 18, 717–733. [Google Scholar]
- Howard-Williams, C. Morphological Variation between Isolated populations of Becium homblei (De Wild) Duvign & Plancke growing on heavy metal soils. Vegetatio 1971, 23, 141–151. [Google Scholar]
- Luzuriaga, A.L.; Escudero, A.; Perez-Garcia, F. Environmental maternal effects on seed morphology and germination in Sinapis arvensis (Cruciferae). Weed Res. 2006, 46, 163–174. [Google Scholar] [CrossRef]
- Farci, D.; Haniewicz, P.; Cocco, E.; De Agostini, A.; Cortis, P.; Kusaka, M.; Loi, M.C.; Piano, D. The Impact of Fruit Etiolation on Quality of Seeds in Tobacco. Front. Plant Sci. 2020, 11, 563971. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Lan, H.; Zhang, F. Variation of seed heteromorphism in Chenopodium album and the effect of salinity stress on the descendants. Ann. Bot. 2010, 105, 1015–1025. [Google Scholar] [CrossRef]
- Bradley, R.; Burt, A.J.; Read, D.J. The biology of mycorrhiza in the Ericaceae. VIII. The role of mycorrhizal infection in heavy metal resistance. New Phytol. 1982, 91, 197–209. [Google Scholar] [CrossRef]
- Gonzalez-Chavez, C.; Harris, P.J.; Dodd, J.; Meharg, A.A. Arbuscular mycorrhizal fungi confer enhanced arsenate resistance on Holcus lanatus. New Phytol. 2002, 155, 163–171. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Melville, L.H.; Ferrol, N.; Lott, J.N.; Azcón-Aguilar, C.; Peterson, R.L. Ultrastructural localization of heavy metals in the extraradical mycelium and spores of the arbuscular mycorrhizal fungus Glomus intraradices. Can. J. Microbiol. 2008, 54, 103–110. [Google Scholar] [CrossRef]
- Joner, E.J.; Briones, R.; Leyval, C. Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant. Soil 2000, 226, 227–234. [Google Scholar] [CrossRef]
- Martino, E.; Turnau, K.; Girlanda, M.; Bonfante, P.; Perotto, S. Ericoid mycorrhizal fungi from heavy metal polluted soils: Their identification and growth in the presence of zinc ions. Mycol. Res. 2000, 104, 338–344. [Google Scholar] [CrossRef]
- Perotto, S.; Girlanda, M.; Martino, E. Ericoid mycorrhizal fungi: Some new perspectives on old acquaintances. Plant. Soil 2002, 244, 41–53. [Google Scholar] [CrossRef]
- Turnau, K.; Dexheimer, J. Acid phosphatase activity in Pisolithus arrhizus mycelium treated with cadmium dust. Mycorrhiza 1995, 5, 205–211. [Google Scholar] [CrossRef]
- Cordero, R.J.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M.; De Rome, L. Biosorption of copper by fungal melanin. Appl. Microbiol. Biotechnol. 1988, 29, 610–617. [Google Scholar] [CrossRef]
- Kumar, M.S.J.; Singh, M.; Singh, S.; Singh, V.P.; Prasad, S.M.; Singh, M. Adaptation Strategies of Plants against Heavy Metal Toxicity: A Short Review. Biochem. Pharmacol. Open Access 2015, 4, 161. [Google Scholar] [CrossRef]
- Chaoui, A.; El Ferjani, E. β-Estradiol Protects Embryo Growth from Heavy-Metal Toxicity in Germinating Lentil Seeds. J. Plant Growth Regul. 2013, 32, 636–645. [Google Scholar] [CrossRef]
- Patra, M.; Bhowmik, N.; Bandopadhyay, B.; Sharma, A. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ. Exp. Bot. 2004, 52, 199–223. [Google Scholar] [CrossRef]
- Li, W.; Khan, M.A.; Yamaguchi, S.; Kamiya, Y. Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Regul. 2005, 46, 45–50. [Google Scholar] [CrossRef]
- Monika, K.; Romana, I. Ovules, Female Gametophytes and Embryos are More Sensitive to Heavy Metal Pollution than Anthers and Pollen of Cardaminopsis Arenosa (L.) Hayek (Brassicaceae), A Member of Calamine Flora. Acta Biol. Cracoviensia Ser. Bot. 2014, 56, 128–137. [Google Scholar] [CrossRef]
- Yang, J.; Cai, L.; Liu, D.; Chen, G.; Gratzfeld, J.; Sun, W. China’s conservation program on Plant Species with Extremely Small Populations (PSESP): Progress and perspectives. Biol. Conserv. 2020, 244, 108535. [Google Scholar] [CrossRef]
- Sulis, E.; Bacchetta, G.; Cogoni, D.; Gargano, D.; Fenu, G. Assessing the global conservation status of the rock rose Helianthemum caput-felis. Oryx 2019, 54, 197–205. [Google Scholar] [CrossRef]
- Fenu, G.; Bacchetta, G.; Christodoulou, C.S.; Cogoni, D.; Fournaraki, C.; Pietro, G.D.G.G.; Gotsiou, P.; Kyratzis, A.; Piazza, C.; Vicens, M.; et al. A Common Approach to the Conservation of Threatened Island Vascular Plants: First Results in the Mediterranean Basin. Diversity 2020, 12, 157. [Google Scholar] [CrossRef]
- Lai, R. Aggiornamento Corologico, Tassonomico, Nomenclaturale Della Flora Orchidologica Della Sardegna. Ph.D. Thesis, University of Cagliari, Cagliari, Italy, 2008. [Google Scholar]
- Lussu, M.; Marignani, M.; Lai, R.; Loi, M.C.; Cogoni, A.; Cortis, P. A Synopsis of Sardinian Studies: Why Is it Important to Work on Island Orchids? Plants 2020, 9, 853. [Google Scholar] [CrossRef]
- De Waele, J.; Pisano, M. Interazione fra attività mineraria ed un acquifero carsico: L’esempio di Barraxiutta (Sardegna sud-occidentale). In Proceedings of the Convegno Nazionale Sull’Inquinamento Delle Grotte e Degli Acquiferi Carsici e Possibili Ricadute Sulla Collettività, Ponte di Brenta, Italy, 26–27 September 1998; pp. 195–209. [Google Scholar]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 14 September 2020).
- Kassambara, A. Ggpubr: ‘Ggplot2’ Based Publication Ready Plots. R Package Version 0.2.4. 2019. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 20 November 2020).
- Peterson, B.G.; Carl, P. PerformanceAnalytics: Econometric Tools for Performance and Risk Analysis, R Package Version 2.0.4. 2020. Available online: https://CRAN.R-project.org/package=PerformanceAnalytics (accessed on 20 November 2020).
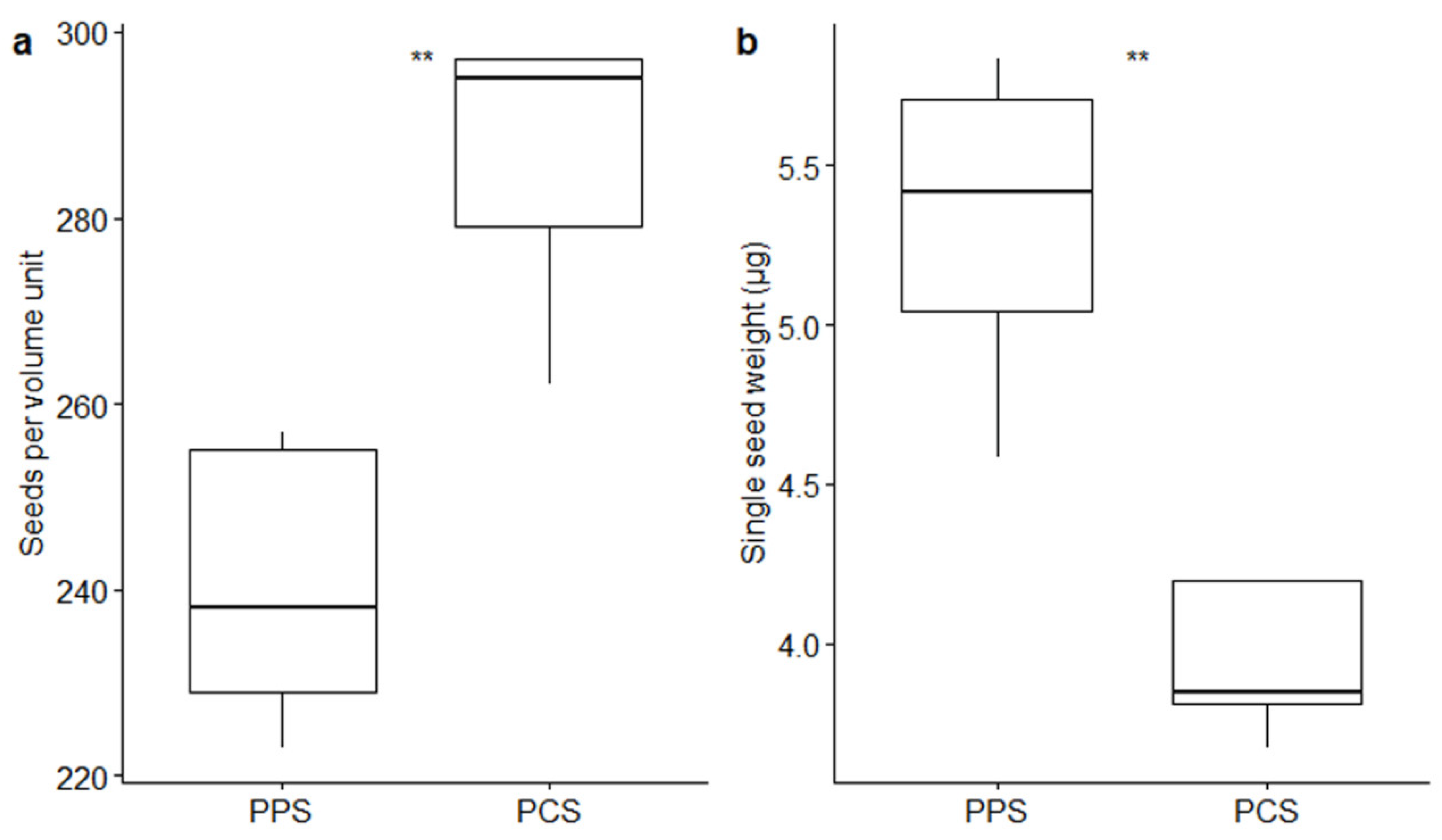
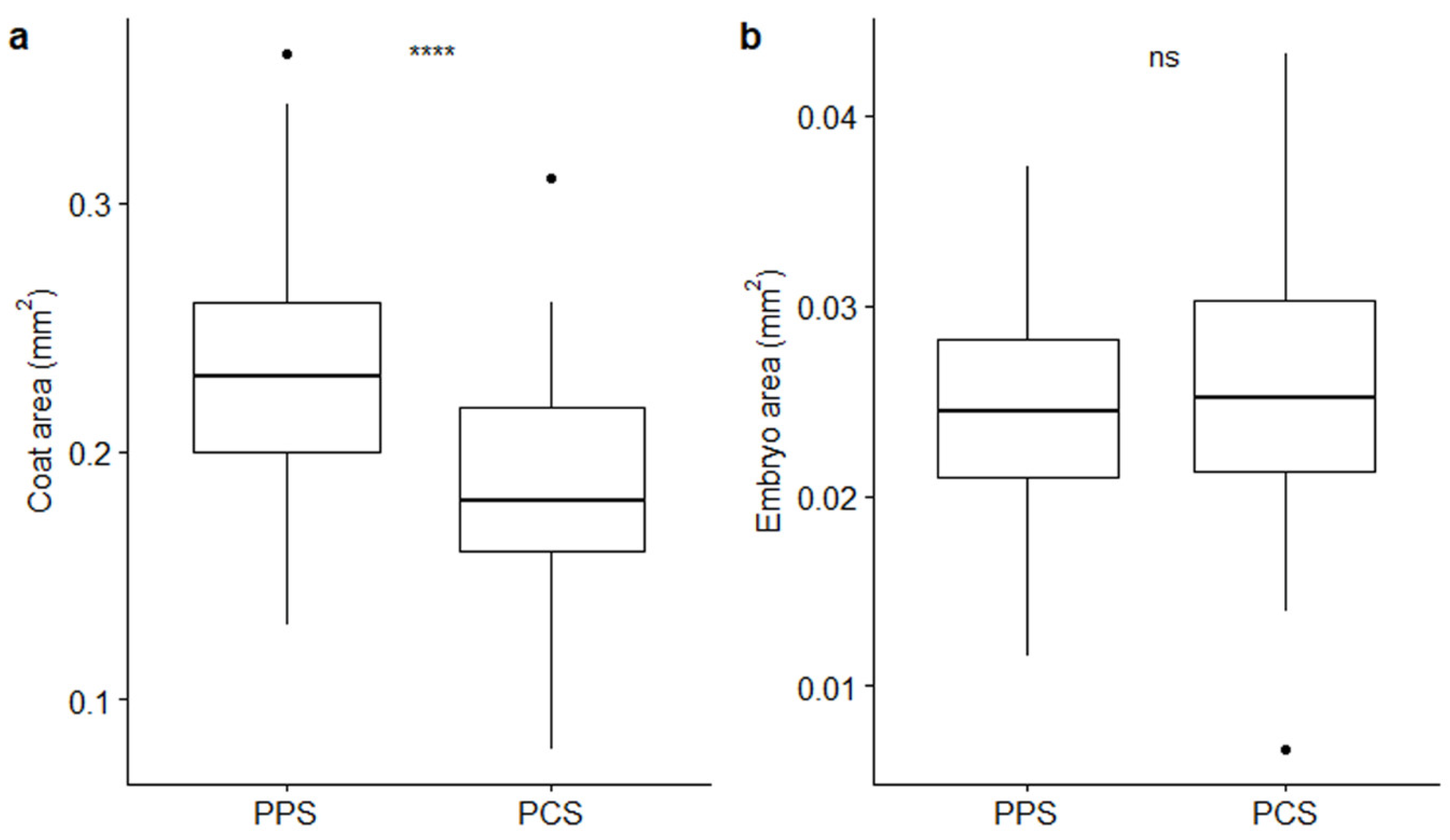
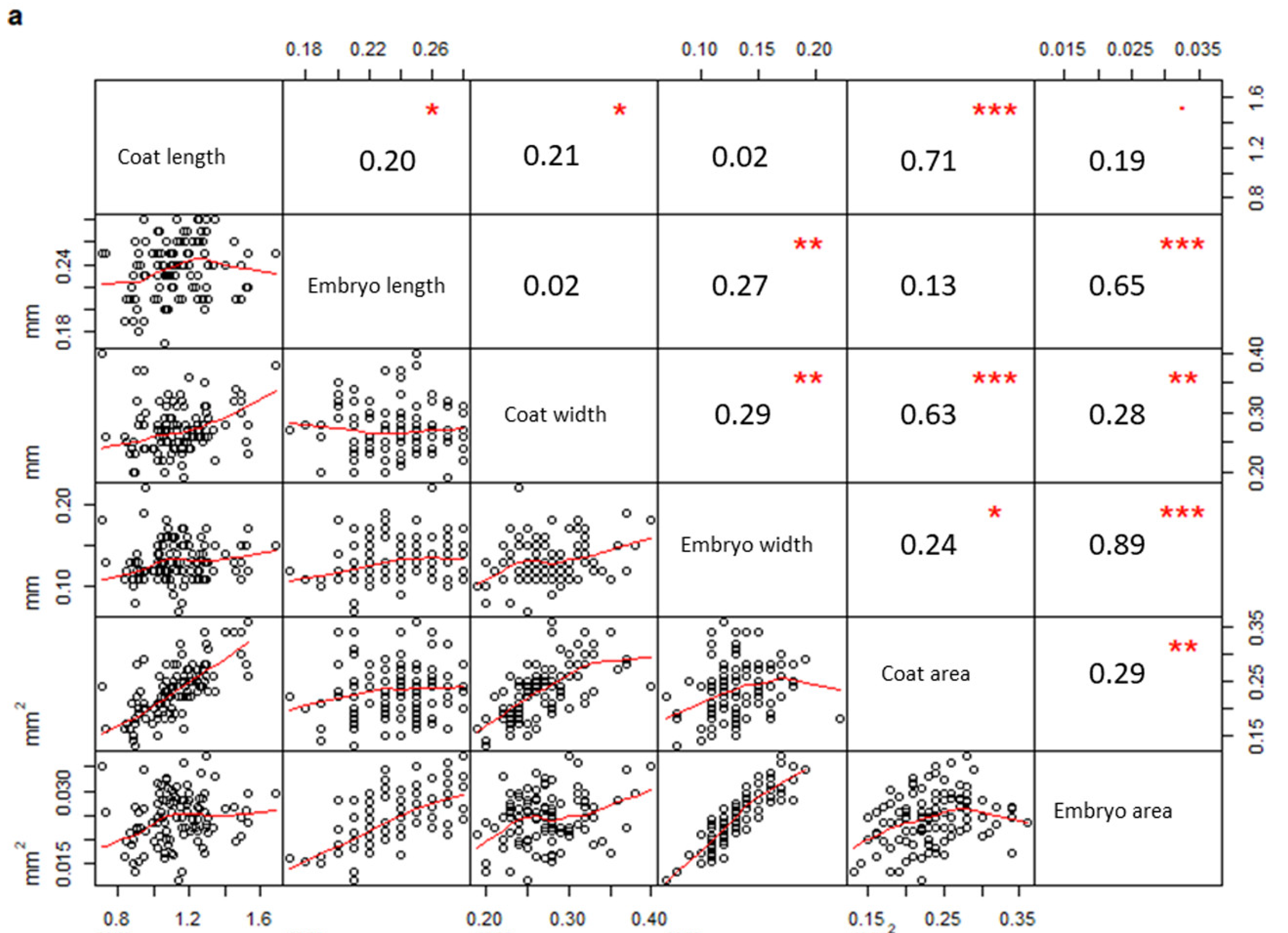
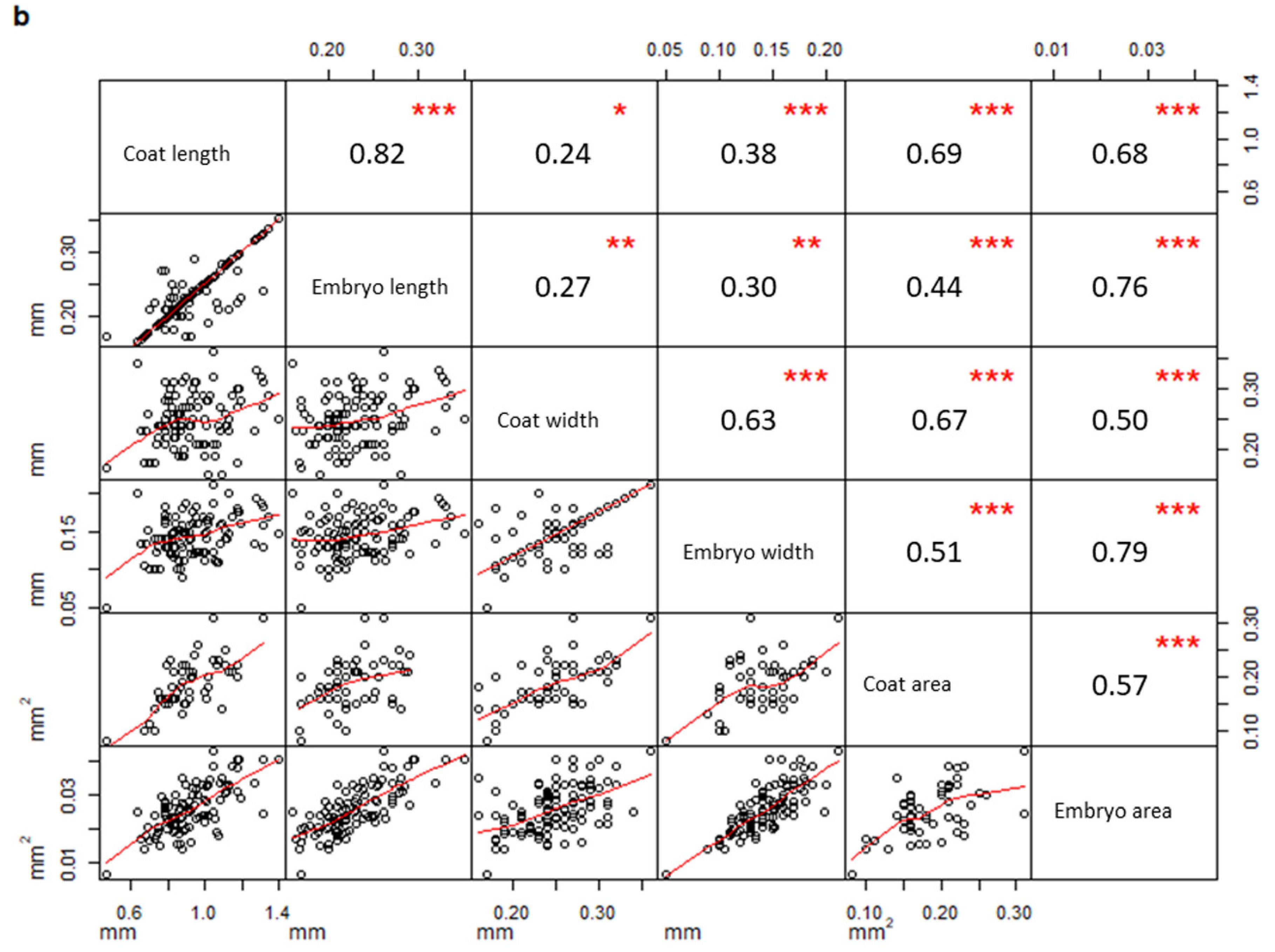
| Measurements | PPS | PCS | Test | Test Results |
|---|---|---|---|---|
| Coat length (mm) | 1.13 ± 0.02 | 0.94 ± 0.02 | Mann–Whitney U test | W = 8846; p = 1.06 × 10−5 **** |
| Embryo length (mm) | 0.24 ± 0.002 | 0.23 ± 0.004 | Mann–Whitney U test | W = 6281.5; p = 0.13, ns |
| Coat width (mm) | 0.27 ± 0.004 | 0.25 ± 0.004 | Mann–Whitney U test | W = 7204.5; p = 3.61 × 10−4 ** |
| Embryo width (mm) | 0.13 ± 0.002 | 0.14 ± 0.003 | Mann–Whitney U test | W = 3904.5; p = 1.21 × 10−4 *** |
| Coat area (mm2) | 0.24 ± 0.005 | 0.19 ± 0.006 | t-test | t = 6.07; p = 5.79 × 10−4 **** |
| Embryo area (mm2) | 0.02 ± 0.001 | 0.03 ± 0.001 | t-test | t = -1.83; p = 0.07, ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Agostini, A.; Cortis, P.; Cogoni, A.; Gargiulo, R.; Fenu, G. Epipactis tremolsii Seed Diversity in Two Close but Extremely Different Populations: Just a Case of Intraspecific Variability? Plants 2020, 9, 1625. https://doi.org/10.3390/plants9111625
De Agostini A, Cortis P, Cogoni A, Gargiulo R, Fenu G. Epipactis tremolsii Seed Diversity in Two Close but Extremely Different Populations: Just a Case of Intraspecific Variability? Plants. 2020; 9(11):1625. https://doi.org/10.3390/plants9111625
Chicago/Turabian StyleDe Agostini, Antonio, Pierluigi Cortis, Annalena Cogoni, Roberta Gargiulo, and Giuseppe Fenu. 2020. "Epipactis tremolsii Seed Diversity in Two Close but Extremely Different Populations: Just a Case of Intraspecific Variability?" Plants 9, no. 11: 1625. https://doi.org/10.3390/plants9111625
APA StyleDe Agostini, A., Cortis, P., Cogoni, A., Gargiulo, R., & Fenu, G. (2020). Epipactis tremolsii Seed Diversity in Two Close but Extremely Different Populations: Just a Case of Intraspecific Variability? Plants, 9(11), 1625. https://doi.org/10.3390/plants9111625







