Exploratory Study of Fatty Acid Profile in Two Filmy Ferns with Contrasting Desiccation Tolerance Reveal the Production of Very Long Chain Polyunsaturated Omega-3 Fatty Acids
Abstract
1. Introduction
2. Results
2.1. Water and Lipid Content
2.2. Fatty Acids Fractions (SFA, MUFA, PUFA)
2.3. Fatty Acids Profile
2.4. Membrane Integrity
3. Discussion
4. Materials and Methods
4.1. Plant Material and Maintenance Conditions
4.2. Treatments and Sample Collection
4.3. Leaf Lipid Extraction
4.4. Fatty Acid Analysis
4.5. Membrane Integrity Evaluation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant. Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nelson, D.E.; Jensenayb, R.G. Adaptations to Environmental Stresses. Plant. Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- De Paula, F.; Thi, A.; De Silva, J.; Justin, A.; Demandre, C.; Mazliak, P. Effects of water stress on the molecular species composition of polar lipids from Vigna unguiculata L. leaves. Plant Sci. 1990, 66, 185–193. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant. Cell 1995, 7, 957–970. [Google Scholar] [CrossRef]
- Gigon, A.; Matos, A.-R.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A.-T. Effect of Drought Stress on Lipid Metabolism in the Leaves of Arabidopsis thaliana (Ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef]
- Pham-Thi, A.T.; Borrel-Flood, C.; Vieira da Silva, J.; Justin, A.M.; Mazliak, P. Effects of water stress on lipid metabolism in cotton leaves. Phytochemistry 1985, 24, 723–727. [Google Scholar] [CrossRef]
- Monteiro de Paula, F.; Pham-Thi, A.T.; Zuily-Fodil, Y.; Ferrari-Iliou, R.; Vieira da Silva, J.; Mazliak, P. Effect of water stress on the biosynthesis and degradation of polyunsaturated lipid molecular species in leaves of Vigna unguiculata. Plant. Physiol. Biochem. 1993, 31, 707–715. [Google Scholar]
- Pham-Thi, A.T.; Borrel-Flood, C.; Vieira da Silva, J.; Justin, A.M.; Mazliak, P. Effects of drought on [1-14C]-oleic and [1-14C]-linoleic acid desaturation in cotton leaves. Physiol. Plant. 1987, 69, 147–150. [Google Scholar] [CrossRef]
- Toldi, O.; Tuba, Z.; Scott, P. Vegetative desiccation tolerance: Is it a goldmine for bioengineering crops? Plant. Sci. 2009, 176, 187–199. [Google Scholar] [CrossRef]
- Gaff, D.F. Mechanisms of desiccation tolerance in resurrection vascular plants. In Mechanisms of Environmental Stress Resistance in Plants; Basra, A.S., Basra, R.K., Eds.; Harwood Academic Publishers: London, UK, 1997; pp. 43–58. [Google Scholar]
- Iwatsuki, K. Hymenophyllaceae. In Pteridophytes and Gymnosperms. The Families and Genera of Vascular Plants; Kramer, K.U., Green, P.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; Volume 1, pp. 157–163. [Google Scholar] [CrossRef]
- Saldaña, A.; Parra, M.J.; Flores-Bavestrello, A.; Corcuera, L.J.; Bravo, L.A. Effects of forest successional status on microenvironmental conditions, diversity and distribution of filmy fern species in a temperate rainforest. Plant Species Biol. 2014, 29, 253–262. [Google Scholar] [CrossRef]
- Proctor, M.C.F. Comparative ecophysiological measurements on the light responses, water relations and desiccation tolerance of the filmy ferns Hymenophyllum wilsonii Hook. and H. tunbrigense (L.) Smith. Ann. Bot. 2003, 91, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.W.; Evans, G.B. Biological Flora of the British Isles. Hymenophyllum. J. Ecol. 1972, 60, 245–268. [Google Scholar] [CrossRef]
- Ebihara, A.; Dubuissin, J.Y.; Iwatsuki, K.; Hennequin, S.; Ito, M. A taxonomic revision of Hymenophyllaceae. Blumea-Biodivers. Evol. Biogeogr. Plants 2006, 51, 221–280. [Google Scholar] [CrossRef]
- Parra, M.J.; Acuña, K.; Corcuera, L.J.; Saldaña, A. Vertical distribution of Hymenophyllaceae species among host tree microhabitats in a temperate rain forest in Southern Chile. J. Veg. Sci. 2009, 20, 588–595. [Google Scholar] [CrossRef]
- Shreve, F. Studies on Jamaican Hymenophyllaceae. Bot. Gaz. 1911, 51, 184–209. [Google Scholar] [CrossRef]
- Gasulla, F.; Vom Dorp, K.; Dombrink, I.; Zahringer, U.; Gisch, N.; Dörmann, P.; Bartels, D. The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: A comparative approach. Plant J. 2013, 75, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, B.; Pham-Thi, A.T.; Monteiro de Paula, F.; Vieira da Silva, J. Effect of dehydration and rehydration on the polar lipid and fatty acid composition of Ramonda species. Can. J. Bot. 1992, 70, 107–113. [Google Scholar] [CrossRef]
- Quartacci, M.F.; Forli, M.; Rascio, N.; Dalla Vecchia, F.; Bochicchio, A.; Navari-Izzo, F. Desiccation-tolerant Sporobolus stapfianus: Lipid composition and cellular ultrastructure during dehydration and rehydration. J. Exp. Bot. 1997, 48, 1269–1279. [Google Scholar] [CrossRef]
- Swanson, E.S.; Anderson, N.H.; Gellerman, J.L.; Schlenk, H. Ultrastructure and lipid composition of mosses. Bryologist 1976, 79, 339–349. [Google Scholar] [CrossRef]
- Tshabuse, F.; Farrant, J.M.; Humbert, L.; Moura, D.; Rainteau, D.; Espinasse, C.; Idrissi, A.; Merlier, F.; Acket, S.; Rafudeen, M.S.; et al. Glycerolipid analysis during desiccation and recovery of the resurrection plant Xerophyta humilis (Back) Dur and Schinz. Plant Cell Environ. 2017, 41, 533–547. [Google Scholar] [CrossRef]
- Jones, K.H.; Senft, J.A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J. Histochem. Cytochem. 1985, 33, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Krömer, T.; Kessler, M. Filmy ferns (Hymenophyllaceae) as high-canopy epiphytes. Ecotropica 2006, 12, 57–63. [Google Scholar]
- Rachmilevitch, S.; DaCosta, M.; Huang, B. Physiological and biochemical indicators for stress tolerance. In Plant-Environment Interactions; Huang, B., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 321–356. [Google Scholar]
- Tuba, Z.; Smirnoff, N.; Csintalan, Z.S.; Szente, K.; Nagy, Z. Respiration during slow desiccation of the poikilochlorophyllous desiccation tolerant plant Xerophyta scabrida at present-day CO2 concentration. Plant Physiol. Biochem. 1997, 35, 381–386. [Google Scholar]
- Sherwin, H.W.; Farrant, J.L. Differences in rehydration of three desiccation-tolerant angiosperm species. Anna. Bot. 1996, 78, 703–710. [Google Scholar] [CrossRef]
- Yobi, A.; Wone, B.W.M.; Xu, W.; Alexander, D.C.; Gou, L.; Ryals, J.A.; Oliver, M.J.; Cushman, J.C. Comparative metabolic profiling between desiccation-sensitive and desiccation-tolerant species of Selaginella reveals insights into the resurrection trait. Plant J. 2012, 72, 983–999. [Google Scholar] [CrossRef]
- Liu, X.; Ma, D.; Zhang, Z.; Wang, S.; Du, S.; Deng, X. Plant lipid remodeling in response to abiotic stresses. Environ. Exp. Bot. 2019, 165, 174–184. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Thrower, N.; Mhaske, V.; Stymne, S.; Baxter, M.; Yang, W.; Liu, J.; Shaw, K.; Shorrosh, B.; Zhang, M.; et al. PlantFAdb: A resource for exploring hundreds of plant fatty acid structures synthesized by thousands of plants and their phylogenetic relationships. Plant J. 2018, 96, 1299–1308. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; Debono, A.; Durrett, T.P.; et al. Acyl-Lipid Metabolism. Arab. Book 2013, 11, e0161. [Google Scholar] [CrossRef]
- Miquel, M.; Browse, J. Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. Biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J. Biol. Chem. 1992, 267, 1502–1509. [Google Scholar]
- Napier, J.A. The production of unusual fatty acids in transgenic plants. Annu. Rev. Plant Biol. 2007, 58, 295–319. [Google Scholar] [CrossRef]
- Bach, L.; Faure, J.D. Role of very-long-chain fatty acids in plant development, when chain length does matter. Comptes Rendus Biol. 2010, 333, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.A.; Velankar, D.A.; Chaugule, B.B. Fatty acid composition of the cold-water-inhabiting freshwater red alga Sirodotia Kylin. J. Appl. Phycol. 2009, 21, 99–102. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2008, 21, 75–80. [Google Scholar] [CrossRef]
- Jamieson, G.R.; Reid, E.H. The fatty acid composition of fern lipids. Phytochemistry 1975, 14, 2229–2232. [Google Scholar] [CrossRef]
- Zank, T.K.; Zähringer, U.; Beckmann, C.; Pohnert, G.; Boland, W.; Holtorf, H.; Reski, R.; Lerchl, J.; Heinz, E. Cloning and functional characterization of and enzyme involved in the elongation of ∆6-polyunsaturated fatty acids from the moss Physcomitrella patens. Plant J. 2002, 31, 255–268. [Google Scholar] [CrossRef]
- Hui, S.W.; Mason, J.T.; Huang, C. Acyl chain interdigitation in saturated mixed-chain phosphatidylcholine bilayer dispersions. Biochemistry 1984, 23, 5570–5577. [Google Scholar] [CrossRef]
- Scheneiter, R.; Hitomi, M.; Ivessa, A.S.; Fasch, E.V.; Kohlwein, S.D.; Tartakoff, A.M. A yeast acetylcoenzyme A carboxylase mutant links very-long-chain fatty acid synthesis to the structure and function of the nuclear membrane-pore complex. Mol. Cell Biol. 1996, 16, 7161–7172. [Google Scholar] [CrossRef]
- Navari-Izzo, F.; Quartacci, M.F.; Pinzino, C.; Rascio, N.; Vazzana, C.; Sgherri, C.L.M. Protein dynamics in thylakoids of the desiccation-tolerant plant Boea hygroscopica during dehydration and rehydration. Plant Physiol. 2000, 124, 1427–1436. [Google Scholar] [CrossRef]
- Wu, J.; Seliskar, D.M.; Gallagher, J.L. Stress tolerance in the marsh plant Spartina patens: Impact of NaCl on growth and root plasma membrane lipid composition. Physiol. Plant. 1998, 102, 307–317. [Google Scholar] [CrossRef]
- Quartacci, M.F.; Glisic, O.; Stevanovic, B.; Navari-Izzo, F. Plasma membrane lipids in the resurrection plant Ramonda serbica following dehydration and rehydration. J. Exp. Bot. 2002, 53, 2159–2166. [Google Scholar] [CrossRef]
- Li, A.; Wang, D.; Yu, B.; Yu, X.; Li, W. Maintenance or collapse: Responses of extraplastidic membrane lipid composition to desiccation in the resurrection plant Paraisometrum mileense. PLoS ONE 2014, 9, e103430. [Google Scholar] [CrossRef] [PubMed]
- Cyril, J.; Powell, G.L.; Duncan, R.R.; Waird, W.V. Changes in membrane polar lipid fatty acids of Seashore paspalum in response to low temperature exposure. Crop. Sci. 2002, 42, 2031–2037. [Google Scholar] [CrossRef]
- Platt, K.A.; Oliver, M.J.; Thomson, W.W. Membranes and organelles of dehydrated Selaginella and Tortula retain their normal configuration and structural integrity. Protoplasma 1994, 178, 57–65. [Google Scholar] [CrossRef]
- Silva-Artur, M.A.; Rienstra, J.; Dennis, T.J.; Farrant, J.M.; Ligterink, W.; Hilhorst, H. Structural plasticity of intrinsically disordered LEA proteins from Xerophyta schlechteri provides protection in vitro and in vivo. Front. Plant Sci. 2019, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Alves-Vieira, E.; da Cruz-Centeno, D.; Freschi, L.; Alves da Silva, E.; Regina-Braga, M. The dual strategy of the bromeliad Pitcairnia burchelli Mez to cope with desiccation. Environ. Exp. Bot. 2017, 143, 135–148. [Google Scholar] [CrossRef]
- Burja, A.M.; Armenta, R.E.; Radianingtyas, H.; Barrow, C.J. Evaluation of fatty acid extraction methods for Thraustochytrium sp. ONC-T18. J. Agric. Food Chem. 2007, 55, 4795–4801. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO 15304 Animal and Vegetable Fats and Oils—Determination of the Content of Trans Fatty Acid Isomers of Vegetable Fats and Oils—Gas Chromatographic Method; International Organization for Standardization: Geneva, Switzerland, 2002; p. 20. [Google Scholar]
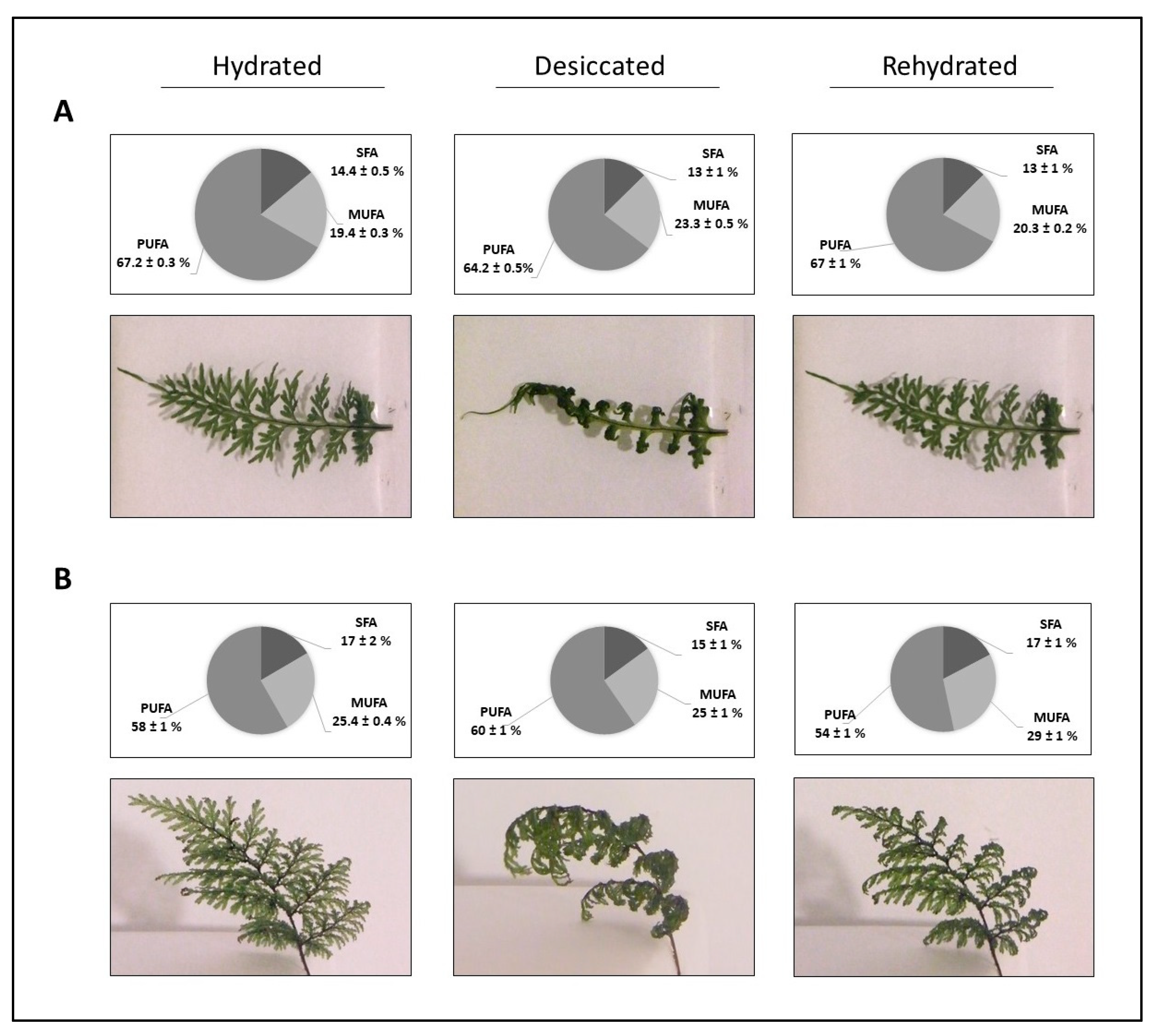
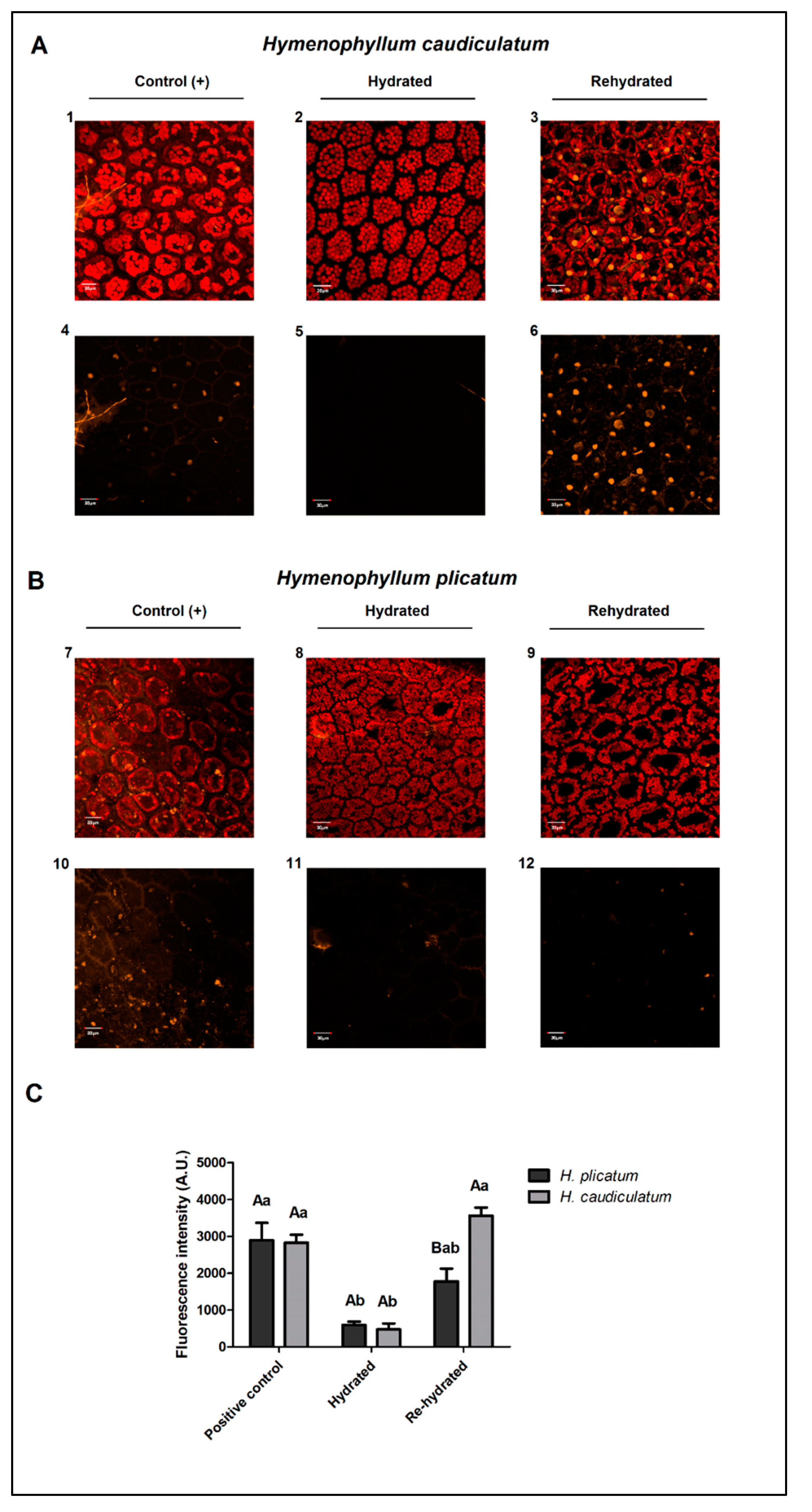
| H. caudiculatum | H. plicatum | |||||
|---|---|---|---|---|---|---|
| Hydrated | Desiccated | Rehydrated | Hydrated | Desiccated | Rehydrated | |
| RWC (%) | 85 ± 1 Aa | 13 ± 1 Ac | 56 ± 5 Bb | 88 ± 1 Aa | 14 ± 2 Ac | 75 ± 2 Ab |
| Lipid Content (mg g−1 Dry Weight) | 36 ± 2 Aa | 29 ± 2 Aab | 20 ± 3 Ab | 29 ± 2 Aa | 30 ± 4 Aa | 26 ± 1 Aa |
| Fatty Acid | H. caudiculatum | H. plicatum | ||||
|---|---|---|---|---|---|---|
| Hydrated | Desiccated | Rehydrated | Hydrated | Desiccated | Rehydrated | |
| C4:0 | 25 ± 3 | 18 ± 2 | 14 ± 2 | 16 ± 3 | 19 ± 2 | 6 ± 1 |
| C6:0 | 8 ± 1 | 9 ± 1 | 7 ± 1 | 7 ± 1 | 2.4 ± 0.2 | 4.6 ± 0.4 |
| C8:0 | 2.7 ± 0.2 | 4.6 ± 0.1 | 4.1 ± 0.1 | 3.3 ± 0.5 | 2.3 ± 0.2 | 2.6 ± 0.2 |
| C11:0 | 5.8 ± 0.5 | 5.5 ± 0.4 | 3.3 ± 0.4 | 3.3 ± 0.2 | n.d. | 2.5 ± 0.2 |
| C14:0 | n.d. | n.d. | n.d. | 4 ± 2 | n.d. | 2.0 ± 0.2 |
| C15:1 | 64 ± 2 | 72 ± 3 | 55 ± 8 | 77 ± 6 | 38 ± 1 | 44 ± 2 |
| C16:0 | 3 ± 1 | 3 ± 1 | 1.4 ± 0.4 | 7± 3 | n.d. | 5 ± 1 |
| C17:1 | 9.4 ± 0.4 | 24 ± 1 | 11 ± 1 | 14 ± 1 | 8 ± 1 | 8.1 ± 0.5 |
| C18:0 | 25 ± 3 | 20 ± 1 | 17 ± 2 | 42 ± 4 | 30 ± 2 | 33 ± 1 |
| C18:1n9c | 47 ± 1 | 48 ± 2 | 39 ± 7 | 57 ± 5 | 64 ± 1 | 45 ± 3 |
| C18:2n6c | n.d. | n.d. | n.d. | 12 ± 1 | 10 ± 1 | 12 ± 2 |
| C18:3n6 | 171 ± 2 | 150 ± 4 | 128 ± 26 | 124 ± 15 | 100 ± 2 | 64 ± 6 |
| C21:0 | 10 ± 1 | 13 ± 2 | 10 ± 2 | 13 ± 1 | 18 ± 3 | 9 ± 1 |
| C20:3n3 | 218 ± 2 | 234 ± 3 | 201 ± 33 | 141 ± 11 | 174 ± 4 | 109 ± 6 |
| C20:3n6 | 18.0 ± 0.3 | 16 ± 1 | 11± 2 | 27 ± 3 | 21 ± 1 | 11 ± 1 |
| C22:1n9 | n.d. | n.d. | n.d. | n.d. | 13 ± 1 | 10 ± 1 |
| C22:6n3 | 9 ± 2 | 11 ± 1 | 10 ± 2 | 41 ± 2 | 8 ± 1 | 5.7 ± 0.3 |
| C24:0 | 5.6 ± 0.2 | 6.9 ± 0.1 | 6 ± 1 | n.d. | 2 ± 1 | 1.1 ± 0.1 |
| C24:1n9 | n.d. | n.d. | n.d. | n.d. | 10 ± 7 | 4.0 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabert, C.; Inostroza, K.; Bravo, S.; Sepúlveda, N.; Bravo, L.A. Exploratory Study of Fatty Acid Profile in Two Filmy Ferns with Contrasting Desiccation Tolerance Reveal the Production of Very Long Chain Polyunsaturated Omega-3 Fatty Acids. Plants 2020, 9, 1431. https://doi.org/10.3390/plants9111431
Rabert C, Inostroza K, Bravo S, Sepúlveda N, Bravo LA. Exploratory Study of Fatty Acid Profile in Two Filmy Ferns with Contrasting Desiccation Tolerance Reveal the Production of Very Long Chain Polyunsaturated Omega-3 Fatty Acids. Plants. 2020; 9(11):1431. https://doi.org/10.3390/plants9111431
Chicago/Turabian StyleRabert, Claudia, Karla Inostroza, Silvana Bravo, Néstor Sepúlveda, and León A. Bravo. 2020. "Exploratory Study of Fatty Acid Profile in Two Filmy Ferns with Contrasting Desiccation Tolerance Reveal the Production of Very Long Chain Polyunsaturated Omega-3 Fatty Acids" Plants 9, no. 11: 1431. https://doi.org/10.3390/plants9111431
APA StyleRabert, C., Inostroza, K., Bravo, S., Sepúlveda, N., & Bravo, L. A. (2020). Exploratory Study of Fatty Acid Profile in Two Filmy Ferns with Contrasting Desiccation Tolerance Reveal the Production of Very Long Chain Polyunsaturated Omega-3 Fatty Acids. Plants, 9(11), 1431. https://doi.org/10.3390/plants9111431







