Abstract
The reclamation of abandoned mining heaps rich in potentially toxic elements (PTEs) is critical for the environment. We carried out a laboratory experiment studying the effects of the addition of four natural sorbents (biochar, bentonite, chicken manure and organo-zeolitic substrate) to soils contaminated with PTEs, predominantly Cu, As and Sb, on the germination and growth of the autochthonous grasses Agrostis capillaris, A. stolonifera, Festuca rubra and Poa pratensis. The experiment used Petri dish tests with water extracts of contaminated soil and soil neutralised with the four sorbents. Standard indexes of the germination process were used (germination percentage, time required for 50% germination index, speed of emergence), and different values were found depending on the plant species and sorbent used. However, the percentage of seeds germinating was lower for each sorbent compared to the control (distilled water). The fresh mass values were positively stimulated by all sorbents. Electrolyte leakage was the highest in seedlings watered with an extract of untreated soil from the heap compared to extracts from treated soils and the control. This can be interpreted as eliminating the harmful effects of increased potentially toxic element (PTE) contents by sorbents, which can be useful in remediation processes.
1. Introduction
Extreme habitats include areas once occupied by the mining industry, which left behind mine heaps and various types of toxic waste. These habitats have very specific plant cover because they are often characterised by a lack of soil and nutrients (including humus) and often a constant lack of moisture [1,2]. An additional problem is the fact that various types of toxic compounds, including many potentially toxic elements (PTEs), remain in the ground on mining heaps. Although many PTEs are indispensable for plants, they can block their development at higher concentrations. This negative effect depends on the PTE’s bioavailability in soil solutions, which is influenced by pH, organic matter content, microbial activity and cation exchange capacity of the soil. The contents of toxic substances is a colonisation barrier for many species of plants, even those with a relatively wide ecological scale [3,4,5,6].
However, there are plants that can tolerate this kind of extreme habitat. They usually colonise small depressions in the mining heap or sites with decaying material in the substrate that are relatively wet. Dead organic matter can gradually build up between stones, which will form humus over time if other unfavourable factors such as surface runoff are avoided. As a result, this can lead to the formation of a specific mosaic of vegetation. According to previously conducted studies of mining areas, perennial plants predominate in the heaps, while annual and biennial plants are relatively rare [7,8,9]. Heaps with untypical metal contents in the substrate are like ecological islands, as they can only be inhabited by species with specific physiological adaptations. In general, they do not differ morphologically from species growing under more favourable conditions, but their physiology is different. These adaptations are the result of evolutionary processes to which this small group of plants was exposed, enabling them to exist in phytotoxic conditions [10,11].
Because of the unusual conditions in mine heaps, plant succession results in new and unique plant communities [12,13,14]. During colonisation, lichens and bryophytes appear on the surfaces of stones and rocks and between the stones, grasses and tolerant dicotyledon species [12,13]. As the heaps usually originated from different periods of mining, the vegetation formed on them occurs at different stages of succession [7,15]. However, the spontaneous succession processes are strongly stretched in time, and there is a great need for the rehabilitation of mining heaps. This would contribute to faster colonisation of these areas and thus reduce the flow of toxic elements to the neighbouring areas. Therefore, studies on the tolerance of common plant species to post-mining soils containing specific components are needed. Another significant problem is the binding of toxins present in the substrate by the inhabited species. However, studies on this issue are generally lacking.
The aim of our experiment was to investigate the effect of the addition of four natural sorbents (biochar, bentonite, chicken manure and organo-zeolitic substrate) to soils contaminated predominantly with copper (Cu) on the germination and early growth of four common grass species, Agrostis capillaris L., A. stolonifera L., Festuca rubra L., Poa pratensis L. The use of sorbents is proving to be an economically and environmentally-friendly form of reducing the content and bioavailability of PTEs in contaminated soils [16,17,18]. Biochar is caused by a decrease in bioavailable fractions of Cd, Cu, Pb and Zn [19], while bentonite Cd, Pb, Zn [20] and chicken manure reduces the content of Pb, Cd, Cr, As [21]. Perlite can sorb Pb, Cu, Ni, Cd [22], calcium carbonate (CaCO3), Pb, Ni and Cd [23]. Adding organo-zeolitic substrate to the soil not only improves the soil texture, but it also acts as a supplement of P, N, Ca and K nutrient elements [24]. Sorbents also have a positive effect on plant growth and a significant increase in biomass yield. Chicken manure was effective for Trifolium pratense L., Dactylis glomerata L., Lolium perenne L., Elytrigia repens (L.) Nevski [25], biochar Triticum aestivum L. [19], bentonite for Oryza sativa L. [26].
We asked the following questions: (i) Does the addition of sorbents have a positive effect on the germination parameters of the tested species? (ii) do sorbents induce a higher increase in the length of seedlings? (iii) do young seedlings achieve higher values of biomass and water content in cells thanks to sorbents? and (iv) does the addition of sorbents reduce environmental stress, reducing electrolyte leakage from the cells of the analysed grasses?
2. Results
2.1. Soil Analyses
The results of soil analyses are presented in Table 1. The copper content exceeds the European Union limit by almost 10 times [27]. The average pH of anthrosol (5.17) indicates strongly acidic soil.

Table 1.
Content of potentially toxic elements (PTEs) (in mg·kg−1) and pH in contaminated soil.
2.2. Germination of Seeds
On the third day of germination, all water extracts inhibited the germination of seeds of the tested species except for Agrostis capillaris, compared to controls. On the seventh day of germination, the germination percentage (GP) was generally higher for seeds watered with distilled water than for extracts. The exception was for Poa pratensis, for which the GPs were clearly higher than for the seeds from the control (Table 2). After the seventh day of the experiment, Festuca pratensis and Poa pratensis had germinated the best (Figure 1); the other two species had a slightly weaker development, regardless of the type of sorbent, but still better than on the soil contaminated with copper and other metals.

Table 2.
Germination percentage (GP) index (%) after 3 and 7 days seeds germinated on water extracts from contaminated soil.
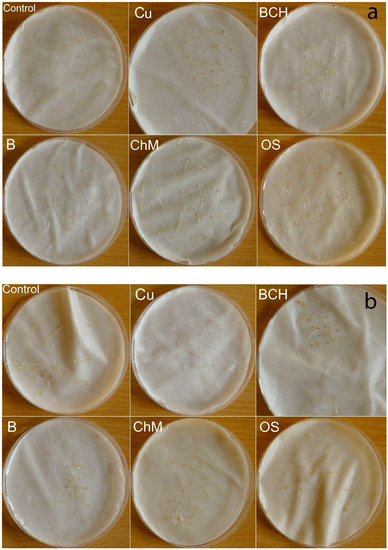
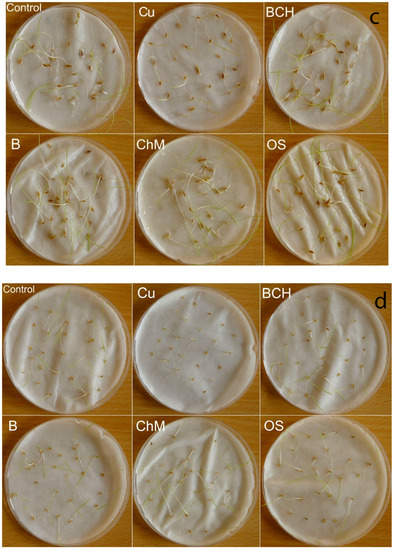
Figure 1.
Petri dishes tests after 7 days of the experiment; (a) Agrostis capillaris, (b) A. stolonifera, (c) Festuca rubra, (d) Poa pratensis; Control—distilled water, Cu—copper soil; copper soil with sorbents: BCH—biochar, B—bentonite, ChM—chicken manure, OS—organo-zeolitic substrate.
The values of the T50 index were similar, regardless of the species and type of extract used (including the control). Statistical analysis showed significant differences only between A. stolonifera and the other three studied species (Table 3).

Table 3.
The time required for 50% germination index (T50) seeds germinated on water extracts from contaminated soil.
The speed of emergence (SE) index in A. capillaris seedlings was not significantly different between the control and the soil extracts. In A. stolonifera, the SE was lowest in seedlings watered with BCH extracts, and highest in ChM, compared to the control. For F. rubra, similar SE values were found in the control and for ChM and OS extracts. The lowest SE values were observed for seedlings watered with B. In P. pratensis, the SE index was lowest in seedlings grown on the ChM extract and highest in the control (Figure 2).
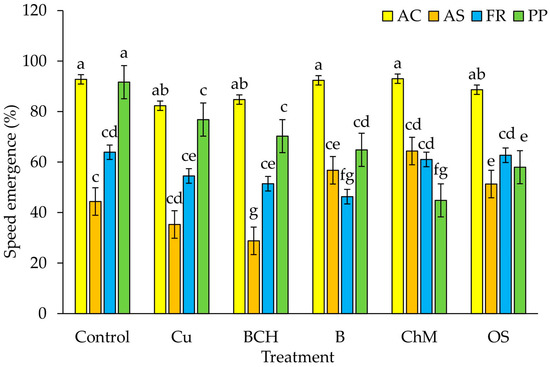
Figure 2.
Speed of emergence (SE) seeds germinated on water extracts from contaminated soil; species: AC—Agrostis capillaris, AS—A. stolonifera, FR—Festuca rubra, PP—Poa pratensis; Control—distilled water, Cu—copper soil; copper soil with sorbents: BCH—biochar, B—bentonite, ChM—chicken manure, OS—organo-zeolitic substrate; mean ± SD (n = 5) different letters differ significantly according to Fisher’s test p ≤ 0.05.
Biometric analysis of seedlings treated with water soil extracts showed a positive effect of sorbents on elongation growth (Table 4). Regardless of species, B and OS stimulated elongation growth relative to seedlings from the control sample. The use of the manure sorbent (ChM) also stimulated elongation growth for A. stolonifera and F. rubra. Compared to the control, only the addition of carbon (BCH) had a negative effect on the growth of all tested seedlings.

Table 4.
Lengths of whole seedlings and inhibition of percentage (IP) of seeds germinated on water extracts from contaminated soil.
The fresh mass of seedlings of the tested grass species on water extracts of soils with sorbents was higher than in the control (Table 5). Each of the water extracts had a positive effect on this parameter. The highest increase in fresh mass was observed for A. capillaris seedlings germinated on the OS extract. The lowest values of fresh mass relative to the control were found for F. rubra watered with the ChM extract. The dry mass of seedlings watered with soil water extracts was significantly higher in A. capillaris and P. pratensis than controls. In the case of A. stolonifera and F. rubra, all extracts significantly reduced the dry mass of seedlings. Tissue water content values of the seedlings were generally similar between the control and the tested soil extracts. Regardless of the species, the highest increase in the percentage of water content was found in seedlings watered with OS, ChM and B, compared to the control.

Table 5.
Fresh, dry masses expressed as % of control and total water content in seedlings treated with water extracts from different contaminated soils.
The percentage of electrolyte leakage of A. capillaris seedlings was higher in each of the tested solutions than in the control (Figure 3). The highest degree of water-ion balance destabilisation was found for seedlings germinated from seeds watered with Cu and BCH extracts. For the remaining three extracts, there was a reduction in electrolyte leakage disturbance, but it was still significantly higher than in seedlings watered with distilled water. For A. stolonifera, electrolyte leakage was similar for soil extracts and the control, with no significant differences found. In the case of seedlings of F. rubra, there were significant decreases in the degree of electrolyte leakage for all water extracts except for seedlings watered with Cu extracts, which were similar to the control. Only slight changes were found in electrolyte leakage for P. pratensis seedlings germinated on BCH and ChM extracts compared to the control and other soil extracts.
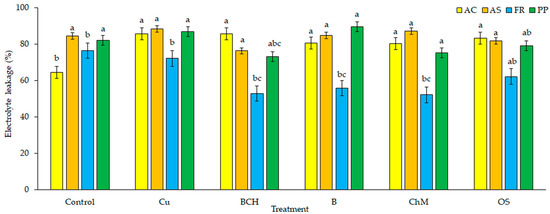
Figure 3.
Electrolyte leakage (EL) in seedlings treated with water extracts from different soils; species: AC—Agrostis capillaris, AS—A. stolonifera, FR—Festuca rubra, PP—Poa pratensis; Control—distilled water, Cu—copper soil; copper soil with sorbents: BCH—biochar, B—bentonite, ChM—chicken manure, OS—organo-zeolitic substrate; mean ± SD (n = 5) different letters differ significantly according to Fisher’s test p ≤ 0.05.
The sources data for pH, germination index, time required for 50% germination index, speed of emergence, length whole seedlings and inhibition of percentage (IP), fresh masses, dry masses and tissue water content, electrolyte leakage are given in Table S1.
3. Discussion
Copper is a micronutrient that plants need, but only in small amounts. It is a component of proteins and enzymes and as such plays an important role in the processes of photosynthesis and respiration, and also participates in the lignification and growth of plants. Without copper, the leaves of plants twist and turn dark green. At low concentrations, it has a stimulating effect, but at high concentrations, it inhibits both seed germination and plant growth, and in extreme cases, leads to complete necrosis [28,29,30,31,32,33]. As mentioned previously, in mining areas, copper deposits are generally accompanied by heavy metals [34], resulting in many negative environmental effects. These heavy metals reduce the productivity of plants and pose a threat to entire ecosystems [35] (Figure 4). They cause environmental stress, influencing biochemical, genetic and physiological changes in plants [36,37]. However, plants have developed potential mechanisms to combat heavy metal toxicity problems. For instance, they produce low molecular mass thiols that have a high affinity for toxic metals [38]. The most important biologically active thiols in this respect are glutathione (GSH) and cysteine. GSH is a substrate for the synthesis of phytochelatin and is of key importance for the detoxification of heavy metals such as cadmium or nickel [39]. Phytochelatins form complexes with toxic metal ions in the cytosol and then transfer them to the vacuole [40]. In this way, they protect plants against the harmful effects of heavy metals, though it is likely that these mechanisms work at different levels in different plant species. According to [41], Cu, As and Sb are the most important toxic metals in terms of environmental risk in the studied locality and pose a serious danger due to the highly toxic nature of their compounds. Already in our research, the contents in the soil are very high, but [42] found even higher (Cu 386–4630, As 37–983, Sb 46.3–1403 in mg·kg−1).

Figure 4.
The copper mine heap of Maximilián in Špania Dolina village, and showing the location in Slovakia (Photo T. Kviatková).
The availability of heavy metals in soils is influenced by the presence of organic matter and soil pH [43]. In neutral and slightly alkaline soil solutions, Cu is present in the form of inorganic complexes [44]. The high content of organic matter and clay minerals reduces the bioavailability of metals while increasing their fraction in the soil [45,46]. pH values > 6.5 reduce the amount of readily soluble forms of metals in the soil and limit their uptake and accumulation by plants [47]. In our study, water extracts with sorbents were characterised by higher pH values compared to non-modified soil extracts contaminated with copper and other metals (Figure 5). Therefore, it can be concluded that the natural sorbents added to the soil probably affected the immobilisation of heavy metals [48].
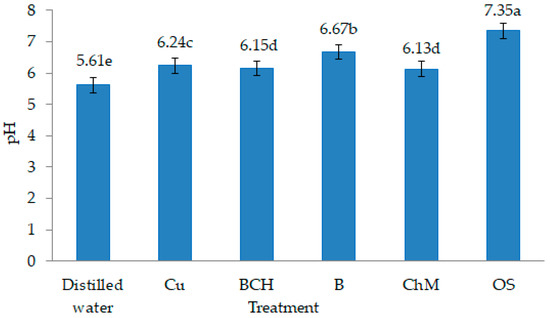
Figure 5.
pH values of water soil extracts: Control—distilled water, Cu—copper soil; copper soil with the sorbents: BCH—biochar, B—bentonite, ChM—chicken manure, OS—organic substrates; mean ± SD (n = 5) different letters differ significantly according to Fisher’s test p ≤ 0.05.
Germination is one of the first stages of seed contact with a stress factor, making it a kind of sensitivity index and measure of tolerance to chemical and physical conditions of the rhizosphere [49,50,51]. During germination, the phytotoxicity of Cu and accompanying heavy metals depends on their concentration levels [52,53,54] (Figure 1). Excesses cause oxidative stress by activating reactive oxygen species and inhibiting catalase activity [55]. Under stress, plant germination and growth are slower as the plant produces smaller cells with thicker cell walls [56], with the germination capacity of seeds reduced with increasing concentrations [57,58]. One of the toxic properties of these elements may be due to a shortage of water in the extracts, which inhibits cell expansion and reduces carbon uptake [59].
The germination percentage (GP) index in our study was higher for seeds watered with distilled water than with extracts from contaminated soil and sorbents (Table 2). The T50 index was significantly different only for Agrostis stolonifera, compared to the other three analysed species (Table 3). However, the values of the speed of emergence (SE) index changed depending on the species and the extract used (Figure 2). These different reactions of the tested seeds to the water extracts with sorbents used may have resulted from their differing protective properties against stress. Seeds are equipped with sensing mechanisms thus that germination will only take place when environmental factors are favourable. In addition to complexes with phytochelatins, plant defence strategies in the fight against heavy metals include reduced absorption by formation, activation of osmolytes substances and antioxidants and changes in enzyme expression [60]. Another protective barrier is the seed coat, which limits the penetration of harmful substances into the seed [33,61]. This is probably related to our observations after the seventh day of the experiment: Festuca pratensis and Poa pratensis, with larger seeds and a thicker seed coat, germinated and developed better (Figure 1).
According to [62], the stage of seedling growth and development is more sensitive to metals than germination. The toxic effects of copper on plant growth have already been confirmed, e.g., for mustard [63], wheat [31], and pine [64]. In our study, the biometric analysis of seedlings showed a stimulating effect of OS extracts and an inhibitory effect of the addition of carbon (BCH) on the growth of all tested species (Table 4). The inhibition of seedling growth most likely resulted from the impairment of the basic seed functions. Copper and heavy metals present in toxic amounts modify the ultrastructure of the cell and disturb its homeostasis. They affect cell nuclei and cell division, causing a reduction in mitotic activity, cytokinesis disturbances, DNA and RNA damage, decreased transcription activity, chromatin condensation, chromosomal aberrations, destruction of the nuclear envelope, reduction of the volume of nuclei and an increase in the number of micronuclei [65]. All these mechanisms ultimately impair plant growth and development.
The effects of heavy metal toxicity on plant mass also depend on their concentrations [66,67]. Our results showed that each of the water extracts with sorbents had a positive effect on the values of the fresh mass of grass species. Seedling dry mass was significantly higher in Agrostis capillaris and P. pratensis, while for A. stolonifera and F. pratensis, an inhibition of mass gain was seen (Table 5; Figure 1). Copper induces the mobilisation of biomass by releasing glucose and fructose, inhibits the decomposition of starch and sucrose in tissues by limiting the activity of alpha-amylase and invertase isoenzymes [55]. Its excess is related to the ability to accumulate free amino acids, e.g., proline and glycine [68,69]. A study by Boroş et al. [56] explained the increase in dry mass by the capturing of metal ions that bind to the cell wall, creating a heavier cell. Reductions of seedling biomass may also be due to limitations in protein formation and disturbances in carbohydrate translocation [70,71]. Thus, copper affects the overall metabolism, water uptake, and, depending on the concentration, limits the synthesis of plant nutrients [72].
In plants, stress leads to anatomical, morphological and physiological changes in root tissues, causing an inhibition of the root’s water and ion transport functions. The specific surface area of the roots depends on the number and size of intercellular spaces and the properties of the cell walls. Monocotyledonous plants have a lower cation-exchange capacity and take up polyvalent ions to a lesser extent. In the case of copper, they show a weaker ability to immobilise it in the cell wall [73]. Limitations on water uptake by plants reduce their turgor, which leads to limits in elongation growth, and the inhibition of root growth is an additional cause of limited water uptake [74]. Our study found the highest values of tissue water content for seedlings watered with OS, ChM and B, compared to the control (Table 5). This demonstrates a neutralisation of the toxic properties of copper on the physiology of seedlings of the studied grass species and a positive effect of natural sorbents.
Cell membranes, which are complex and dynamic structures, participate in the regulation of water transport. They play an important role in the resistance of plant cells to environmental stresses [64]. They constitute selective permeability barriers that regulate the molecular and ionic composition of the intracellular environment through specific channels, conveyors and pumps. Undamaged cell membranes allow water molecules to enter the cell interior and are barriers for molecules of substances dissolved in the cell sap. Copper and heavy metals contribute to changes in the permeability properties of cell membranes. Due to lipid peroxidation, they disrupt their function and structure [75,76]. They can also displace basic metal ions, which usually leads to a reduction or loss of enzymatic activity, the generation and accumulation of reactive oxygen species, and even programmed cell death [77,78]. In our experiment, the percentage of electrolyte leakage was the highest in seedlings watered with Cu soil extracts without any sorbents added. Regardless of the species, the addition of natural sorbents had a positive effect on the membranous structures of cells (Figure 3). This indicates that the influence of copper on changes in the structures of cell membranes is limited, and thus the loss of semi-permeable properties is reduced.
In summary, in our experiment, developmental changes in seedlings most likely resulted from disturbances arising at the stage of embryogenesis and were the result of the direct contact of seeds with extracts, which to a varying degree, eliminated the harmful effects of toxic metals. Further physiological studies of various plant species growing under toxic conditions will allow us to better understand their biochemistry, learn the details of defence strategies and ways to overcome stress, which at the same time will contribute to increasing their productivity. This will enable the identification of species resistant to pollution and their use in the more rapid restoration of vegetation in highly contaminated areas.
4. Materials and Methods
4.1. Study Area
The soil for testing was taken from the Maximilián heap (48°48′29″ N, 19°07′59″ E), located in the village of Špania Dolina (Central Slovakia; Figure 4). The area of the Špania Dolina ore deposit was one of the most recognisable copper deposits in the history of mining in Europe, with the mineralisation creating a vein 4 km long and 1.5 km wide [79]. The first written reports of ore mining in the area of Staré Hory and Špania Dolina date back to the 11th century (from 1006), although the nearby Piesky deposit was probably already exploited in the Late Stone Age [80,81]. The maximum extraction of copper ore, along with its extremely valuable silver content, took place in the years 1496–1546 (58,234 t Cu, 111,280 kg Ag). From the 17th century onwards, mining gradually decreased until it completely disappeared in the early 20th century [82].
In the Maximilián heap, the anthrosol remnants of the mining activity were contaminated by a high content of PTEs. Most Cu comes from chalcopyrite (CuFeS2), and As and Sb from tetrahedrite. The vegetation cover of the heap was very sporadic and sparse, with growth and development made difficult by an excess of coarse-grained tailings, a lack of soil matrix, water, nutrients, increased contents of PTEs, and other environmental and ecological factors.
4.2. Seeds Material with Short Characteristic of Species
Four common grass species typical for Eurasian areas were selected for the experiment: Agrostis capillaris L., A. stolonifera L., Festuca rubra L., Poa pratensis L. Of these, P. pratensis had the highest utility value as a forage grass, while the other species were of lesser importance for forage [83]. However, they were suitable for land reclamation, creating dense turfs and grasslands. For this experiment, the seeds were bought from DLF SeeDs (Hladké Životice, Czech Republic).
All 4 species were certainly native to most of Eurasia [84,85]. They can be found growing in a variety of habitats, including grasslands and meadows, wetlands, fields, roadsides, forest edges, etc., and as a pioneer species on disturbed sites [86]. Agrostis capillaris, A. stolonifera and Festuca rubra were considered as facultative metallophytes [87]. Agrostis capillaris and Poa pratensis were also used in erosion control due to its dense, vigorous turf forming habitat [88].
4.3. Soil Analysis and Modification
The 3 soil samples with the weight of 2–3 g were analysed in the Geochemical and Assaying Laboratories of Bureau Veritas Commodities Canada Ltd. in Vancouver, by inductively coupled plasma emission spectrometry (ICP-ES) method. Samples were digested in aqua regia and acid digestion by HNO3, HClO4 and HF heated until dryness, to decompose most minerals, including silicates to metal salts. The residues were dissolved in concentrated HCl.
The contaminated soil from heap Maximilián was subsequently mixed with natural sorbents, which were able to immobilize PTEs. Water extracts were prepared from soil mixtures (Table 6).

Table 6.
Soil modifications are used to prepare water extracts to experiment.
4.4. Water Extract and pH Values
100 g of each type of soil treatment was flooded with 150 mL of distilled water, thoroughly mixed, wrapped in aluminium foil, and put on a shaker for 2 h. The water solutions were left at room temperature for 24 h in order to extract the chemical substances they contained. Then, the water soil solutions were filtered through filter paper and stored in a refrigerator for the duration of the experiment. pH values of each of the solutions were determined (pH-meter CX-701, Elmetron, Zabrze, Poland), and the pH of water extracts of soils with the addition of natural sorbents ranged from 6.15 to 7.35. The highest values were for OS, and the lowest for BCH (Figure 5).
4.5. Germination Conditions
Grass seeds (each separately) were sterilised in 1% acetone for 5 min, then rinsed 3 times with distilled water. 25 seeds of each species were placed on separate sterilised Petri dishes (Ø 9 cm) with 3 layers of Whatman filter paper (Grade 1:11 μm—medium flow filter paper). On the first day, seeds were watered with 5 mL of water soil extracts. Every other day, the seeds on Petri dishes were wetted with 3 mL of each extract, respectively. The control group consisted of seeds watered with distilled water. For the duration of the experiment, seeds were placed in the dark, at room temperature. After 3 and 7 days, seeds were counted. Germinated seeds were considered those whose germinal root was equal to half the size of the seed. A germination experiment was performed in 3 repetitions for each of the extracts and control samples.
4.6. Germination Parameters
The effect on the germination capacity of seeds was evaluated by the germination percentage—GP [89], the time required for 50% germination—T50 [90], and speed of emergence—SE [91] according Table 7.

Table 7.
Formulas of germination indexes used in the experiment.
4.7. Biometric Analysis
The effect of water extracts on seedlings length was determined using a caliper (Topex 31C615, Grupa Topex, Warszawa, Poland), with an accuracy of 1 mm. The inhibition percentage (IP) expressed as a percentage of control seedlings was measured according to Islam et al. [92].
where: LE—seedling length (cm) treated with the aqueous extract, LC—seedling length (cm) treated with the distilled water (control group). Inhibition percentage (IP) of growth (expressed as control %)—a minus (−) value indicates an increase of seedlings length, and a plus (+) value indicates a decrease of elongation of seedlings.
IP = (1 − (LE/LC)) × 100
4.8. Fresh and Dry Mass and Tissue Water Content
Fresh mass (FM) of seedlings was determined on a scale (Ohaus Adventurer Pro, OHAUS CORPORATION, NJ, USA). The plant material was dried for 48 h at 105 °C in a dryer (WAMED SUP 10, WAMED Wytwórnia Aparatury Medycznej SSP, Warszawa, Poland) to obtain the dry mass (DM). The total water content was determined according to the formula:
H2O (%) = 100 − ((DM × 100)/FM),
4.9. Electrolyte Leakage
Electrolyte leakage was measured in 7 days of seeds germination according to the method used by [64]. A single grass seedling was placed in polypropylene vials with 30 mL distilled water and shaken for 3 h on a shaker (Labnet International Inc., Edison, NJ, USA) to determine the leakage of the electrolyte from live cells (E1) by conductivity meter CX-701 (Elmetron, Zabrze, Poland) with the electrode (K = 1.02) (Elmetron, Zabrze, Poland). Then plant material was frozen at −75 °C to macerate the tissues. After 24 h, the seedling in water was defrosted and subjected to the same procedure (E2) as samples with live material. On the basis of the results, the total electrolyte leakage was determined according to the formula:
EL (%) = (E1/E2) × 100
4.10. Statistical Analysis
Statistical analysis was performed using the multivariate-way ANOVA analysis of variance test. The obtained results were subjected to multivariate analysis examined the relationship between a given parameter and species and extracts. At the same time, all species were analysed in order to verify how they respond to stress factors in the form of water extracts from soil. Which species was the least and which was the most sensitive. The differences between the means ± SD (n = 5) were measured by Fisher’s test at p ≤ 0.05 in the program StatSoft, Inc. 2018 (Warszawa, Poland). STATISTICA (data analysis software system), version 13.1.
5. Conclusions
Sorbents, used as an additive to neutralise copper (Cu) and other PTEs in soils, have a significant effect on the germination and early growth of grass seeds. Reactions seeds on soil water extracts depend on grass species and type of extracts. In general, the addition of any of the sorbents to the contaminated soil is beneficial. Soils with B and ChM had the most positive effect on seeds germination percentage (GP) (i).
In the case of the elongation growth of seedlings, a negative effect of BCH extracts and a positive effect of OS extract were demonstrated for all seed species (ii).
The fresh mass values were stimulated by all sorbent extracts. The dry mass of seedlings was higher in Agrostis capillaris and Poa pratensis, and lower in A. stolonifera and Festuca rubra. The tissue water content of the tested seedlings was higher for those grown with OS, ChM and B sorbents, compared to the control (iii).
Electrolyte leakage was highest in seedlings watered with Cu soil extracts for Agrostis capillaris. For the other studied species, although a decrease in the degree of destabilisation of cell membranes was observed, significant differences in the values of this parameter were not found compared to the control (iv).
The experiment confirmed the hypothesis that the addition of natural sorbents (especially organic substrates, chicken manure and bentonite) eliminates the toxic properties of copper and heavy metals contained in the soil, which is an important conclusion from the point of view of the reclamation of copper heaps. Sorbents have a positive effect on germination and early seed growth and reduce the destabilisation of seedling cell membranes. However, differences in plant responses to extracts depend on the species and the modifications applied to the extracts.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/11/1591/s1, Table S1: Sources data for (a) pH; (b) germination index (GI); (c) time required for 50% germination index (T50); (d) speed of emergence (SE); (e) length whole seedlings and inhibition of percentage (IP); (f) fresh masses, dry masses and tissue water content (TWC); (g) electrolyte leakage.
Author Contributions
Conceptualisation, I.T. and K.M.; methodology, I.T., K.M. and B.B.-K.; validation, I.T., T.K., K.M. and B.B.-K.; formal analysis, B.B.-K.; investigation, K.M.; resources, T.K. and B.B.-K.; data curation, I.T. and B.B.-K.; writing—original draft preparation, K.M. and B.B.-K.; writing—review and editing, I.T. and T.K.; visualization, T.K., K.M. and B.B.-K.; supervision, I.T.; project administration, I.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Science under grant VEGA 2/0040/17 and VEGA 1/0291/19.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frazeková, D.; Boguská, Z.; Fazekaš, J.; Škvareninová, J.; Chovancová, J. Contamination of vegetation growing on soils and substrates in the unhygienic region of Central Spiš (Slovakia) polluted by heavy metals. J. Environ. Biol. 2016, 37, 1335–1340. [Google Scholar]
- Rahmonov, O.; Krzysztofik, R.; Srodek, D.; Smolarek-Lach, J. Vegetation and Environmental Changes on Non-Reclaimed Spoil Heaps in Southern Poland. Biology 2020, 9, 164. [Google Scholar] [CrossRef]
- Baker, A.; Brooks, R.; Reeves, R. Growing for gold and copper and zinc. New Sci. 1988, 10, 44–48. [Google Scholar]
- Chojnacka, K.; Chojnacki, A.; Gorecka, H.; Górecki, H. Bioavailability of heavy metals from polluted soils to plants. Sci. Total Environ. 2005, 337, 175–182. [Google Scholar] [CrossRef]
- Soltani, N.; Keshavaryi, B.; Moore, F.; Sorooshian, A.; Ahmadi, R.M. Distribution of potentially toxic elements (PTEs) in tailings, soils, and plants around Gol-E-Gohar iron mine, a case study in Iran. Environ. Sci. Pollut. Res. 2017, 24, 18798–18816. [Google Scholar] [CrossRef]
- Palansooriya, N.K.; Shaheen, M.S.; Chen, S.S.; Tsang, C.W.D.; Hashimoto, Y.; Hou, D.; Bolan, S.N.; Rinklebe, Z.Y.; Ok, S.Y. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Inter. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Roman, A.; Gafta, D.; Ursu, T.M.; Cristea, V. Plant Assemblages of Abandoned Ore Mining Heaps: A Case Study from Roşia Montană Mining Area, Romania. In Geographical Changes in Vegetation and Plant Functional Types; Greller, M.A., Fujiwara, K., Pedrotti, F., Eds.; Springer International Publishing: New York, NY, USA, 2018; pp. 303–332. [Google Scholar] [CrossRef]
- Banásová, V. Vegetácia medených a antimónových háld. (Vegetation of copper and antimony mine heaps). Biol. Práce 1976, 22, 1–109. (In Slovak) [Google Scholar]
- Kompala-Bala, A.; Sierka, E.; Dyderski, K.M.; Bierza, W.; Magurno, F.; Besenyei, L.; Blońska, A.; Ryś, K.; Jagodziński, M.A.; Woźniak, G. Do the dominant plant species impact the substrate and vegetation composition of post-coal mining spoil heaps? Ecol. Eng. 2020, 143, 105685. [Google Scholar] [CrossRef]
- Mithofer, A.; Schulze, B.; Boland, W. Biotic and heavy metal stress response in plants: Evidence for common signals. FEBS Lett. 2004, 566, 1–5. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Turisová, I.; Štrba, T.; Andráš, P.; Aschenbrenner, Š. Floristic composition on the abandoned copper heaps in central Slovakia. Rom. J. Miner. Depos. 2014, 87, 61–64. [Google Scholar]
- Širka, P.; Turisová, I.; Petrášová, A. Bryophytes of Cu-mine heaps in the vicinity of Banská Bystrica (Central Slovakia). AUPC Studia Nat. 2016, 1, 7–23. [Google Scholar]
- Banásová, V.; Horak, O.; Čiamporová1, M.; Nadubinská, M.; Lichtscheidl, I. The vegetation of metalliferous and non-metalliferous grasslands in two former mine regions in Central Slovakia. Biológia 2006, 61, 433–439. [Google Scholar]
- Woch, M.W.; Stefanowicz, M.A.; Stanek, M. Waste heaps left by historical Zn-Pb ore mining are hotspots of species diversity of beech forest understory vegetation. Sci. Total Environ. J. 2017, 599–600, 32–41. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Peng, W.; Li, X.; Xiao, S.; Fan, W. Review of remediation technologies for sediments contaminated by heavy metals. J. Soils Sediments 2018, 18, 1701–1719. [Google Scholar] [CrossRef]
- Sun, W.; Ji, B.; Khoso, A.S.; Tang, H.; Liu, R.; Hu, Y. An extensive review on restoration technologies for mining tailings. Environ. Sci. Pollut. Res. 2018, 25, 33911–33925. [Google Scholar] [CrossRef]
- Ali, A.; Guo, D.; Jeyasundar, A.S.G.P.; Li, Y.; Xiao, R.; Du, J.; Li, R.; Zhang, Z. Application of wood biochar in polluted soils stabilized the toxic metals and enhanced wheat (Triticum aestivum) growth and soil enzymatic activity. Ecotoxicol. Environ. Saf. 2019, 184, 109636. [Google Scholar] [CrossRef]
- Wasilkowski, D.; Nowak, A.; Michalska, J.; Mrozik, A. Ecological restoration of heavy metal-contaminated soil using Na-bentonite and green compost coupled with the cultivation of the grass Festuca Arundinacea. Ecol. Eng. 2019, 138, 420–433. [Google Scholar] [CrossRef]
- Wan, Y.; Huang, Q.; Wang, Q.; Yu, Y.; Su, D.; Qiao, Y.; Li, H. Accumulation and bioavailability of heavy metals in an acid soil and their uptake by paddy rice under continuous application of chicken and swine manure. J. Hazard. Mater. 2020, 384, 121293. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Raja, D.F. Experimental characterisation and evaluation of perlite as a sorbent for heavy metal ions in single and quaternary solutions. J. Water Process. Eng. 2014, 4, 179–184. [Google Scholar] [CrossRef]
- Mourid, S.S. Carbonate Content on Potential Toxic Heavy Metals Adsorption in Calcareous Soils. Curr. Sci. Int. 2014, 3, 141–149. [Google Scholar]
- Damian, G.; Andráš, P.; Damian, F.; Turisová, I.; Iepure, G. The role of organo-zeolitic material in supporting phytoremediation of a copper mining waste dump. Int. J. Phytoremediat. 2018, 20, 1307–1316. [Google Scholar] [CrossRef]
- Vrînceanu, O.N.; Motelică, M.D.; Dumitru, M.; Calciu, I.; Tănase, V.; Preda, M. Assessment of using bentonite, dolomite, natural zeolite and manure for the immobilization of heavy metals in a contaminated soil: The Copsa Mică case study (Romania). Catena 2019, 176, 336–342. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Xu, Y.; Liang, X.; Wang, L. In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Appl. Clay Sci. 2015, 105–106, 200–206. [Google Scholar] [CrossRef]
- Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture. Available online: http://europa.eu.int/servlet/portail/RenderServlet?search=DocNumber&lg=en&nb_docs=25&domain=Legislation&coll=&in_force=NO&an_doc=1986&nu_doc=278&type_doc=Directive (accessed on 11 July 2020).
- Xu, J.K.; Yang, L.X.; Wang, Z.Q.; Dong, G.C.; Huang, J.Y.; Wang, Y.L. Effects of soil copper concentration on growth, development and yield formation of rice (Oryza sativa). Rice Sci. 2005, 12, 125–132. [Google Scholar]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Tanyolaç, D.; Ekmekçi, Y.; Ünalan, S. Changes in photochemical and antioxidant enzyme activities in maize (Zea mays L.) leaves exposed to excess copper. Chemosphere 2007, 67, 89–98. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, G.; Shi, G.; Pan, F. Toxicity of Cu, Pb, and Zn on seed germination and young seedlings of wheat (Triticum aestivum L.). In Proceedings of the 4th Conference on Computer and Computing Technologies in Agriculture (CCTA), Nanchang, China, 22–25 October 2010; pp. 231–240. [Google Scholar]
- Kabata-Pendias, A.; Szteke, B. Pierwiastki Śladowe w Geo- i Biosferze; IUNG-PIB: Puławy, Poland, 2012; p. 270. ISBN 978-83-7562-120-4. (In Polish) [Google Scholar]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Banásová, V.; Hajdúk, J. Príspevok k vegetácií banských háld z malokarpatských rudných ložísk. Bull. Slov. Bot. Spoločnosti 2006, 28, 203–210. (In Slovak) [Google Scholar]
- Zandi, P.; Yang, J.; Możdżeń, K.; Barabasz-Krasny, B. A review of copper speciation and transformation in plant and soil/wetland systems. Adv. Agric. 2020, 160, 249–293. [Google Scholar] [CrossRef]
- Khan, A.; Kuek, C.; Chaudhry, T.; Khoo, C.; Hayes, W. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 2000, 41, 197–207. [Google Scholar] [CrossRef]
- Gall, J.E.; Rajakaruna, N. The physiology, functional genomics, and applied ecology of heavy metal-tolerant Brassicaceae. In Brassicaceae: Characterization, Functional Genomics and Health Benefits; Lang, M., Ed.; Nova: New York, NY, USA, 2013; pp. 121–148. ISBN 978-1-62808-856-4. [Google Scholar]
- Bricker, T.J.; Pichtel, J.; Brown, H.J.; Simmons, M. Phytoextraction of Pb and Cd from superficial soil: Effects of amendments and croppings. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng 2001, 36, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; Persans, M.W.; Nieman, K.; Albrecht, C.; Peer, W.; Pickering, I.J.; Salt, D.E. Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Cell 2004, 16, 2176–2191. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E.; Prince, R.C.; Pickering, I.J.; Raskin, I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol. 1995, 109, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Andráš, P.; Lichý, A.; Rusková, J.; Matúšková, L. Heavy metal contamination of the landscape at the Ľubietová deposit (Slovakia). World Academy of Science. Eng. Technol. 2008, 34, 97–100. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.193.6112&rep=rep1&type=pd (accessed on 13 July 2020).
- Andráš, P.; Ladomerský, J.; Samešová, D.; Rusko, M.; Dirner, V.; Krnáč, J. Comparison of Cu, As and Sb speciation at dump-fields in the areas of abandoned cu-deposits Ľubietová and Špania Dolina. In Proceedings of the Manažérstvo Životného Prostredia 2012—Management of Environment 2012, 2nd International Scientific Conference, Bratislava, Slovak Republic, 19–20 November 2012; pp. 173–183. Available online: https://www.sszp.eu/wp-content/uploads/2012_konf_MaZP_C01_Andras-et-al.pdf (accessed on 10 July 2020).
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 15, 84–91. [Google Scholar] [CrossRef]
- Gruca-Królikowska, S.; Wacławek, W. Metals in the environment part II. Effect of heavy metals on plants. Chem. Dydakt. Ekol. Metrol. 2006, 11, 1–2. [Google Scholar]
- Bolan, N.S.; Duraisamy, V. Role of inorganic and organic soil amendments on immobilisation and phyto availability of heavy metals: A review involving specific case studies. Aust. J. Soil Res. 2003, 41, 533–555. [Google Scholar] [CrossRef]
- Castaldi, P.; Alberti, G.; Merella, R.; Melis, P. Study of the organic matter evolution during municipal solid waste composting aimed at identifying suitable parameters for the evaluation of compost maturity. Waste Manag. 2005, 25, 209–213. [Google Scholar] [CrossRef]
- Usman, A.; Kuzyakov, Y.; Stahr, K. Effect of clay minerals on immobilization of heavy metals and microbial activity in a sewage sludge-contaminated soil. J. Soils Sediments 2005, 5, 245–252. [Google Scholar] [CrossRef]
- Herath, I.; Kumarathilaka, P.; Navaratne, A.; Rajakaruna, N.; Vithanage, M. Immobilization and phytotoxicityreduction of heavy metals in serpentine soil using biochar. J. Soils Sediments 2015, 15, 126–138. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. BioRecovery 1989, 1, 81–126. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055. [Google Scholar] [CrossRef]
- Solanki, R.; Dhankhar, R. Biochemical changes and adaptive strategies of plants under heavy metal stress. Biologia 2011, 66, 195–204. [Google Scholar] [CrossRef]
- Singh, D.; Nath, K.; Sharma, Y.K. Response of wheat seed germination and seedling growth under copper stress. J. Environ. Biol. 2007, 28, 409–414. [Google Scholar]
- Sfaxi-Bousbih, A.; Chaoui, A.; El Ferjani, E. Copper affects the cotyledonary carbohydrate status during the germination of bean seed. Biol. Trace Elem. Res. 2010, 137, 110–116. [Google Scholar] [CrossRef]
- Pavel, V.L.; Sobariu, D.L.; Diaconu, M.; Stătescu, F.; Gavrilescu, M. Effects of heavy metals on Lepidium sativum germination and growth. Environ. Eng. Manag. J. 2013, 12, 727–733. [Google Scholar] [CrossRef]
- Pena, L.B.; Azpilicueta, C.E.; Gallego, S.M. Sunflower cotyledons cope with copper stress by inducing catalase subunits less sensitive to oxidation. J. Trace Elem. Med. Biol. 2011, 25, 125–129. [Google Scholar] [CrossRef]
- Boroş, M.N.; Micle, V. Effects of copper-induced stress on seed germination of maize (Zea mays L.). Agricultura 2015, 95, 95–96. [Google Scholar] [CrossRef]
- Hema, C.; Subramani, A. Phytotoxic effect of copper and chromium on seed germination percentage of Vigna radiate L. Int. J. Curr. Res. Rev. 2013, 5, 95–100. [Google Scholar]
- Liu, H.Q.; Mo, Y.F.; Kong, X.Z.; Liu, Y.; Liu, H.P. Effects of copper on germination of watermelon seeds and growth of watermelon seedlings. In Proceedings of the 3rd International Conference on Application of Materials Science and Environmental Materials (AMSEM2015), Phuket Island, Thailand, 1–3 October 2015; Xu, Q., Ed.; Morehead State University: Morehead, KY, USA, 2016; pp. 108–113. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, D.-G.; Lee, S.-H.; Kang, K.Y.; Lee, J.J.; Kim, P.J.; Yoon, H.-S.; Kim, J.-S.; Lee, B.-H. Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere 2007, 67, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Ociepa-Kubicka, A.; Ociepa, E. The toxic effects of heavy metals on plants, animals and humans. Inżynieria I Ochr. Środowiska 2012, 15, 169–180. [Google Scholar]
- Możdżeń, K.; Rzepka, A. Rola łupiny nasiennej podczas kiełkowania i wzrostu nasion bobu (Vicia faba L.) w obecności siarczanu ołowiu. Agron. Sci. 2016, 71, 55–65. (In Polish) [Google Scholar]
- Kozlov, M.V. Pollution resistance of mountain birch, Betula pubescens subsp. czerepanovii, near the copper nickel smelter: Natural selection or phenotypic acclimation? Chemosphere 2005, 59, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Fargašová, A. Toxicity comparison of some possible toxic metals (Cd, Cu, Pb, Se, Zn) on young seedlings of Sinapis alba L. Plant Soil Environ. 2004, 50, 33–38. [Google Scholar] [CrossRef]
- Możdżeń, K.; Wanic, T.; Rut, G.; Łaciak, T.; Rzepka, A. Toxic effects of high copper content on physiological processes in Pinus sylvestris L. Photosynthetica 2017, 55, 193–200. [Google Scholar] [CrossRef]
- Siwek, M. Plants in postindustrial sites, contaminated with heavy metals. Part I. Uptake, transport and toxicity of heavy (trace) metals. Wiad. Bot. 2008, 52, 7–22. [Google Scholar]
- Lou, L.-Q.; Schen, Z.-G.; Li, X.-D. The copper tolerance mechanisms of Elsholtzia haichowensis, a plant from copper-enriched soils. Environ. Exp. Bot. 2004, 51, 111–120. [Google Scholar] [CrossRef]
- Pinto, A.P.; Mota, A.M.; de Verennes, A.; Pinto, F.C. Influence of organic matter on the uptake of cadmium, zinc, copper, and iron by sorghum plants. Sci. Total Environ. 2004, 326, 239–247. [Google Scholar] [CrossRef]
- Waditee, R.; Bhuiyan, M.N.H.; Rai, V.; Aoki, K.; Tanaka, Y.; Hibino, T.; Suzuki, S.; Takano, J.; Jagendorf, A.; Takabe, T.; et al. Genes for direct methylation of glycine provide high levels of glycine betaine and abiotic-stress tolerance in Synechococcus and Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.E.; Hasaneen, M.N.A.; Tourky, S.M.N. Plant growth, metabolism and adaptation in relation to stress conditions. XXIV. Salinity-biofertility interactive effects on proline, glycine and various antioxidants in Lactuca sativa. Plant Omics J. 2009, 2, 197–205. [Google Scholar]
- Wani, P.; Khan, M.S.; Zaidi, A. Effect of metal tolerant plant growth promoting Bradyrhizobium sp. (vigna) on growth, symbiosis, seed yield and metal uptake by green gram plants. Chemosphere 2007, 70, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.E.; Tourky, S.M.N.; Elsharkawy, S.E.A. Element content, growth and metabolic changes in Cu- and Cd- stressed Phaseolus vulgaris plants. J. Plant Environ. Res. 2018, 3, 9. [Google Scholar] [CrossRef]
- Zhang, H.; Lian, C.; Shen, Z. Proteomic identification of small, copper-responsive proteins in germinating embryos of Oryza sativa. Ann. Bot. 2009, 103, 923–930. [Google Scholar] [CrossRef]
- Szatanik-Kloc, A. Zmiany właściwości powierzchniowych korzeni wybranych roślin jednoliściennych i dwuliściennych determinowane fitotoksycznością glinu i miedzi. Acta Agrophys. 2010, 176, 135. (In Polish) [Google Scholar]
- Grzyś, E. The Effect of Some Biologically Active Substances on Maize Grown under Stress Conditions; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu ELMA: Wrocław, Poland, 2012; p. 100. ISBN 978-83-7717-085-4. [Google Scholar]
- Sun, B.-Y.; Kan, S.-H.; Zhang, Y.-Z.; Deng, S.-H.; Wu, J.; Yuan, H.; Qi, H.; Yang, G.; Li, L.; Zhang, X.-H.; et al. Certain antioxidant enzymes and lipid peroxidation of radish (Raphanus sativus L.) as early warning biomarkers of soil copper exposure. J. Hazard. Mater. 2010, 183, 833–838. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Panda, P.; Mazumdar, P.; Kumar, D.; Sharma, G.; Sahoo, L.; Sanjib, P. Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol. Biochem. 2012, 53, 33–39. [Google Scholar] [CrossRef]
- Shakya, K.; Chettri, M.K.; Sawidis, T. Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch. Environ. Contam. Toxicol. 2008, 54, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Michňová, J.; Ozdín, D. Primárna hydrotermálna mineralizácia na lokalite Polkanová. Miner. Slovaca 2010, 42, 69–78. (In Slovak) [Google Scholar]
- Točík, A.; Bublová, M. Príspevok k zaniknutej ťažbe medi na Slovensku. Študijné Zvesti Archeol. Ústavu Sav 1985, 21, 47–135. (In Slovak) [Google Scholar]
- Koděra, M. Topografická Mineralógia Slovenska I–III; Veda: Bratislava, Slovakia, 1990; p. 488. ISBN 80-224-0102-1. [Google Scholar]
- Jeleň, S.; Galvánek, J.; Andráš, P.; Bendík, A.; Beláček, B.; Bozalková, I.; Gaál, Ľ.; Gajdoš, A.; Háber, M.; Konečný, V.; et al. Náučno-Poznávací Sprievodca po Geologických a Geografických Lokalitách Stredného Slovenska; Geologický ústav SAV: Banská Bystrica, Slovakia, 2009; p. 320. ISBN 987–80–970413–4–2. [Google Scholar]
- Filipek, J. Projekt klasyfikacji roślin łąkowych i pastwiskowych na podstawie liczby wartości użytkowej. Postępy Nauk Rol. 1973, 4, 59–68. (In Polish) [Google Scholar]
- CABI. Invasive Species Compendium; CAB International: Wallingford, UK, 2020; Available online: www.cabi.org/isc (accessed on 4 November 2020).
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.N.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea. Volume 5: Alismataceae to Orchidaceae (Monocotyledoneae); Cambridge University Press: Cambridge, UK, 1980; 452+xxxviip. [Google Scholar]
- Global Invasive Species Database. Available online: http://www.issg.org/database (accessed on 4 November 2020).
- Otte, M.L.; Jacob, D.L. Mine area remediation. In Ecological Engineering. Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Elsevier: Oxford, UK, 2008; Volume 3, pp. 2397–2402. [Google Scholar]
- Hubbard, C.E. Grasses: A Guide to Their Structure, Identification, Uses, and Distribution in the British Isles, 3rd ed.; Penguin Books Ltd.: Harmondsworth, UK, 1984; 476p. [Google Scholar]
- TeKrony, D.M. Seed Vigour Testing; Handbook on Seed Testing, Contribution No. 32; Association of Official Seed Analysts: Ithaca, NY, USA, 1983. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Afzal, I.; Khaliq, A. Optimization of hydropriming techniques for rice seed invigoration. Seed Sci. Technol. 2006, 34, 507–512. [Google Scholar] [CrossRef]
- Islam, A.K.M.A.; Anuar, N.; Yaakob, Z. Effect of genotypes and pre-sowing treatments on seed germination behavior of Jatropha. Asian J. Plant Sci. 2009, 8, 433–439. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Kato-Noguchi, H. Allelopathic potentiality of medicinal plant Leucas aspera. Int. J. Agric. Sustain. 2012, 4, 1–7. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).