Comparison of Morphological Characteristics and Determination of Different Patterns for Rubber Particles in Dandelion and Different Rubber Grass Varieties
Abstract
1. Introduction
2. Results
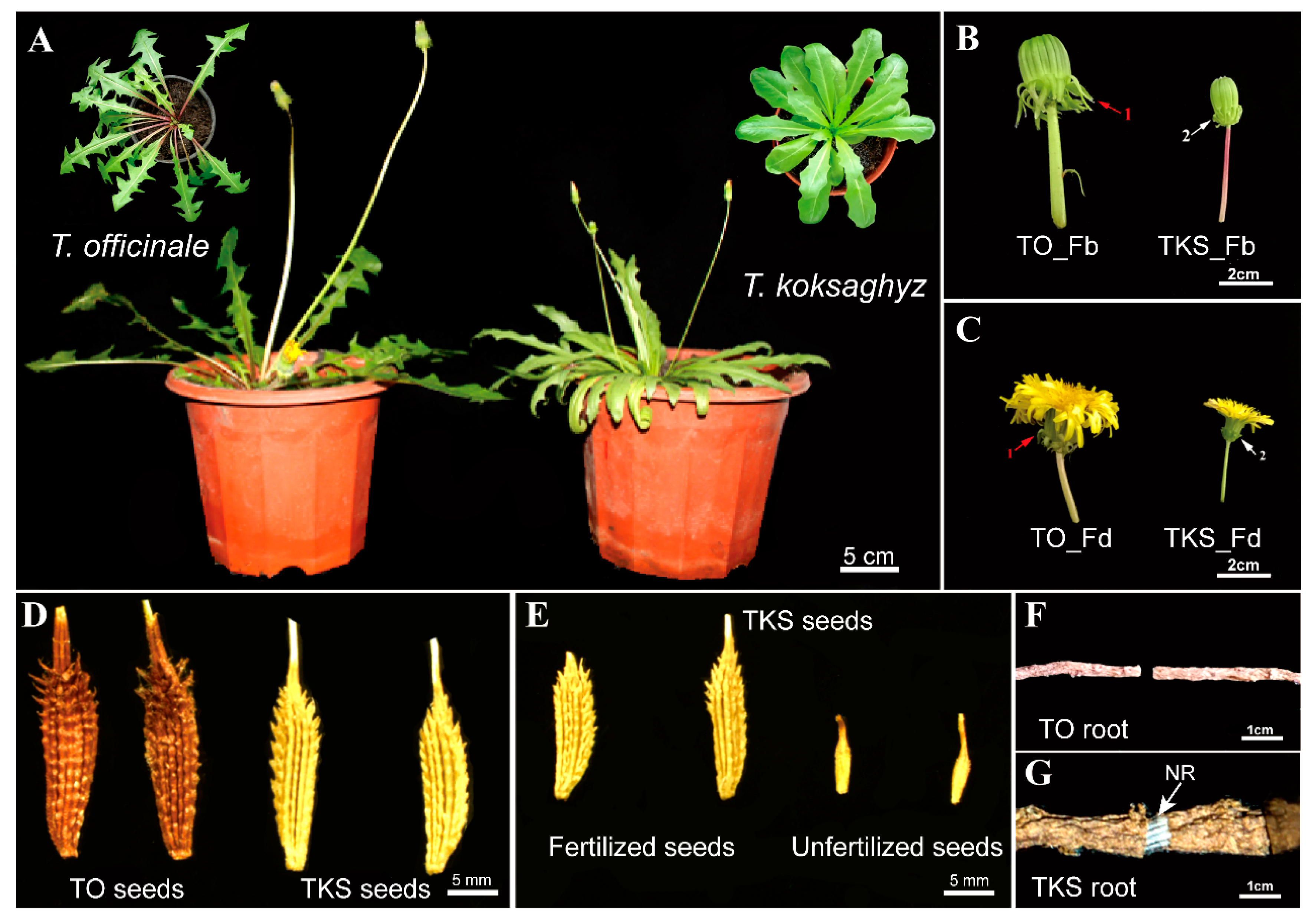
2.1. Comparison of Morphological Characteristics of Common Dandelion, T. officinale, and Russian Dandelion, T. koksaghyz
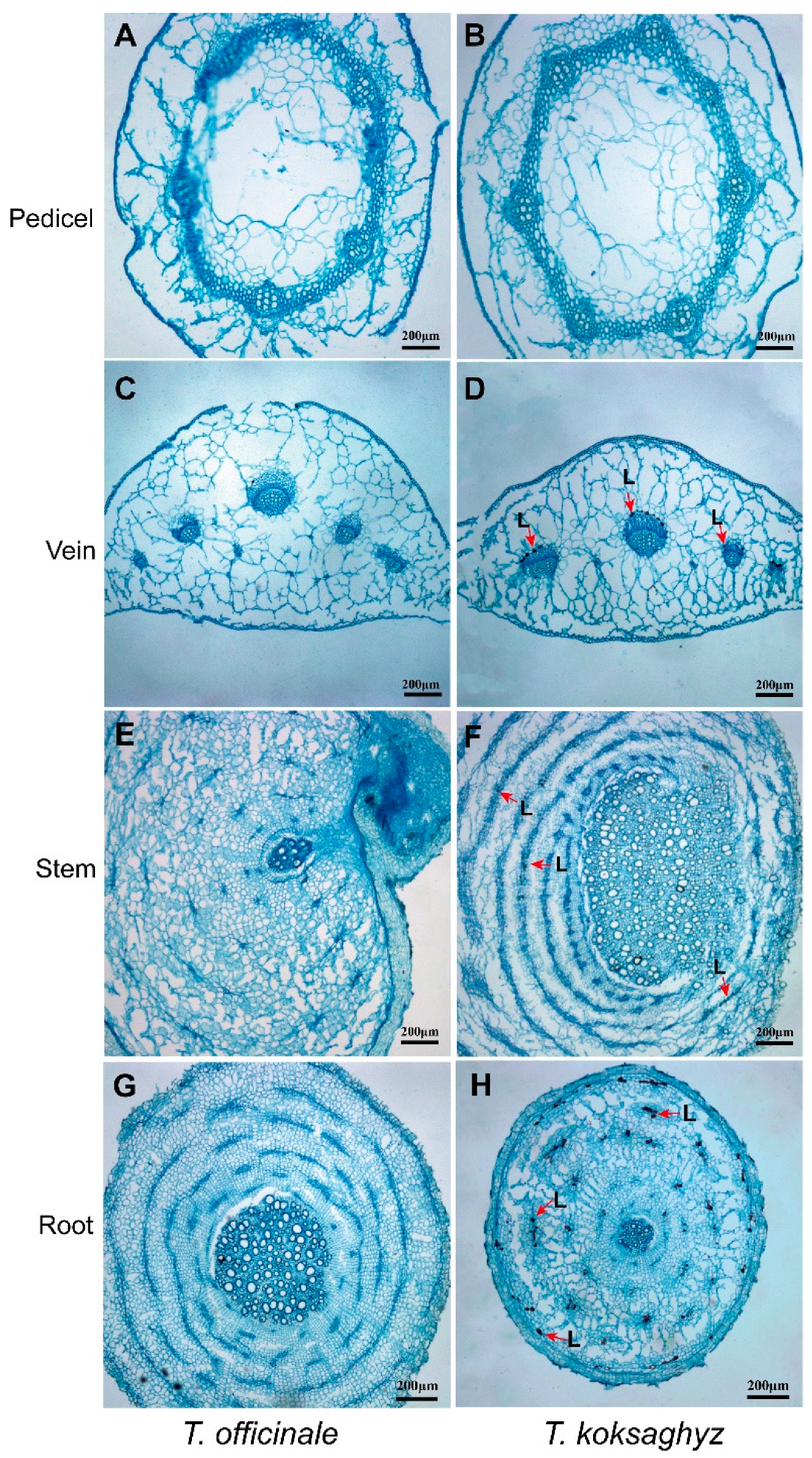
2.2. Determination and Statistical Analysis of Laticifer Cells in Different Tissues of T. koksaghyz
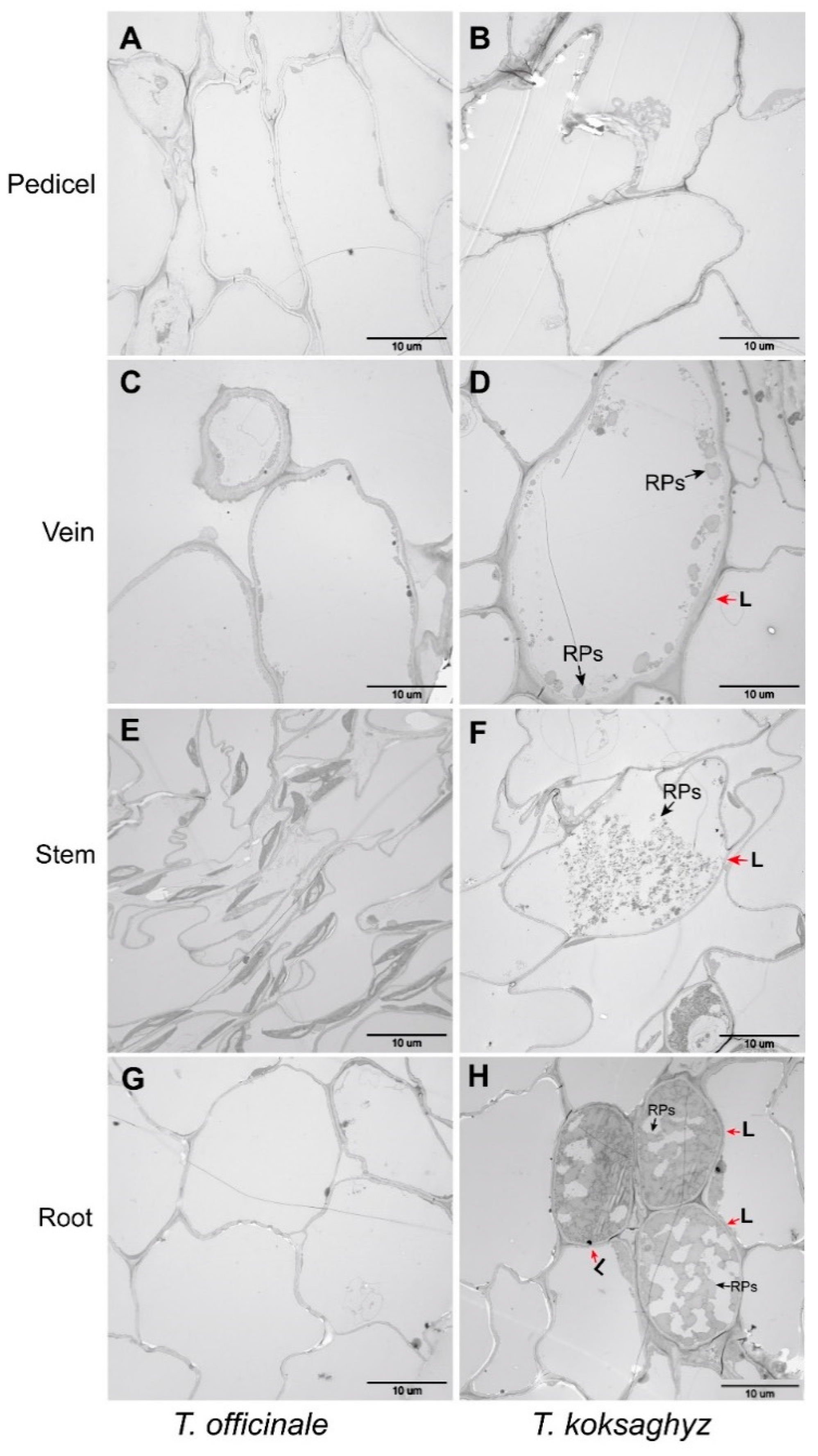
2.3. Morphological Analysis of Laticifer Cells and Rubber Particles in the Nine-Month-Old Roots of T. kok-saghyz from Different Varieties and the Rubber Tree H. brasiliensis
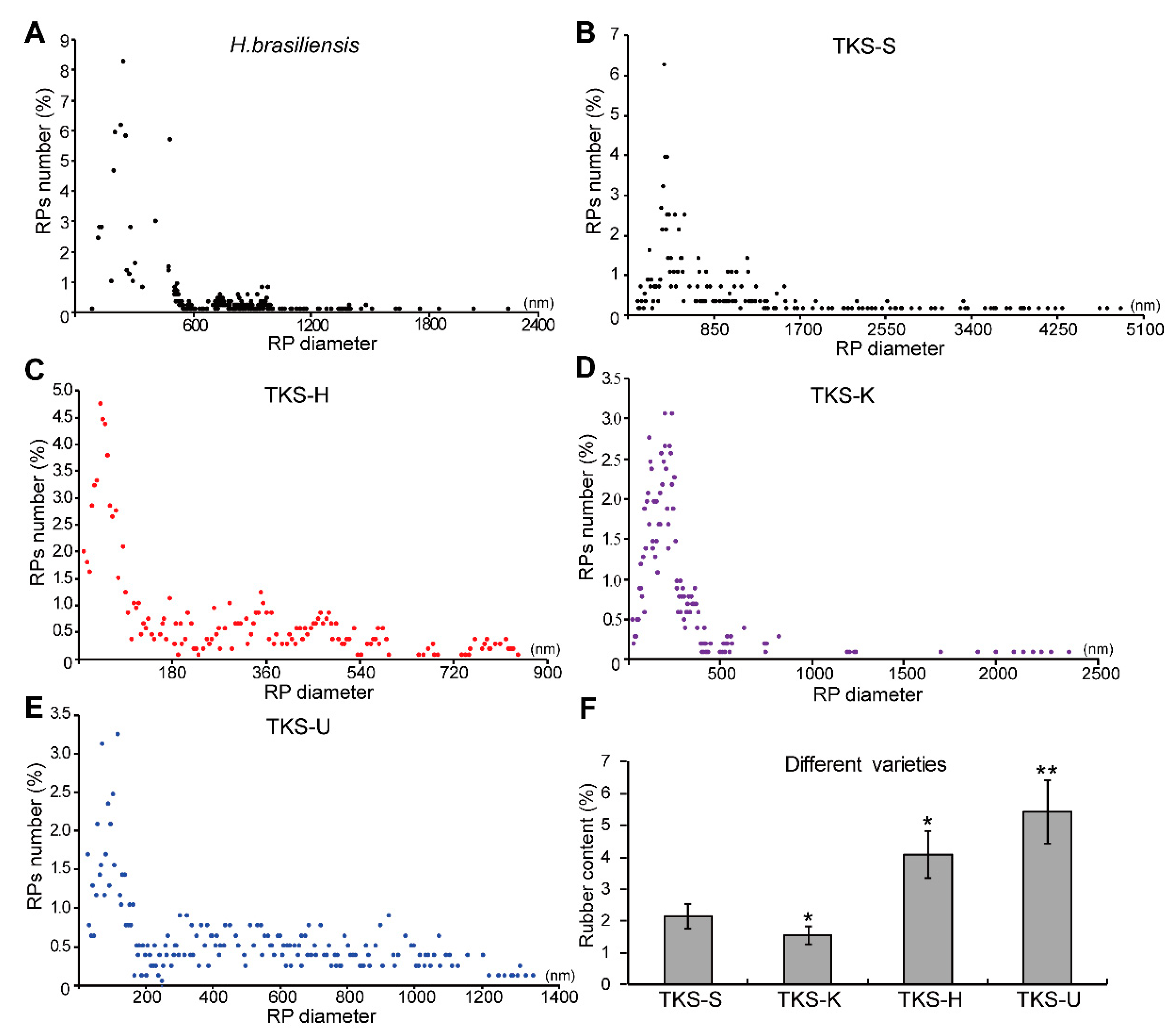
2.4. Comparison of the Accumulation Pattern of Different Rubber Particles and the Natural Rubber Content in Different Rubber-Producing Plants
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Morphological Observation of Laticifer Cells in Different Tissues
4.3. Morphological Analysis of Large and Small Rubber Particles
4.4. Determination of Natural Rubber Content
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schuetz, K.; Carle, R.; Schieber, A. Taraxacum-A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Roberts, T.H.; Matthews, K.R.; Bezerra, C.F.; Morais-Braga, M.F.; Coutinho, H.D.; Sharopov, F.; Salehi, B.; Yousaf, Z.; Sharifi-Rad, M.; et al. Ethnobotany of the genus Taraxacum-Phytochemicals and antimicrobial activity. Phytother. Res. 2018, 32, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Xu, X.; Ruan, J.; Liu, S.Z.; Wu, S.G.; Shao, X.J.; Wang, X.B.; Gan, L.; Qin, B.; Yang, Y.S.; et al. Genome analysis of Taraxacum kok-saghyz Rodin provides new insights into rubber biosynthesis. Natl. Sci. Rev. 2018, 5, 78–87. [Google Scholar] [CrossRef]
- Arias, M.; Herrero, J.; Ricobaraza, M.; Hernandez, M.; Ritter, E. Evaluation of root biomass, rubber and inulin contents in nine Taraxacum koksaghyz Rodin populations. Ind. Crops Prod. 2016, 83, 316–321. [Google Scholar] [CrossRef]
- Krotkov, G. A review of literature on Taraxacum Koksaghyz. Rod. Bot Rev. 1945, 11, 417–461. [Google Scholar] [CrossRef]
- Cornish, K. Alternative natural rubber crops: Why should we care? Technol. Innov. 2017, 18, 245–256. [Google Scholar] [CrossRef]
- Yamashita, S.; Takahashi, S. Molecular mechanisms of natural rubber biosynthesis. Annu. Rev. Biochem. 2020, 14, 13. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Q.L.; Sun, Y.; Tong, Z.; Chang, L.L.; Yu, L.; Zhang, X.; Yuan, B.; He, P.; Jin, X.; et al. Proteomic landscape has revealed small rubber particles are crucial rubber biosynthetic machines for ethylene-stimulation in natural rubber production. Int. J. Mol. Sci. 2019, 20, 5082. [Google Scholar] [CrossRef]
- Esau, K. Plant Anatomy, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1965; pp. 308–335. [Google Scholar]
- Schmidt, T.; Lenders, M.; Hillebrand, A.; van Deenen, N.; Munt, O.; Reichelt, R.; Eisenreich, W.; Fischer, R.; Prüfer, D.; Gronover, C.S. Characterization of rubber particles and rubber chain elongation in Taraxacum koksaghyz. BMC Biochem. 2010, 11, 11. [Google Scholar] [CrossRef]
- Cornish, K.; Kopicky, S.L.; Mcnulty, S.K.; Amstutz, N.; Chanon, A.M.; Walker, S.; Kleinhen, M.D.; Miller, A.R.; Streeter, J.G. Temporal diversity of Taraxacum kok-saghyz plants reveals high rubber yield phenotypes. Biodiversitas 2016, 17, 847–856. [Google Scholar] [CrossRef]
- Xie, Q.L.; Ding, G.H.; Zhu, L.P.; Yu, L.; Yuan, B.X.; Gao, X.; Wang, D.; Sun, Y.; Liu, Y.; Li, H.; et al. Proteomic landscape of the mature roots in a rubber-producing grass Taraxacum kok-saghyz. Int. J. Mol. Sci. 2019, 20, 2596. [Google Scholar] [CrossRef] [PubMed]
- Yeang, H.Y.; Arif, S.M.; Yusof, F.; Sunderasan, E. Allergenic proteins of natural rubber latex. Methods 2002, 27, 32–45. [Google Scholar] [CrossRef]
- Ramirez-Cadavid, D.A.; Valles-Ramirez, S.; Cornish, K.; Michel, F.C. Simultaneous quantification of rubber, inulin, and resins in Taraxacum kok-saghyz roots by sequential solvent extraction. Plant Biotechnol. J. 2018, 122, 647–656. [Google Scholar] [CrossRef]
- Tang, C.R.; Yang, M.; Fang, Y.J.; Luo, Y.F.; Gao, S.H.; Xiao, X.H.; An, Z.; Zhou, B.; Zhang, B.; Tan, X.; et al. The rubber tree genome reveals new insights into rubber production and species adaptation. Nat. Plants 2016, 2, 16073. [Google Scholar] [CrossRef]
- Liu, J.; Shi, C.; Shi, C.C.; Li, W.; Zhang, Q.J.; Zhang, Y.; Li, K.; Lu, H.F.; Shi, C.; Zhu, S.T.; et al. The chromosome-based rubber tree genome provides new insights into spurge genome evolution and rubber biosynthesis. Mol. Plant 2020, 13, 336–350. [Google Scholar] [CrossRef]
- Mooibroek, H.; Cornish, K. Alternative sources of natural rubber. Appl. Microbiol. Biotechnol. 2000, 53, 355–365. [Google Scholar] [CrossRef]
- Van Beilen, J.B.; Poirier, Y. Establishment of new crops for the production of natural rubber. Trends Biotechnol. 2007, 25, 522–529. [Google Scholar] [CrossRef]
- Kirschner, J.; Stepanek, J.; Cerny, T.; Heer, P.; Dijk, P.J. Available ex situ germplasm of the potential rubber crop Taraxacum kok-saghyz belongs to a poor rubber producer, T. brevicorniculatum (Compositae–Crepidinae). Genet. Resour. Crop Evol. 2013, 2, 455–471. [Google Scholar] [CrossRef]
- Kreuzberger, M.; Hahn, T.; Zibek, S.; Schiemann, J.; Thiele, K. Seasonal pattern of biomass and rubber and inulin of wild Russian dandelion (Taraxacum kok-saghyz L. Rodin) under experimental field conditions. Eur. J. Agron. 2016, 80, 66–77. [Google Scholar] [CrossRef]
- Stolze, A.; Wanke, A.; van Deenen, N.; Geyer, R.; Prüfer, D.; Gronover, C.S. Development of rubber-enriched dandelion varieties by metabolic engineering of the inulin pathway. Plant Biotechnol. J. 2017, 6, 740–753. [Google Scholar] [CrossRef]
- Hagel, J.M.; Yeung, E.C.; Facchini, P.J. Got milk? The secret life of laticifers. Trends Plant Sci. 2008, 13, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.F.; Cornish, K. Microstructure of purified rubber particles. Int. J. Plant Sci. 2000, 161, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Wi, S.G.; Chung, G.C.; Kim, Y.S.; Kang, H. The micromorphology and protein characterization of rubber particles in Ficus carica, Ficus benghalensis and Hevea brasiliensis. J. Exp. Bot. 2003, 54, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Ulmann, M. Wertvolle Kautschukpflanzen des Gemäßigten Klimas Dargestellt Aufgrund Sowjetischer Forschungsarbeiten; Akademie-Verlag GmbH: Berlin, Germany, 1951. [Google Scholar]
- Van Dijk, P.; Kirschner, J.; Stepanek, J.; Omarovisch, I.; Cerny, T. Taraxacum koksaghyz Rodin definitely is not an example of over-collecting in the past. J. Appl. Bot. Food Qual. 2010, 83, 217–219. [Google Scholar]
- Warmke, H.E. Macrosporogenesis, fertilization, and early embryology of Taraxacum kok-saghyz. Bull. Torrey Bot. Club. 1943, 70, 164–173. [Google Scholar] [CrossRef]
- Abbe, L.B. A rapid histological technic for staining latex in roots of Taraxacum Kok-Saghyz. Stain Technol. 1946, 21, 19–22. [Google Scholar] [CrossRef]
- Hao, B.Z.; Wu, J.L. Laticifer differentiation in Hevea brasiliensis induction by exogenous jasmonic acid and linolenic acid. Ann. Bot. 2000, 85, 37–43. [Google Scholar] [CrossRef]
- Cherian, S.; Ryu, S.B.; Cornish, K. Natural rubber biosynthesis in plants, the rubber transferase complex, and metabolic engineering progress and prospects. Plant Biotechnol. J. 2019, 17, 2041–2061. [Google Scholar] [CrossRef]
- Wang, X.C.; Wang, D.; Sun, Y.; Yang, Q.; Chang, L.L.; Wang, L.M.; Meng, X.; Huang, Q.; Jin, X.; Tong, Z. Comprehensive proteomics analysis of laticifer latex reveals new insights into ethylene stimulation of natural rubber production. Sci. Rep. 2015, 5, 13778. [Google Scholar] [CrossRef]
- Yamashita, S.; Yamaguchi, H.; Waki, T.; Aoki, Y.; Mizuno, M.; Yanbe, F.; Ishii, T.; Funaki, A.; Tozawa, Y.; Miyagi-Inoue, Y.; et al. Identification and reconstitution of the rubber biosynthetic machinery on rubber particles from Hevea Brasiliensis. eLife 2016, 5, e19022. [Google Scholar] [CrossRef]
- Puskas, J.E.; Gautriaud, E.; Deffieux, A.; Kennedy, J.P. Natural rubber biosynthesis-A living carbocationic polymerization? Prog. Polym. Sci. 2006, 31, 533–548. [Google Scholar] [CrossRef]
- Kirby, J.; Keasling, J.D. Biosynthesis of plant isoprenoids perspectives for microbial engineering. Annu. Rev. Plant Biol. 2009, 60, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, M.A.; Meulia, T.; Cornish, K. Laticifer and Rubber Particle Ontogeny in Taraxacum kok-saghyz (Rubber Dandelion) Roots. Microsc. Microanal. 2016, 22, 1034–1035. [Google Scholar] [CrossRef]
- McAssey, E.V.; Gudger, E.G.; Zuellig, M.P.; Burke, J.M. Population genetics of the rubber-producing Russian dandelion (Taraxacum kok-saghyz). PLoS ONE 2016, 11, e0146417. [Google Scholar] [CrossRef] [PubMed]
- Hodgson-Kratky, K.M.; Wolyn, D.J. Cytoplasmic male sterility in Russian dandelion. J. Am. Soc. Hort. Sci. 2015, 140, 580–586. [Google Scholar] [CrossRef]
- Wang, X.C.; Shi, M.J.; Wang, D.; Chen, Y.Y.; Cai, F.G.; Zhang, S.X.; Wang, L.; Tong, Z.; Tian, W.M. Comparative proteomics of primary and secondary lutoids reveals that chitinase and glucanase play a crucial combined role in rubber particle aggregation in Hevea Bras. J. Proteome Res. 2013, 12, 5146–5159. [Google Scholar] [CrossRef]
- Yu, L.; Yuan, B.X.; Wang, L.L.; Sun, Y.; Ding, G.H.; Souleymane, O.A.; Zhang, X.Y.; Xie, Q.L.; Wang, X.C. Identification and characterization of glycoproteins and their responsive patterns upon ethylene stimulation in the rubber latex. Int. J. Mol. Sci. 2020, 21, 5282. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, T.; Xiang, T.; Dong, Y.Y.; Zhang, J.C.; Zhang, L.Q. Quantitation of isoprenoids for natural rubber biosynthesis in natural rubber latex by liquid chromatography with tandem mass spectrometry. J. Chromatogr. A 2018, 1558, 115–119. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Ding, G.; Ma, J.; Wang, L.; Yu, L.; Ruan, X.; Zhang, X.; Zhang, W.; Wang, X.; Xie, Q. Comparison of Morphological Characteristics and Determination of Different Patterns for Rubber Particles in Dandelion and Different Rubber Grass Varieties. Plants 2020, 9, 1561. https://doi.org/10.3390/plants9111561
Yuan B, Ding G, Ma J, Wang L, Yu L, Ruan X, Zhang X, Zhang W, Wang X, Xie Q. Comparison of Morphological Characteristics and Determination of Different Patterns for Rubber Particles in Dandelion and Different Rubber Grass Varieties. Plants. 2020; 9(11):1561. https://doi.org/10.3390/plants9111561
Chicago/Turabian StyleYuan, Boxuan, Guohua Ding, Junjun Ma, Lingling Wang, Li Yu, Xueyu Ruan, Xueyan Zhang, Wangfeng Zhang, Xuchu Wang, and Quanliang Xie. 2020. "Comparison of Morphological Characteristics and Determination of Different Patterns for Rubber Particles in Dandelion and Different Rubber Grass Varieties" Plants 9, no. 11: 1561. https://doi.org/10.3390/plants9111561
APA StyleYuan, B., Ding, G., Ma, J., Wang, L., Yu, L., Ruan, X., Zhang, X., Zhang, W., Wang, X., & Xie, Q. (2020). Comparison of Morphological Characteristics and Determination of Different Patterns for Rubber Particles in Dandelion and Different Rubber Grass Varieties. Plants, 9(11), 1561. https://doi.org/10.3390/plants9111561






