Allelopathic Responses of Rice Seedlings under Some Different Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice Seeds and Indicator Plants
2.2. Paddy Soils Preparation
2.3. Phenolic Standards and Reagents
2.4. Other Chemicals
2.5. Extracts and Root Exudates of Rice Seedlings under the Stress Conditions
2.6. Allelopathic Response of the Extracts and Root Exudates from Rice Seedlings
2.6.1. Evaluating Allelopathic Response of the Extracts and Root Exudates in Laboratory Condition
2.6.2. Evaluating Allelopathic Responses of the Extracts and Root Exudates against the Growth of Barnyardgrass in Greenhouse
2.7. Determination of Total Phenolic and Flavonoid Contents
2.8. Identification of Phenolic Contained in the Extracts and Root Exudates under the Stress Conditions
2.9. Statistical Analyses
3. Results
3.1. Allelopathic Responses in Bioassay
3.1.1. Allelopathic Responses of the Extracts on the Indicator Plants
3.1.2. The Effects of Root Exudates on Indicator Plants
3.2. Allelopathic Response of Extracts and Root Exudates on the Growth of Barnyardgrass in Greenhouse
Allelopathic Response of the Extracts and Exudates on the Emergence and Growth of Natural Weeds
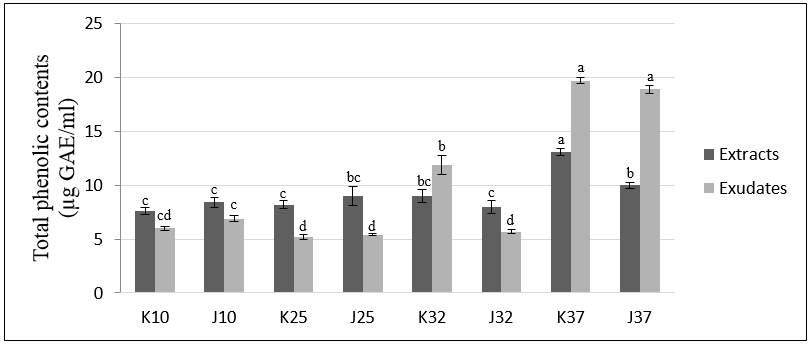
3.3. Determination of Total Phenolic and Flavonoid Contents of Extracts and Exudates from Rice Seedlings
3.4. Identification of Phenolic Components
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the role of allelochemicals in plant defences. In Advances in Botanical Research, How Plants Communicate with Their Biotic Environment; Becard, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 82, p. 384. [Google Scholar]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press Inc.: Orlando, FL, USA, 1984; p. 422. [Google Scholar]
- Khanh, T.D.; Chung, I.M.; Tawata, S.; Xuan, T.D. Allelopathy for weed management in sustainable agriculture. Health 2007, 1, 2. [Google Scholar] [CrossRef]
- Chen, B.M.; Liao, H.X.; Chen, W.B.; Wei, H.J.; Peng, S.L. Role of allelopathy in plant invasion and control of invasive plants. Allelopathy J. 2017, 41, 155–166. [Google Scholar] [CrossRef]
- Kong, C.H.; Chen, X.H.; Hu, F.; Zhang, S.Z. Breeding of commercially acceptable allelopathic rice cultivars in China. Pest Manag. Sci. 2011, 67, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.D.; Minh, T.N.; Trung, K.H.; Khanh, T.D. Allelopathic potential of sweet potato varieties to control weeds: Imperata cylindrica, Bidens pilosa and Ageratum conyzoides. Allelopathy J. 2016, 38, 41–54. [Google Scholar]
- Subudhi, P.K.; Sasaki, T.; Khush, G.S. Rice. In Genome Mapping and Molecular Breeding in Plants, Volume 1 Cereals and Millets; Kole, C., Ed.; Spinger: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Khanh, T.D.; Xuan, T.D.; Chung, I.M. Rice allelopathy and the possibility for weed management. Ann. Appl. Biol. 2007, 151, 325–339. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Peters, R.J. The role of momilactones in rice allelopathy. J. Chem. Ecol. 2013, 39, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Olofsdotter, M.; Rebulanan, M.; Madrid, A.; Dali, W.; Navarez, D.; Olk, D.C. Why phenolic acids are unlikely primary allelochemicals in rice. J. Chem. Ecol. 2002, 28, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Patra, B.; Schluttenhofer, C.; Wu, Y.; Pattanaik, S.; Yuan, L. Transcriptional regulation of secondary metabolite biosynthesis in plants. Bochim. Biophys. Acta 2013, 1829, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Ballhorn, D.J.; Kautz, S.; Heil, M.; Hegeman, A.D. Cyanogenesis of wild lima bean (Phaseolus lunatus L.) is an efficient direct defence in nature. Plant Signal. Behav. 2009, 4, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.B.; dos Santos, R.C.; Lima, L.M.; de Albuquerque, M.F.P.; Custodio, N.R.J.M.; da Camara, C.A.G.; de Rezende, R.A. Allelopathy, an alternative tool to improve cropping systems: A review. Agron. Sustain. Dev. 2011, 31, 379–395. [Google Scholar] [CrossRef]
- Jassey, V.E.J.; Chiapusio, G.; Gilbert, D.; Buttler, A.; Toussaint, M.L.; Binet, P. Experimental climate effect on seasonal variability of poly phenol/phenol oxidase interplay along a narrow fen-bog ecological gradient in Sphagnum fallax. Glob. Chang. Biol. 2011, 17, 2945–2957. [Google Scholar] [CrossRef]
- Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Schweitzer, J.A. Plant soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Seigler, D.S. Plant Secondary Metabolism; Chapman and Hall (Kluwer Academic Publishers): Boston, MA, USA, 1998; p. 711. [Google Scholar]
- Khanh, T.D.; Hong, N.H.; Xuan, T.D.; Chung, I.M. Paddy weed control by medicinal and leguminous plants from Southeast Asia. Crop Prot. 2005, 24, 421–431. [Google Scholar] [CrossRef]
- Linh, T.H.; Linh, L.H.; Cuc, D.T.K.; Ham, L.H.; Khanh, T.D. Improving submergence tolerance of Vietnamese rice cultivar by molecular breeding. J. Plant Breed. Genet. 2013, 1, 157–168. [Google Scholar]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; DaSilva, W.P.; Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef]
- Xuan, T.D.; Tsuzuki, E.; Matsuo, M.; Khanh, T.D. Correlation between inhibitory exhibition and suspected allelochemicals in alfalfa (Medicago sativa L.). Plant Prod. Sci. 2003, 6, 165–171. [Google Scholar] [CrossRef]
- Belz, R.G. Allelopathy in crop/weed interaction—An update. Pest Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Ku, Y.C.; Kim, K.H.; Chung, I.M. Allelopathic potential of rice germplasm against barnyardgrass. Allelopathy J. 2004, 13, 17–28. [Google Scholar]
- Ashry, M.A.; Zein, A.A.; Nady, M.F.EI.; Abdel-Dayem, Sh.M. Effect of potential allelopathic Egyptian rice cultivars against Echinochloa crus-galli and Echinochloa colonum. J. Plant Prot. Pathol. 2012, 3, 629–644. [Google Scholar]
- Xuan, T.D.; Minh, T.N.; Anh, L.H.; Khanh, T.D. Allelopathic momilactones A and B are implied in rice drought and salinity tolerance, not weed resistance. Agron. Sustain. Dev. 2016, 36, 52–60. [Google Scholar] [CrossRef]
- Khang, D.T.; Ha, P.T.T.; Lang, N.T.; Tuyen, P.T.; Minh, L.T.; Minh, T.N.; Bach, D.T.; Xuan, T.D. Involvement of phenolic compounds in anaerobic flooding germination of rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2016, 56, 73–81. [Google Scholar] [CrossRef]
- Gautam, P.; Lal, B.; Raja, R.; Baig, M.J.; Haldar, D.; Rath, L.; Shahid, M.; Tripathi, R.; Mohanty, S.; Bhattacharyya, P.; et al. Post-flood nitrogen and basal phosphorus management affect survival, metabolic changes and anti-oxidant enzyme activities of submerged rice (Oryza sativa). Funct. Plant Biol. 2014, 41, 1284–1294. [Google Scholar] [CrossRef]
- Inderjit, R.d.M. Is separating resource competition from allelopathy realistic? Bot. Rev. 1997, 63, 221–230. [Google Scholar] [CrossRef]
- Maruyama, K.; Urano, K.; Yoshiwara, K.; Morishita, Y.; Sakurai, N.; Suzuki, H.; Kojima, M.; Sakakibara, H.; Shibata, D.; Saito, K.; et al. Integrated analysis of the effects of cold and dehydration on rice metabolites, phytohormones, and gene transcripts. Plant. Physiol. 2014, 164, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Yang, Z.; Okazaki, Y.; Nakabayashi, R.; Fukushima, A.; Saito, K. Using metabolomic approaches to explore chemical diversity in rice. Mol. Plant. 2015, 8, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Shi, J.; Quan, S.; Cui, B.; Kleessen, S.; Nikoloski, Z.; Tohge, T.; Alexander, D.; Guo, L.; Lin, H.; et al. Metabolic variation between japonica and indica rice cultivars as revealed by non-targeted metabolomic. Sci. Rep. 2014, 4, 5067. [Google Scholar] [CrossRef] [PubMed]
- Kulichikhin, K.; Yamauchi, T.; Watanabe, K.; Nakazono, M. Biochemical and molecular characterization of rice (Oryza sativa L.) roots formng a barrier to radial oxygen loss. Plant Cell Environ. 2014, 37, 2406–2420. [Google Scholar] [PubMed]
- Einhellig, F.A. Allelopathy: Current status and future goals. In Allelopathy: Organisms, Processes and Applications; ACS Symposium Series 583; Dakshini, I.K.M.M., Einhellig, F.A., Eds.; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Xu, G.F.; Shen, S.C.; Zhang, F.D.; Zhang, Y.H. Allelopathic response to different temperature conditions of wide rice (Oryza longistaminata) and its descendants. Chin. J. Rice Sci. 2016, 30, 559–566. [Google Scholar]
- Vartapetia, B.B.; Andreeva, I.N.; Maslova, I.P. Ultrastricture of mitochondria in roots under conditions of anoxia and elevated temperatures, Fiziol. Ras. (Moscow). Sov. Plant Physiol. Engl. Transl. 1972, 19, 1105–1111. [Google Scholar]
- Zobayed, S.M.A.; Kozai, F.A.T. Temperature stress can alter the photosynthetic efficiency and secondary metabolites concentrations in St. John’s wort. Plant Physiol. Biochem. 2005, 43, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Einhellig, F.A. An integrated view of allelochemicals amid multiple stresses. In Principles and Practices in Plant Ecology: Allelochemical Interactions; Dakhini, I.K.M.M., Foy, C.L., Eds.; CRC Press: Boca Taton, FL, USA, 1999; pp. 479–494. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Weidner, S.; Chrzanowski, S.; Karamac, M.; Król, A.; Badowiec, A.; Mostek, A.; Amarowicz, R. Analysis of phenolic compounds and antioxidant abilities of extracts from germinating Vitis californica seeds submitted to cold stress conditions and recovery after the stress. Int. J. Mol. Sci. 2014, 15, 16211–16225. [Google Scholar] [CrossRef] [PubMed]
- Dalton, B.R. The occurrence and behavior of plant phenolic acids in soil environments and their potential involvement in allelochemical interference interactions: Methodological limitations in establishing conclusive proof of allelopathy. In Principles and Practices in Plant Ecology: Allelochemical Interactions; Dakshini, I.K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 57–74. [Google Scholar]
- Wu, H.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Allelochemicals in wheat (Triticum aestivum L.): Cultivar difference in the exudation of phenolic acids. J. Agric. Food Chem. 2001, 49, 3742–3745. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J. Changes in the levels of plant secondary metabolites under water stress and nutrient stress. Recent Adv. Phytochem. 1984. [Google Scholar] [CrossRef]
- Zeinali, A.; Esmaeili, M.; Heidarzade, A. Salicylic acid and abiotic stress influence allelochemicals and inhibitory potential of root exudates of two rice (Oryza sativa) cultivars against barnyardgrass (Echinochloa crus-galli L.). Int. J. Farm. Allied Sci. 2013, 19, 779–784. [Google Scholar]
- Xuan, T.D.; Tawata, S.; Khanh, T.D.; Chung, I.M. Decomposition of allelopathic plants in soil. J. Agron. Crop Sci. 2005, 191, 162–171. [Google Scholar] [CrossRef]
- Xuan, T.D.; Khang, D.T. Effects of exogenous application of protocatechuic acid and vanillic acid to chlorophylls, phenolics and antioxidant enzymes of rice (Oryza sativa L.) in submergence. Molecules 2018, 23, 620. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.T.; Xuan, T.D. Foliar application of vanillic and p-hydroxybenzoic acids enhance drought tolerance and formation of phytoalexin momilactones in rice. Arch. Agron. Soil Sci. 2018. [Google Scholar] [CrossRef]

| Variety | Treatment | |||
|---|---|---|---|---|
| 10 °C | 25 °C | 32 °C | 37 °C | |
| Koshihikari (K) | K10 | K25 | K32 | K37 |
| Jasmine (J) | J10 | J25 | J32 | J37 |
| Extracts | Germination Rate | Survival Rate | Shoot Height | Root Length | Dry Weight | AI |
|---|---|---|---|---|---|---|
| (1 mg/mL) | (%) | (%) | (cm) | (cm) | (mg) | (%) |
| Lettuce | ||||||
| K10 | 73.3 ab (−21.4) | 86.3 a (−13.7) | 1.1 bc (−3.9) | 2.8 cd (−24.2) | 5.0 ab (−9.0) | −14.4 |
| J10 | 86.7 ab (−7.1) | 96.3 a (−3.7) | 1.2 abc (+0.5) | 3.2 b (−13.5) | 5.6 a (+1.8) | −4.4 |
| K25 | 73.3 ab (−21.4) | 90.3 a (−9.7) | 1.1 c (−7.5) | 3.3 b (−11.9) | 5.3 ab (−4.8) | −11.1 |
| J25 | 80.0 ab (−14.3) | 78.9 a (−21.1) | 1.3 a (+7.7) | 3.5 ab (−6.7) | 5.8 a (+4.8) | −5.9 |
| K32 | 70.0 b (−25.0) | 95.3 a (−4.8) | 0.9 d (−18.8) | 2.82 cd (−24.6) | 4.6 ab (−16.3) | −17.9 |
| J32 | 76.7 ab (−17.9) | 77.4 a (−22.6) | 1.2 ab (+4.1) | 3.15 bc (−15.8) | 5.5 a (0.0) | −10.4 |
| K37 | 66.7 b (−28.6) | 95.8 a (−4.2) | 1.1 c (−5.9) | 2.2 e (−41.9) | 3.9 b (−28.9) | −21.9 |
| J37 | 80.0 ab (−14.3) | 95.8 a (−4.2) | 1.1 bc (−3.7) | 2.6 d (−31.2) | 5.4 ab (−2.4) | −11.2 |
| Control | 93.3 a (0.0) | 100.0 a (0.0) | 1.2 abc (0.0) | 3.7 a (0.0) | 5.5 a (0.0) | |
| AI (%) | −18.8 | −10.5 | −3.4 | −21.2 | −6.9 | |
| P Variety | * | ns | * | * | * | |
| P Temperature | ns | ns | * | * | ns | |
| P Variety*Temperature | ns | ns | * | * | ns | |
| Radish | ||||||
| K10 | 83.3 a (−3.8) | 64.4 b (−35.6) | 2.4 a (+63.8) | 1.5 de (−50.4) | 8.3 ab (−9.5) | −7.1 |
| J10 | 80.0 a (−7.7) | 92.6 ab (−7.4) | 2.4 a (+62.4) | 2.2 b (−24.1) | 8.7 ab (−4.4) | +3.8 |
| K25 | 73.3 ab (−15.4) | 91.7 ab (−8.3) | 2.4 a (+60.0) | 1.7 cd (−41.8) | 8.3 ab (−9.5) | −3.0 |
| J25 | 76.7 ab (−11.5) | 69.6 ab (−30.4) | 2.3 a (+54.8) | 2.0 bc (−33.6) | 7.8 ab (−14.2) | −7.0 |
| K32 | 53.3 b (−38.5) | 88.9 ab (−11.1) | 2.2 a (+48.6) | 1.2 e (−60.9) | 6.6 b (−27.4) | −17.9 |
| J32 | 70.0 ab (−19.2) | 80.8 ab (−19.2) | 2.2 a (+45.9) | 1.6 cde (−46.5) | 7.7 ab (−16.1) | −11.0 |
| K37 | 73.3 ab (−15.4) | 77.8 ab (−22.2) | 2.1 a (+40.1) | 1.5 de (−50.5) | 6.6 b (−27.7) | −15.1 |
| J37 | 66.7 ab (−23.1) | 90.5 ab (−9.5) | 2.2 a (+50.7) | 1.9 bcd (−36.7) | 7.3 ab (−19.7) | −7.7 |
| Control | 86.7 a (0.0) | 100.0 a (0.0) | 1.5 b (0.0) | 3.0 a (0.0) | 9.1 a (0.0) | |
| AI (%) | −16.8 | −18.0 | +53.3 | −43.1 | −16.1 | |
| P Variety | ns | ns | ns | * | ns | |
| P Temperature | * | ns | * | * | * | |
| P Variety*Temperature | ns | * | ns | * | ns | |
| Barnyardgrass | ||||||
| K10 | 96.7 a (−3.3) | 100.0 a (0.0) | 4.5 ab (+38.4) | 4.8 de (−19.3) | 10.5 b (−15.1) | +0.1 |
| J10 | 96.7 a (−3.3) | 100.0 a (0.0) | 4.9 a (+51.6) | 4.6 de (−22.8) | 9.9 b (−19.5) | +1.2 |
| K25 | 93.3 a (−6.7) | 100.0 a (0.0) | 4.0 ab (+24.4) | 5.0 cd (−15.9) | 10.5 b (−15.1) | −2.7 |
| J25 | 86.7 a (−13.3) | 100.0 a (0.0) | 4.7 ab (+44.6) | 5.6 bc (−5.9) | 10.0 b (−18.9) | +1.3 |
| K32 | 93.3 a (−6.7) | 100.0 a (0.0) | 4.2 abc (+29.2) | 4.2 e (−29.0) | 9.3 b (−24.3) | −6.2 |
| J32 | 96.7 a (−3.3) | 100.0 a (0.0) | 4.7 ab (+46.0) | 4.8 de (−19.1) | 9.7 b (−21.1) | +0.5 |
| K37 | 90.0 a (−10.0) | 100.0 a (0.0) | 3.7 bc (+15.6) | 6.7 a (+11.8) | 13.8 a (+11.6) | +5.8 |
| J37 | 93.3 a (−6.7) | 96.3 a (−3.7) | 4.7 ab (+45.0) | 6.7 a (+11.8) | 10.4 b (−15.4) | +6.2 |
| Control | 100.0 a (0.0) | 100.0 a (0.0) | 3.2 c (0.0) | 6.0 ab (0.0) | 12.3 a (0.0) | |
| AI (%) | −6.7 | −0.5 | +36.9 | −11.1 | −14.7 | |
| P Variety | ns | ns | * | * | * | |
| P Temperature | ns | ns | * | * | * | |
| P Variety*Temperature | ns | ns | ns | * | * | |
| Exudates | Germination Rate | Survival Rate | Shoot Height | Root Length | Dry Weight | AI |
|---|---|---|---|---|---|---|
| (50%) | (%) | (%) | (cm) | (cm) | (mg) | (%) |
| Lettuce | ||||||
| K10 | 93.3 a (−3.4) | 96.7 a (−3.3) | 1.3 cd (+21.4) | 3.0 c (−22.5) | 6.7 a (+19.8) | +2.4 |
| J10 | 93.3 a (−3.4) | 96.3 a (−3.7) | 1.3 cd (+21.2) | 3.3 bc (−14.2) | 6.7 a (+19.8) | +3.9 |
| K25 | 93.3 a (−3.4) | 96.7 a (−3.3) | 1.3 bc (+26.0) | 3.2 c (−18.3) | 6.6 a (+19.2) | +4.0 |
| J25 | 100.0 a (+3.4) | 100.0 a (0.0) | 1.2 de (+10.1) | 3.3 c (−16.2) | 6.6 a (+18.6) | +3.2 |
| K32 | 96.7 a (0.0) | 100.0 a (0.0) | 1.4 ab (+35.6) | 4.1 a (+5.6) | 6.9 a (+23.4) | +12.9 |
| J32 | 100.0 a (+3.4) | 100.0 a (0.0) | 1.3 bc (+26.1) | 3.2 c (−16.6) | 6.2 a (+11.4) | +4.9 |
| K37 | 96.7 a (0.0) | 100.0 a (0.0) | 1.5 a (+39.6) | 4.2 a (+7.2) | 8.3 a (+48.5) | +19.1 |
| J37 | 93.3 a (−3.4) | 100.0 a (0.0) | 1.3 bc (+23.9) | 3.1 c (−19.2) | 6.9 a (+24.6) | +5.2 |
| Control | 96.7 a (0.0) | 100.0 a (0.0) | 1.1 e (0.0) | 3.9 ab (0.0) | 5.6 a (0.0) | |
| AI (%) | −0.9 | −1.3 | +25.5 | −11.8 | +23.2 | |
| P Variety | ns | ns | * | * | ns | |
| P Temperature | ns | ns | * | * | ns | |
| P Variety*Temperature | ns | ns | * | * | ns | |
| Radish | ||||||
| K10 | 96.7 a (−3.3) | 100.0 a (0.0) | 1.9 bc (+39.0) | 3.9 a (−14.1) | 12.3 a (+5.4) | +5.4 |
| J10 | 100.0 a (0.0) | 96.7 a (−3.3) | 1.5 d (+11.0) | 4.3 a (−5.7) | 10.0 a (−14.6) | −2.5 |
| K25 | 96.7 a (−3.3) | 100.0 a (0.0) | 1.7 cd (+20.7) | 4.2 a (−6.8) | 10.8 a (−7.7) | +0.6 |
| J25 | 96.7 a (−3.3) | 100.0 a (0.0) | 1.5 d (+9.8) | 4.3 a (−6.6) | 10.1 a (−13.4) | −2.7 |
| K32 | 100.0 a (0.0) | 90.0 a (−10.0) | 2.0 b (+44.2) | 3.8 a (−17.6) | 10.9 a (−6.3) | +2.1 |
| J32 | 100.0 a (0.0) | 96.7 a (−3.3) | 1.8 bc (+33.7) | 2.7 b (−40.1) | 10.6 a (−8.9) | −3.7 |
| K37 | 96.7 a (−3.3) | 100.0 a (0.0) | 2.6 a (+89.0) | 3.7 ab (−19.5) | 11.8 a (+1.4) | +13.5 |
| J37 | 96.7 a (−3.3) | 100.0 a (0.0) | 1.8 bc (+33.0) | 3.7 ab (−18.9) | 9.8 a (−16.0) | −1.0 |
| Control | 100.0 a (0.0) | 100.0 a (0.0) | 1.4 d (0.0) | 4.6 ab (0.0) | 11.7 a (0.0) | |
| AI (%) | −2.1 | −2.1 | +35.1 | −16.2 | −7.5 | |
| P Variety | ns | ns | * | ns | ns | |
| P Temperature | ns | ns | * | * | ns | |
| P Variety*Temperature | ns | ns | * | * | ns | |
| Barnyardgrass | ||||||
| K10 | 90.0 a (−3.6) | 100.0 a (+8.0) | 4.4 bcd (−7.3) | 6.1 cd (−25.5) | 16.0 abc (+1.9) | −5.3 |
| J10 | 93.3 a (0.0) | 96.3 a (+4.0) | 3.9 e (−16.8) | 5.7 de (−29.9) | 11.3 c (−28.2) | −14.2 |
| K25 | 93.3 a (0.0) | 92.6 a (0.0) | 5.1 a (+8.2) | 6.9 b (−16.0) | 16.6 ab (+5.3) | −0.5 |
| J25 | 90.0 a (−3.6) | 100.0 a (+8.0) | 4.6 bc (−3.3) | 6.8 bc (−17.3) | 17.6 a (+12.1) | −0.8 |
| K32 | 93.3 a (0.0) | 96.3 a (+4.0) | 4.2 cde (−11.7) | 6.2 bcd (−23.6) | 12.2 bc (−22.7) | −10.8 |
| J32 | 96.7 a (+3.6) | 100.0 a (+8.0) | 3.8 e (−18.9) | 5.2 ef (−37.0) | 13.9 abc (−11.4) | −11.1 |
| K37 | 86.7 a (−7.1) | 96.3 a (+4.0) | 4.1 de (−12.3) | 4.8 fg (−41.3) | 12.1 bc (−23.3) | −16.0 |
| J37 | 83.3 a (−10.7) | 100.0 a (+8.0) | 4.1 de (−12.2) | 4.2 g (−48.8) | 11.9 bc (−24.2) | −17.6 |
| Control | 93.3 a (0.0) | 92.6 a (0.0) | 4.7 ab (0.0) | 8.2 a (0.0) | 15.7 abc (0.0) | |
| AI (%) | −2.7 | +5.5 | −9.3 | −29.9 | −11.3 | |
| P Variety | ns | ns | * | * | ns | |
| P Temperature | ns | ns | * | * | * | |
| P Variety*Temperature | ns | ns | * | * | * | |
| Sample | Shoot Height | Root Length | Dry Weight | AI |
|---|---|---|---|---|
| (cm) | (cm) | (g) | (%) | |
| Extracts (1 mg/mL) | ||||
| K10 | 45.9 a (−5.4) | 18.2 b (−39.7) | 1.7 bc (−23.0) | −22.7 |
| J10 | 43.5 a (−10.3) | 24.3 ab (−19.4) | 1.7 bc (−20.2) | −16.6 |
| K25 | 42.8 a (−11.7) | 26.4 a (−12.6) | 1.4 c (−33.8) | −19.4 |
| J25 | 43.8 a (−9.8) | 25.2 a (−16.6) | 1.6 bc (−28.6) | −18.3 |
| K32 | 44.7 a (−7.8) | 26.4 a (−12.6) | 1.8 ab (−15.7) | −12.0 |
| J32 | 45.5 a (−6.2) | 24.3 ab (−19.4) | 1.7 bc (−23.2) | −16.3 |
| K37 | 42.3 a (−12.9) | 24.9 ab (−17.6) | 1.5 bc (−30.0) | −20.2 |
| J37 | 44.9 a (−7.4) | 24.6 ab (−18.4) | 1.7 bc (−23.7) | −16.5 |
| Control | 48.5 a (0.0) | 30.2 a (0.0) | 2.2 a (0.0) | |
| AI (%) | −8.9 | −19.5 | −24.8 | |
| P Variety | ns | ns | ns | |
| P Temperature | ns | * | * | |
| P Variety*Temperature | ns | * | ns | |
| Exudates (50%) | ||||
| K10 | 42.6 bc (−11.3) | 20.0 abc (−8.4) | 3.3 a (+3.8) | −5.3 |
| J10 | 42.4 c (−11.8) | 19.0 bcd (−13.4) | 3.0 e (−5.2) | −10.1 |
| K25 | 44.6 abc (−7.2) | 17.3 cd (−20.9) | 2.5 g (−23.0) | −17.0 |
| J25 | 48.0 a (−0.2) | 16.2 d (−25.9) | 3.0 e (−5.1) | −10.4 |
| K32 | 43.5 bc (−9.5) | 18.8 cd (−14.1) | 3.2 c (−0.7) | −8.1 |
| J32 | 46.9 ab (−2.4) | 17.3 cd (−20.9) | 2.7 f (−17.1) | −13.5 |
| K37 | 46.1 abc (−4.0) | 18.4 cd (−16.2) | 3.1 d (−3.8) | −8.0 |
| J37 | 42.0 c (−12.6) | 22.4 a (+2.5) | 3.2 c (−0.8) | −3.6 |
| Control | 48.1 a (0.0) | 21.9 a (0.0) | 3.2 b (0.0) | |
| AI (%) | −7.4 | −14.7 | −6.5 | |
| P Variety | ns | ns | * | |
| P Temperature | * | * | * | |
| P Variety*Temperature | * | * | * | |
| Sample | Number of Weeds | Dry Weight of Weeds (mg) | AI | ||||
|---|---|---|---|---|---|---|---|
| Monocot | Dicotyledon | Total Weeds | Monocot | Dicotyledon | Total Weeds | (%) | |
| Extracts (1 mg/mL) | |||||||
| K10 | 24.3 abc (+14.1) | 22.7 bc (−19.0) | 47.0 a (−4.7) | 21.1 cd (+22.4) | 7.8 def (−11.0) | 28.9 cd (+11.2) | +3.3 |
| J10 | 20.7 c (−3.1) | 19.0 cd (−32.1) | 39.7 bc (−19.6) | 22.4 cd (+30.0) | 10.1 c (+15.6) | 32.5 bcd (+25.1) | +2.8 |
| K25 | 24.7 abc (+15.6) | 19.3 cd (−31.0) | 44.0 abc (−10.8) | 34.1 ab (+97.7) | 8.0 cdef (−8.7) | 42.1 ab (+61.8) | +25.5 |
| J25 | 26.0 ab (+21.9) | 22.3 bc (−20.2) | 48.3 a (−2.0) | 35.7 a (+107.2) | 12.9 b (+47.5) | 48.6 a (+87.1) | +85.1 |
| K32 | 21.7 bc (+1.6) | 26.0 ab (−7.1) | 47.7 a (−3.4) | 27.7 abc (+60.9) | 9.8 cd (+11.4) | 37.5 bcd (+44.2) | +20.4 |
| J32 | 27.3 a (+28.1) | 18.0 cd (−35.7) | 45.3 ab (−8.1) | 25.4 bcd (+47.2) | 7.3 ef (−16.7) | 32.7 bcd (+25.6) | +8.8 |
| K37 | 25.3 abc (+18.8) | 18.0 cd (−35.7) | 43.3 abc (−12.2) | 20.7 cd (+19.9) | 16.1 a (+84.0) | 36.8 bc (+41.5) | +14.7 |
| J37 | 21.3 bc (0.0) | 17.0 d (−39.3) | 38.3 c (−22.3) | 10.5 e (−38.9) | 5.9 f (−33.1) | 16.4 e (−36.9) | −29.6 |
| Control | 21.3 bc (0.0) | 28.0 a (0.0) | 49.3 a (0.0) | 17.2 de (0.0) | 8.8 cde (0.0) | 26.0 de (0.0) | |
| AI (%) | +12.1 | −27.5 | −10.4 | +43.3 | +11.1 | +32.5 | |
| P Variety | ns | * | * | ns | * | * | |
| P Temperature | ns | * | * | * | * | * | |
| P Variety*Temperature | * | * | * | * | * | * | |
| Root Exudates (50%) | |||||||
| K10 | 4.3 bcd (−38.1) | 17.0 ab (+18.6) | 21.3 a (0.0) | 2.0 de (−88.3) | 1.1 cd (−21.4) | 3.1 d (−83.8) | −41.9 |
| J10 | 4.3 bcd (−38.1) | 16.3 ab (+14.0) | 20.7 a (−3.1) | 3.2 bcd (−86.1) | 1.8 ab (+28.6) | 5.0 c (−73.4) | −38.3 |
| K25 | 2.3 d (−66.7) | 18.7 a (+30.2) | 21.0 a (−1.6) | 1.3 e (−92.7) | 1.3 c (−9.5) | 2.5 de (−86.5) | −42.5 |
| J25 | 5.0 abc (−28.6) | 10.7 c (−25.6) | 15.7 b (−26.6) | 1.2 e (−92.9) | 1.3 c (−4.8) | 2.6 de (−86.3) | −56.5 |
| K32 | 2.3 d (−66.7) | 6.3 e (−55.8) | 8.7 d (−59.4) | 1.0 e (−94.0) | 0.2 e (−85.7) | 1.2 e (−93.4) | −76.4 |
| J32 | 4.7 bc (−33.3) | 6.7 de (−53.5) | 11.3 cd (−46.9) | 3.5 bc (−79.7) | 2.1 a (+50.0) | 5.6 bc (−70.0) | −58.5 |
| K37 | 6.3 ab (−9.5) | 7.7 de (−46.5) | 14.0 bc (−34.4) | 4.6 b (−73.7) | 2.1 a (+52.4) | 6.7 b (−64.3) | −49.4 |
| J37 | 3.7 cd (−47.6) | 9.3 cd (−34.9) | 13.0 bc (−39.1) | 2.3 cde (−86.6) | 0.8 d (−42.9) | 3.1 d (−83.3) | −61.2 |
| Control | 7.0 a (0.0) | 14.3 b (0.0) | 21.3 a (0.0) | 17.4 a (0.0) | 1.4 bc (0.0) | 18.8 a (0.0) | |
| AI (%) | −41.1 | −19.2 | −26.4 | −86.8 | −4.2 | −80.1 | |
| P Variety | ns | * | * | * | * | * | |
| P Temperature | * | * | * | * | * | * | |
| P Variety*Temperature | * | * | * | * | * | * | |
| Sample | GA | PA | Ca | ChA | p-HA | VA | SyA | Vn | FA | SA | p-CA | BA | EA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K10 (Ext) | - | - | - | - | - | - | - | 2.651 cde | - | - | - | 3.222 b | - |
| J10 (Ext) | - | - | - | - | - | - | - | 2.543 def | - | 1.269 b | - | 2.273 bc | - |
| K25 (Ext) | - | 0.684 a | - | - | - | - | - | 2.441 f | - | 1.160 b | - | - | - |
| J25 (Ext) | - | - | - | - | - | - | - | - | - | 1.443 b | 1.340 a | - | 2.101 b |
| K32 (Ext) | - | - | - | - | 0.572 ab | - | - | - | - | - | - | 2.206 bc | 1.665 b |
| J32 (Ext) | - | 0.658 c | - | - | 0.372 c | - | - | 2.733 bcd | - | 2.152 a | - | 3.388 b | - |
| K37 (Ext) | - | - | - | 6.969 a | - | - | - | - | 1.285 a | - | - | 1.046 c | 1.825 b |
| J37 (Ext) | - | - | - | - | - | - | - | 3.371 a | - | - | - | 5.347 a | - |
| K10 (Exu) | - | - | - | - | - | - | - | 2.907 b | - | - | - | 1.109 c | - |
| J10 (Exu) | - | - | - | - | - | - | - | 2.459 ef | - | - | 1.141 b | 3.102 b | - |
| K25 (Exu) | - | 0.674 b | - | - | - | - | - | 2.778 bc | - | - | - | - | - |
| J25 (Exu) | - | - | - | - | - | - | - | 2.832 bc | - | - | - | - | - |
| K32 (Exu) | - | - | - | - | 0.360 c | 0.045 a | 3.052 a | - | - | 1.309 b | - | 5.543 a | - |
| J32 (Exu) | - | - | - | - | 0.620 a | - | - | 2.536 def | - | - | - | 1.553 c | - |
| K37 (Exu) | - | - | 0.638 a | 5.912 c | 0.513 ab | - | - | 2.544 def | 1.198 a | - | - | - | 1.871 b |
| J37 (Exu) | 0.804 a | - | 0.379 b | 6.300 b | 0.455 bc | - | - | 2.351 f | 0.976 b | 2.374 a | - | - | 2.871 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanh, T.D.; Anh, L.H.; Nghia, L.T.; Huu Trung, K.; Bich Hien, P.; Minh Trung, D.; Dang Xuan, T. Allelopathic Responses of Rice Seedlings under Some Different Stresses. Plants 2018, 7, 40. https://doi.org/10.3390/plants7020040
Khanh TD, Anh LH, Nghia LT, Huu Trung K, Bich Hien P, Minh Trung D, Dang Xuan T. Allelopathic Responses of Rice Seedlings under Some Different Stresses. Plants. 2018; 7(2):40. https://doi.org/10.3390/plants7020040
Chicago/Turabian StyleKhanh, Tran Dang, La Hoang Anh, La Tuan Nghia, Khuat Huu Trung, Pham Bich Hien, Do Minh Trung, and Tran Dang Xuan. 2018. "Allelopathic Responses of Rice Seedlings under Some Different Stresses" Plants 7, no. 2: 40. https://doi.org/10.3390/plants7020040
APA StyleKhanh, T. D., Anh, L. H., Nghia, L. T., Huu Trung, K., Bich Hien, P., Minh Trung, D., & Dang Xuan, T. (2018). Allelopathic Responses of Rice Seedlings under Some Different Stresses. Plants, 7(2), 40. https://doi.org/10.3390/plants7020040








