Qualitative and Quantitative Analysis of Polyphenols in Lamiaceae Plants—A Review
Abstract
1. Introduction
2. Extraction and Purification
3. Chromatographic Techniques with Ultraviolet/Visible (UV/Vis) Based Detection
4. Hyphenated Chromatographic Techniques
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AcOEt | ethyl acetate |
| APCI | atmospheric pressure chemical ionization |
| ASE | accelerated solvent extraction |
| BHA | butylated hydroxyanisole |
| BHT | butylated hydroxytoluene |
| CE | capillary electrophoresis |
| CO2 | carbon dioxide |
| DAD | diode array |
| DPPH• | 2,2-diphenyl-1-picrylhydrazyl radical |
| ESI | electrospray ionization |
| EtOH | ethanol |
| FC | flash chromatography |
| FID | flame ionization detection |
| GC | gas chromatography |
| hr | hours |
| H2O | water |
| HCl | hydrochloric acid |
| HESI | heated electrospay ionization |
| HPLC | high-performance liquid chromatography |
| HRMS | high resolution mass spectrometry |
| i.d. | internal diameter |
| LC | liquid chromatography |
| LOD | limit of detection |
| LOQ | limit of quantification |
| LPSE | low pressure solvent extraction |
| LTQ | linear ion trap quadrupole |
| QqQLIT | hybrid linear ion trap |
| MAE | microwave assisted extraction |
| ME | matrix effects |
| MeCN | acetonitrile |
| MeOH | methanol |
| MRM | multiple reaction monitoring |
| MS | mass spectrometry |
| MSn | multi-stage mass spectrometry |
| MS/MS | tandem mass spectrometry |
| MSD | mass selective detector |
| MS-O | mass spectrometry-olfactometry |
| m/zn/a | mass-to-charge ratio not available |
| NaOH | sodium hydroxide |
| N2 | Nitrogen |
| NMR | nuclear magnetic resonance |
| NP/PEG | natural products-polyethylene glycol reagent |
| O2 | Oxygen |
| P & T | purge and trap |
| PDA | photodiode array |
| p.s. | particle size |
| r | linear regression |
| R2 | correlation/determination coefficient |
| RP | reversed phase |
| RSC | radical scavenging capacity |
| SFE | supercritical fluid extraction |
| SIM | selected ion monitoring mode |
| SPE | solid phase extraction |
| mTLC | micro-thin layer chromatography |
| T | temperature |
| t | Time |
| TFA | trifluoroacetic acid |
| TLC | thin layer chromatography |
| TOF | time-of-flight |
| TQS | triple quadrupole spectrometer |
| UAE | ultrasound-assisted extraction |
| UHPLC | ultra-high performance liquid chromatography |
| UV | ultraviolet |
| Vis v/v w/v | visible volume/volume weight/volume |
| 2D | two dimensional |
References
- Hinneburg, I.; Dorman, H.D.; Hiltunen, R. Antioxidant activities of extracts selected culinary herbs and spices. Food Chem. 2006, 97, 122–129. [Google Scholar] [CrossRef]
- Damašius, J.; Venskutonis, P.; Kaškoniene, V.; Maruška, A. Fast screening of the main phenolic acids with antioxidant properties in common spices using on-line HPLC/UV/DPPH radical scavenging assay. Anal. Methods 2014, 6, 2774–2779. [Google Scholar] [CrossRef]
- Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Phytochemical composition of fractions isolated from ten Salvia species by supercritical carbon dioxide and pressurized liquid extraction methods. Food Chem. 2017, 224, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Babovic, N.; Djilas, S.; Jadranin, M.; Vajs, V.; Ivanovic, J.; Petrovic, S.; Zizovic, I. Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. Innov. Food Sci. Emerg. Technol. 2010, 11, 98–107. [Google Scholar] [CrossRef]
- Klejdus, B.; Kováčik, J. Quantification of phenols in cinnamon: A special focus on “total phenols” and phenolic acids including DESI-Orbitrap MS detection. Ind. Crop. Prod. 2016, 83, 774–780. [Google Scholar] [CrossRef]
- Leja, K.B.; Czaczyk, K. The industrial potential of herbs and spices—A mini review. Acta Sci. Pol. Technol. Aliment. 2016, 15, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Calucci, L.; Pinzino, C.; Zandomeneghi, M.; Capocchi, A.; Ghiringhelli, S.; Saviozzi, F.; Tozzi, S.; Galleschi, L. Effects of γ-irradiation on the free radical and antioxidant contents in nine aromatic herbs and spices. J. Agric. Food Chem. 2003, 51, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Aghakhani, F.; Kharazian, N.; Gooini, Z.L. Flavonoid Constituents of Phlomis (Lamiaceae) Species Using Liquid Chromatography Mass Spectrometry. Phytochem. Anal. 2017, 29, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Kovačević, D.B.; Penić, M.; Fegeš, M.; Dragović-Uzelac, V. Microwave-assisted extraction (MAE) of dalmatian sage leaves for the optimal yield of polyphenols: HPLC-DAD identification and quantification. Food Anal. Methods 2016, 9, 2385–2394. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Engel, R.; Szabó, K.; Abrankó, L.S.; Rendes, K.; Füzy, A.; Takács, T.N. Effect of Arbuscular Mycorrhizal Fungi on the Growth and Polyphenol Profile of Marjoram, Lemon Balm, and Marigold. J. Agric. Food Chem. 2016, 64, 3733–3742. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; Gan, R.Y.; Corke, H. The phenolic composition and antioxidant capacity of soluble and bound extracts in selected dietary spices and medicinal herbs. Int. J. Food Sci. Technol. 2016, 51, 565–573. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ribeiro-Santos, R.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. UHPLC-DAD Multi-Method for Determination of Phenolics in Aromatic Plants. Food Anal. Methods 2018, 11, 440–450. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Boudet, A.M. Studies on quinic acid biosynthesis in Quercus pedunculata Ehrh. Seedlings. Plant Cell Physiol. 1980, 21, 785–792. [Google Scholar] [CrossRef]
- Kalili, K.M.; de Villiers, A. Recent developments in the HPLC separation of phenolic compounds. J. Sep. Sci. 2011, 34, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; El-Ahmady, S.H.; Abou-Shoer, M.I.; Al-Azizi, M.M. Application of Chemometrics in Authentication of Herbal Medicines: A Review. Phytochem. Anal. 2013, 24, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.; Costa, H.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food. Sci. Technol. 2015, 45, 336–354. [Google Scholar] [CrossRef]
- Popova, I.E.; Hall, C.; Kubátová, A. Determination of lignans in flaxseed using liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Ganzera, M.; Sturm, S. Recent advances on HPLC/MS in medicinal plant analysis—An update covering 2011–2016. J. Pharm. Biomed. Anal. 2018, 147, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Tubaon, R.M.; Haddad, P.R.; Quirino, J.P. Sample Clean-up Strategies for ESI Mass Spectrometry Applications in Bottom-up Proteomics: Trends from 2012 to 2016. Proteomics 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Dobiáš, P.; Pavlíková, P.; Adam, M.; Eisner, A.; Beňová, B.; Ventura, K. Comparison of pressurised fluid and ultrasonic extraction methods for analysis of plant antioxidants and their antioxidant capacity. Cent. Eur. J. Chem. 2010, 8, 87–95. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Alonso-Carrillo, N.; de los Ángeles Aguilar-Santamaría, M.; Vernon-Carter, E.J.; Jiménez-Alvarado, R.; Cruz-Sosa, F.; Román-Guerrero, A. Extraction of phenolic compounds from Satureja macrostema using microwave-ultrasound assisted and reflux methods and evaluation of their antioxidant activity and cytotoxicity. Ind. Crop. Prod. 2017, 103, 213–221. [Google Scholar] [CrossRef]
- Santos, D.T.; Veggi, P.C.; Angela, M.; Meireles, A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Optimisation of accelerated solvent extraction of antioxidant compounds from rosemary (Rosmarinus officinalis L.), marjoram (Origanum majorana L.) and oregano (Origanum vulgare L.) using response surface methodology. Food Chem. 2011, 126, 339–346. [Google Scholar] [CrossRef]
- Laroze, L.; Soto, C.; Zúñiga, M.L. Raspberry phenolic antioxidants extraction. J. Biotechnol. 2008, 136, 717–742. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 5, 16–40. [Google Scholar] [CrossRef]
- Moraes, M.N.; Zabot, G.L.; Prado, J.M.; Meireles, M.A.A. Obtaining antioxidants from botanic matrices applying novel extraction techniques. Food Public Health 2013, 3, 195–214. [Google Scholar] [CrossRef][Green Version]
- Mojzer, E.B.; Hrnčič, M.K.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Drioli, E.; Cassano, A. Membrane-based agro-food production processes for polyphenol separation, purification and concentration. Curr. Opin. Food Sci. 2017, in press, Corrected Proof. [Google Scholar] [CrossRef]
- Sadeghi, A.; Hakimzadeh, V.; Karimifar, B. Microwave Assisted Extraction of Bioactive Compounds from Food: A Review. Int. J. Food Sci. Nutr. Eng. 2017, 7, 19–27. [Google Scholar] [CrossRef]
- Ekezie, F.G.C.; Sun, D.W.; Han, Z.; Cheng, J.H. Microwave-Assisted Food Processing Technologies for Enhancing Product Quality and Process Efficiency: A Review of Recent Developments. Trends Food Sci. Technol. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, X.H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Aires, A. Phenolics in Foods: Extraction, Analysis and Measurements. In Phenolic Compounds—Natural Sources, Importance and Applications, 1st ed.; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.R., Eds.; InTech: London, UK, 2017; pp. 61–88. ISBN 978-953-51-2958-5. Available online: https://www.intechopen.com/books/phenolic-compounds-natural-sources-importance-and-applications (accessed on 25 March 2018).
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Pocheville, A.; Angulo, I.; Paseiro-Losada, P.; Cruz, J.M. Fractionation and Purification of Bioactive Compounds Obtained from a Brewery Waste Stream. Biomed. Res. Int. 2013, 2013, 408491. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Ligor, T.; Ulanowska, A. Determination of volatile organic compounds: Enrichment and analysis. In Handbook of Trace Analysis, 1st ed.; Buszewski, B., Ed.; Springer International Publishing: Basel, Switzerland, 2016; pp. 403–430. ISBN 978-3-319-19614-5. [Google Scholar]
- Roberto, R.M.; García, N.P.; Hevia, A.G.; Valles, B.S. Application of purge and trap extraction and gas chromatography for determination of minor esters in cider. J. Chromatogr. A 2005, 1069, 245–251. [Google Scholar] [CrossRef]
- Kaczyński, P. Clean-up and matrix effect in LC-MS/MS analysis of food of plant origin for high polar herbicides. Food Chem. 2017, 230, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313. [Google Scholar] [CrossRef] [PubMed]
- Fecka, I.; Turek, S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: Thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008, 108, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Žugić, A.; Đorđević, S.; Arsić, I.; Marković, G.; Živković, J.; Jovanović, S.; Tadić, V. Antioxidant activity and phenolic compounds in 10 selected herbs from Vrujci Spa, Serbia. Ind. Crop. Prod. 2014, 52, 519–527. [Google Scholar] [CrossRef]
- Maher, H.M.; Al-Zoman, N.Z.; Al-Shehri, M.M.; Al-Showiman, H.; Al-Taweel, A.M.; Fawzy, G.A.; Perveen, S. Determination of luteolin and apigenin in herbs by capillary electrophoresis with diode array detection. Instrum. Sci. Technol. 2015, 43, 611–625. [Google Scholar] [CrossRef]
- Hawrył, M.A.; Niemiec, M.A.; Słomka, K.; Waksmundzka-Hajnos, M.; Szymczak, G. Two-Dimensional Micro-TLC Phenolic Fingerprints of Selected Mentha sp. on Cyano-Bonded Polar Stationary Phase. J. Chromatogr. Sci. 2015, 54, 64–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arceusz, A.; Wesolowski, M. Quality consistency evaluation of Melissa officinalis L. commercial herbs by HPLC fingerprint and quantitation of selected phenolic acids. J. Pharm. Biomed. Anal. 2013, 83, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Alvarenga, J.F.R.; Martinez-Huelamo, M.; Leal, L.N.; Lamuela-Raventos, R.M. Characterization of the phenolic and antioxidant profiles of selected culinary herbs and spices: Caraway, turmeric, dill, marjoram and nutmeg. Food Sci. Technol. 2015, 35, 189–195. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Chandra, P.; Kumar, B.; Dutt, B.; Sharma, K.R. A rapid and highly sensitive method for simultaneous determination of bioactive constituents in leaf extracts of six Ocimum species using ultra high performance liquid chromatography-hybrid linear ion trap triple quadrupole mass spectrometry. Anal. Methods 2016, 8, 333–341. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Švarc-Gajić, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds and antioxidant activity of a Mediterranean plant: The case of Satureja montana subsp. kitaibelii. J. Funct. Foods 2015, 18, 1167–1178. [Google Scholar] [CrossRef]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.A.; Nieman, K.M.; Dall’Asta, C.; Del Rio, D. Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef] [PubMed]
- Fatiha, B.; Didier, H.; Naima, G.; Khodir, M.; Martin, K.; Léocadie, K.; Caroline, S.; Mohamed, C.; Pierre, D. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae). Ind. Crop. Prod. 2015, 74, 722–730. [Google Scholar] [CrossRef]
- Jesionek, W.; Majer-Dziedzic, B.; Choma, I.M. Separation, Identification, and Investigation of Antioxidant Ability of Plant Extract Components Using TLC, LC-MS, and TLC-DPPH. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1147–1153. [Google Scholar] [CrossRef]
- Pereira, O.R.; Peres, A.M.; Silva, A.M.; Domingues, M.R.; Cardoso, S.M. Simultaneous characterization and quantification of phenolic compounds in Thymus x citriodorus using a validated HPLC-UV and ESI-MS combined method. Food Res. Int. 2013, 54, 1773–1780. [Google Scholar] [CrossRef]
- Hossain, M.B.; Camphuis, G.; Aguiló-Aguayo, I.; Gangopadhyay, N.; Rai, D.K. Antioxidant activity guided separation of major polyphenols of marjoram (Origanum majorana L.) using flash chromatography and their identification by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2014, 37, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Leto, C.; Saija, A.; Trombetta, D.; Tomaino, A.; Speciale, A.; Napoli, E.M.; et al. Biomolecular Characterization of Wild Sicilian Oregano: Phytochemical Screening of Essential Oils and Extracts, and Evaluation of Their Antioxidant Activities. Chem. Biodivers. 2013, 10, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.M.; Siracusa, L.; Saija, A.; Speciale, A.; Trombetta, D.; Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Leone, R.; et al. Wild Sicilian Rosemary: Phytochemical and Morphological Screening and Antioxidant Activity Evaluation of Extracts and Essential Oils. Chem. Biodivers. 2015, 12, 1075–1094. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Speciale, A.; Trombetta, T.; Leto, C.; Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Bonsangue, G.; Gennaro, M.C.; et al. Phytochemical, Ecological and Antioxidant Evaluation of Wild Sicilian Thyme: Thymbra capitata (L.) Cav. Chem. Biodivers. 2016, 13, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Characterization of aroma-active and phenolic profiles of wild thyme (Thymus serpyllum) by GC-MS-Olfactometry and LC-ESI-MS/MS. J. Food Sci. Technol. 2016, 53, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S.; Konczak, I.; Zhao, J. Identification and quantification of phenolics in Australian native mint (Mentha australis R. Br.). Food Chem. 2016, 192, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Atwi, M.; Weiss, E.K.; Loupassaki, S.; Makris, D.P.; Ioannou, E.; Roussis, V.; Kefalas, P. Major antioxidant polyphenolic phytochemicals of three Salvia species endemic to the island of Crete. J. Herbs Spices Med. Plants 2016, 22, 27–34. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef] [PubMed]
- Çelik, S.E.; Tufan, A.N.; Bekdeşer, B.; Özyürek, M.; Güçlü, K.; Apak, R. Identification and Determination of Phenolics in Lamiaceae Species by UPLC-DAD-ESI-MS/MS. J. Chromatogr. Sci. 2017, 55, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Zabot, G.L.; Moraes, M.N.; Rostagno, M.A.; Meireles, M.A.A. Fast analysis of phenolic terpenes by high-performance liquid chromatography using a fused-core column. Anal. Methods 2014, 6, 7457–7468. [Google Scholar] [CrossRef]
- Milevskaya, V.V.; Temerdashev, Z.A.; Butyl’skaya, T.S.; Kiseleva, N.V. Determination of phenolic compounds in medicinal plants from the Lamiaceae family. J. Anal. Chem. 2017, 72, 342–348. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Murkovic, M. Phenolic Compounds: Occurrence, Classes, and Analysis. In The Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Kidlington/Oxford, UK, 2016; Volume 4, pp. 346–351. ISBN 978-0-12-384953-3. [Google Scholar]
- Oh, Y.; Lee, J.; Yoon, S.; Oh, C.; Choi, D.S.; Choe, E.; Jung, M. Characterization and quantification of anthocyanins in grape juices obtained from the grapes cultivated in Korea by HPLC/DAD, HPLC/MS, and HPLC/MS/MS. J. Food Sci. 2008, 73, C378–C389. [Google Scholar] [CrossRef] [PubMed]
- Kylli, P. Berry Phenolics: Isolation, Analysis, Identification, and Antioxidant Properties. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 26 August 2011. [Google Scholar]
- Bansal, A.; Chhabra, V.; Rawal, R.K.; Sharma, S. Chemometrics: A new scenario in herbal drug standardization. J. Pharm. Anal. 2014, 4, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Areias, F.M.; Valentão, P.; Andrade, P.B.; Moreira, M.M.; Amaral, J.; Seabra, R.M. HPLC/DAD Analysis of Phenolic Compounds from Lavender and its application to quality control. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 2563–2572. [Google Scholar] [CrossRef]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.E.; Komaitis, M. RP-HPLC Analysis of the Phenolic Compounds of Plant Extracts. Investigation of Their Antioxidant Capacity and Antimicrobial Activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, G.S.; Mandić, A.I.; Čanadanović-Brunet, J.M.; Djilas, S.M.; Tumbas, V.T. HPLC Screening of Phenolic Compounds in Winter Savory (Satureja montana L.) Extracts. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 293–306. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid levels in commercial basil (Ocimum basilicum) and Echinacea purpurea products. J. Funct. Foods 2010, 2, 77–84. [Google Scholar] [CrossRef]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Šaponjac, V.T.; Čanadanović-Brunet, J.; Ćetković, G.; Djilas, S. Detection of Bioactive Compounds in Plants and Food Products. In Emerging and Traditional Technologies for Safe, Healthy and Quality Food, 1st ed.; Nedović, V., Raspor, P., Lević, J., Tumbas Šaponjac, V., Barbosa-Cánovas, G.V., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 81–109. ISBN 978-3-319-24040-4. [Google Scholar]
- Yan, R.; Cao, Y.; Yang, B. HPLC-DPPH screening method for evaluation of antioxidant compounds extracted from Semen Oroxyli. Molecules 2014, 19, 4409–4417. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Adholeya, A.; Conlan, X.A.; Cahill, D.M. Acidic Potassium Permanganate Chemiluminescence for the Determination of Antioxidant Potential in Three Cultivars of Ocimum basilicum. Plant Foods Hum. Nutr. 2016, 71, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Schappler, J.; Veuthey, J.L.; Guillarme, D. Current and future trends in UHPLC. TrAC Trends Anal. Chem. 2014, 63, 2–13. [Google Scholar] [CrossRef]
- Yuk, J.; Patel, D.N.; Isaac, G.; Smith, K.; Wrona, M.; Olivos, H.J.; Yu, K. Chemical Profiling of Ginseng Species and Ginseng Herbal Products Using UPLC/QTOF-MS. J. Braz. Chem. Soc. 2016, 27, 1476–1483. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Redruello, B.; Ladero, V.; Del Rio, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. A UHPLC method for the simultaneous analysis of biogenic amines, amino acids and ammonium ions in beer. Food Chem. 2017, 217, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Clausen, A.M. Fundamental and practical aspects of ultrahigh pressure liquid chromatography for fast separations. J. Sep. Sci. 2007, 30, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, X.; Guo, Z.; Feng, J.; Zeng, J.; Xue, X.; Liang, X. Separation and identification of flavonoids from complex samples using off-line two-dimensional liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2012, 1220, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kivilompolo, M.; Hyötyläinen, T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J. Chromatogr. A 2007, 1145, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kumar, A.; Malik, A.K. Flavonoids biosynthesis in plants and its further analysis by capillary electrophoresis. Electrophoresis 2016, 38, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Arries, W.J.; Tredoux, A.G.; Beer, D.; Joubert, E.; Villiers, A. Evaluation of capillary electrophoresis for the analysis of rooibos and honeybush tea phenolics. Electrophoresis 2017, 38, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Patel, J.K.; Patel, M.P.; Rajput, G.C.; Patel, H.A. Introduction to hyphenated techniques and their applications in pharmacy. Pharm. Methods 2010, 1, 2–13. [Google Scholar] [CrossRef]
- Santos, J.; Oliveira, M.B.P.P. Chromatography. In Food Authentication: Management, Analysis and Regulation, 1st ed.; Georgiou, C.A., Danazis, G.P., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 199–232. ISBN 978-1-118-81026-2. [Google Scholar]
- Gross, J.H. Mass Spectrometry: A Textbook, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–17. ISBN 978-3-642-10711-5. [Google Scholar]
- Clarke, W. Mass spectrometry in the clinical laboratory: Determining the need and avoiding pitfalls. In Mass Spectrometry for the Clinical Laboratory, 1st ed.; Nair, H., Clarke, W., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 1–15. ISBN 978-0-12-800871-3. [Google Scholar]
- Rubert, J.; Zachariasova, M.; Hajslova, J. Advances in high-resolution mass spectrometry based on metabolomics studies for food—A review. Food Addit. Contam. Part A 2015, 32, 1685–1708. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, Á. Mass spectrometry instrumentation and techniques. In Medical Applications of Mass Spectrometry, 1st ed.; Vékey, K., Telekes, A., Vertes, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 93–140. ISBN 978-0-444-51980-1. [Google Scholar]
- Boros, B.; Jakabová, S.; Dörnyei, Á.; Horváth, G.; Pluhár, Z.; Kilár, F.; Felinger, A. Determination of polyphenolic compounds by liquid chromatography-mass spectrometry in Thymus species. J. Chromatogr. A 2010, 1217, 7972–7980. [Google Scholar] [CrossRef] [PubMed]
- Nagy, T.O.; Solar, S.; Sontag, G.; Koenig, J. Identification of phenolic components in dried spices and influence of irradiation. Food Chem. 2011, 128, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Downey, F.; Ng, C.K.Y. Comparative analysis of bioactive phytochemicals from Scutellaria baicalensis, Scutellaria lateriflora, Scutellaria racemosa, Scutellaria tomentosa and Scutellaria wrightii by LC-DAD-MS. Metabolomics 2011, 7, 446–453. [Google Scholar] [CrossRef]
- Torras-Claveria, L.; Jauregui, O.; Bastida, J.; Codina, C.; Viladomat, F. Antioxidant Activity and Phenolic Composition of Lavandin (Lavandula x intermedia Emeric ex Loiseleur) Waste. J. Agric. Food Chem. 2007, 55, 8436–8443. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef] [PubMed]
- Chaimbault, P. The Modern Art of Identification of Natural Substances in Whole Plants. In Recent Advances in Redox Active Plant and Microbial Products: From Basic Chemistry to Widespread Applications in Medicine and Agriculture, 1st ed.; Jacob, C., Kirsch, G., Slusarenko, A., Winyard, P.G., Burkholz, T., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 31–94. ISBN 978-94-017-8953-0. [Google Scholar]
- Brice, R.W.; Zhang, X.; Colón, L.A. Fused-core, sub-2 μm packings, and monolithic HPLC columns: A comparative evaluation. J. Sep. Sci. 2009, 32, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.; Quispe, C.; Areche, C. Sepúlveda, B. Phenolic Compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) Analyzed by UHPLC–Q/Orbitrap/MS/MS and Its Antioxidant Properties. Molecules 2016, 21, 245. [Google Scholar] [CrossRef] [PubMed]
- Aceña, J.; Stampachiacchiere, S.; Pérez, S.; Barceló, D. Advances in liquid chromatography–high-resolution mass spectrometry for quantitative and qualitative environmental analysis. Anal. Bioanal. Chem. 2015, 407, 6289–6299. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A. Combining UHPLC and high-resolution MS: A viable approach for the analysis of complex samples? TrAC Trends Anal. Chem. 2014, 63, 113–128. [Google Scholar] [CrossRef]
- Kennedy, J.; Shanks, K.G.; Van Natta, K.; Conaway, M.C.P.; Wiseman, J.M.; Laughlin, B.; Kozak, M. Rapid screening and identification of novel psychoactive substances using PaperSpray interfaced to high resolution mass spectrometry. Clin. Mass Spectrom. 2016, 1, 3–10. [Google Scholar] [CrossRef]
- Peterman, S.M.; Duczak, N., Jr.; Kalgutkar, A.S.; Lame, M.E.; Soglia, J.R. Application of a Linear Ion Trap/Orbitrap Mass Spectrometer in Metabolite Characterization Studies: Examination of the Human Liver Microsomal Metabolism of the Non-Tricyclic Anti-Depressant Nefazodone Using Data-Dependent Accurate Mass Measurements. J. Am. Soc. Mass Spectrom. 2006, 17, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Schmitz, O.J. Comprehensive two-dimensional liquid chromatography tandem diode array detector (DAD) and accurate mass QTOF-MS for the analysis of flavonoids and iridoid glycosides in Hedyotis diffusa. Anal. Bioanal. Chem. 2015, 407, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Senyuva, H.Z.; Gökmen, V.; Sarikaya, E.A. Future perspectives in Orbitrap™-high-resolution mass spectrometry in food analysis: A review. Food Addit. Contam. Part A 2015, 32, 1568–1606. [Google Scholar] [CrossRef] [PubMed]
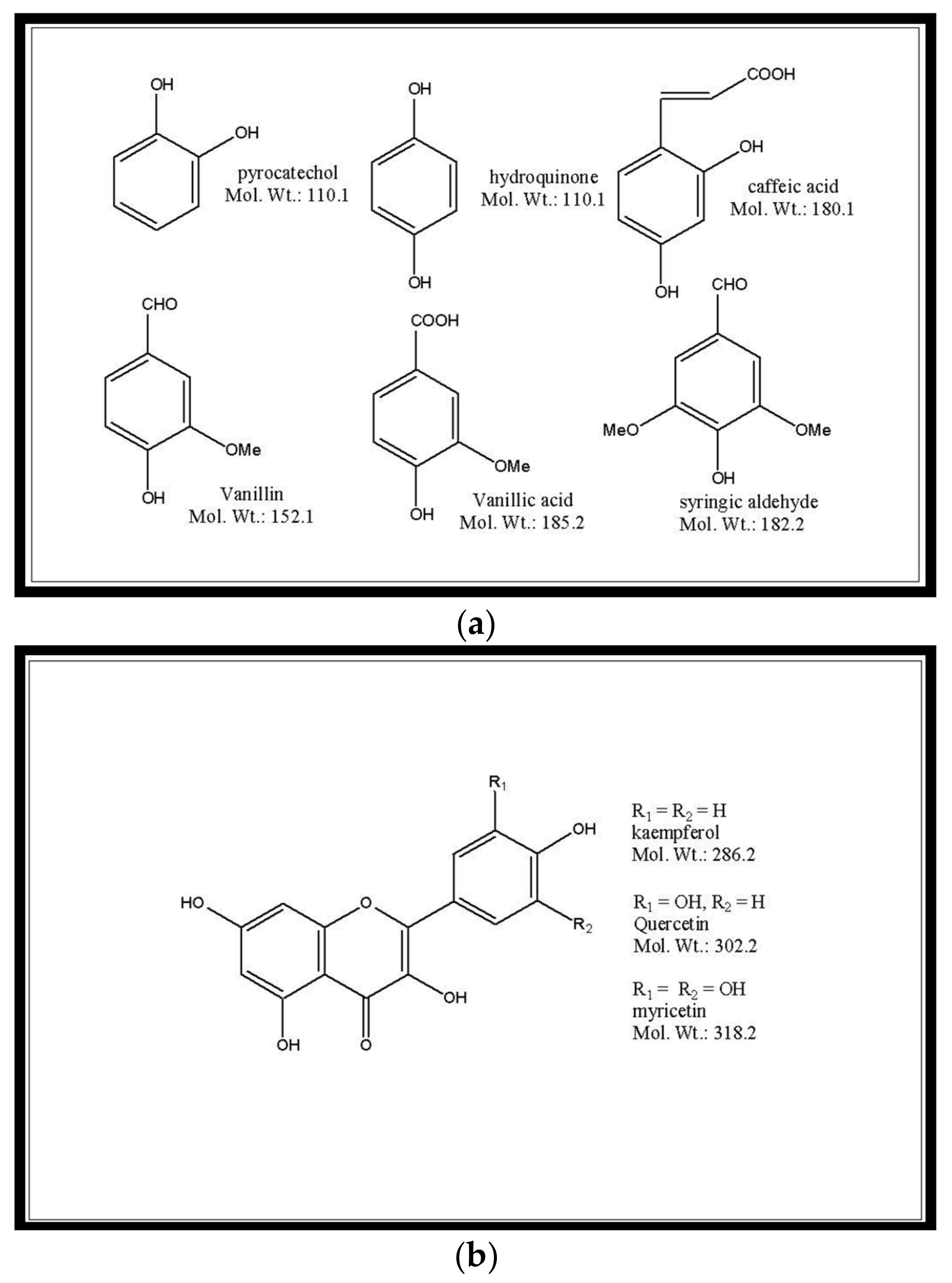
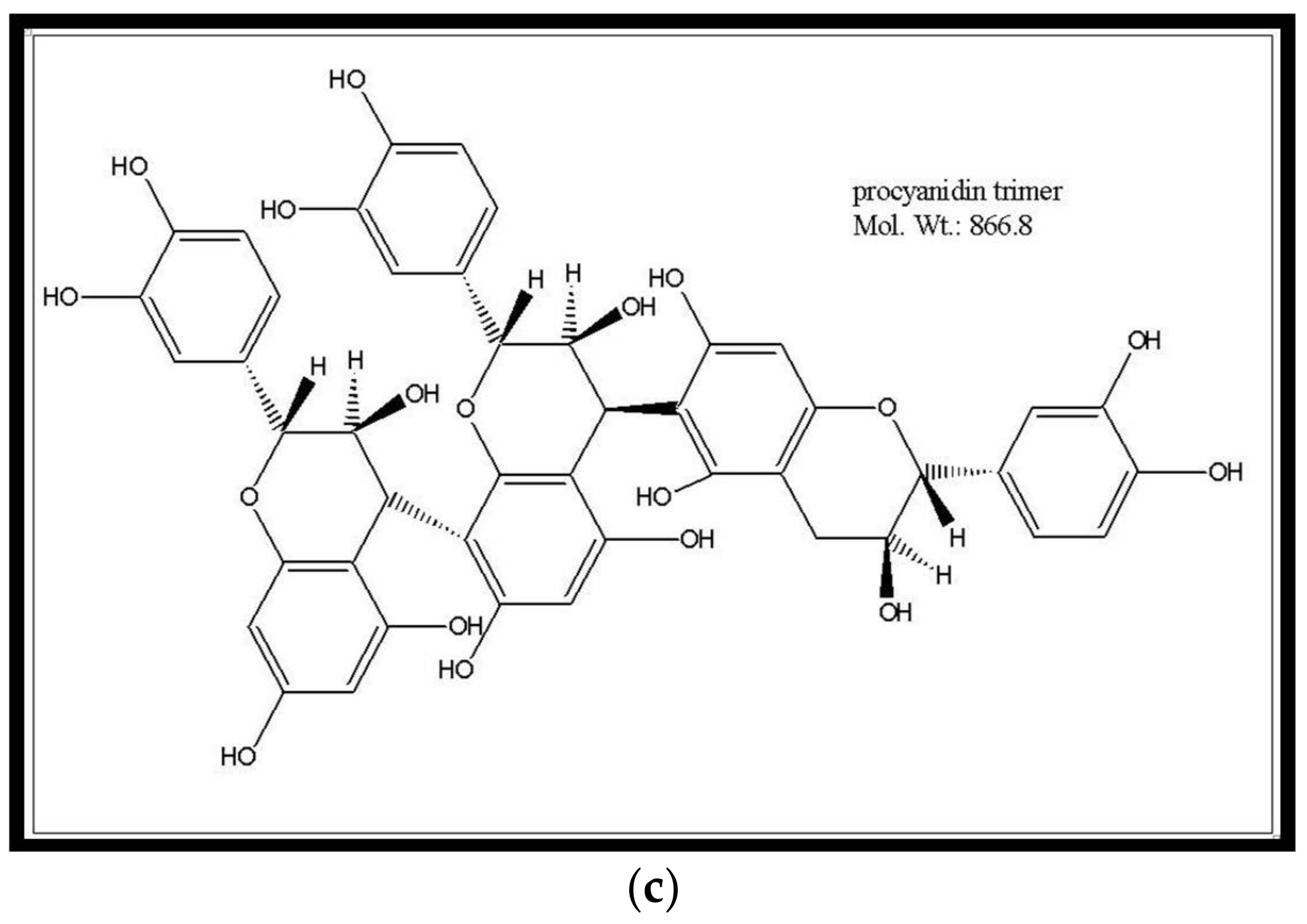
| i) Lamiaceae Species ii) Plant Part | Extraction Process | Polyphenol Classes | i) Solvent ii) Solute: Solvent Ratio | i) Time (t) ii) Temperature (T) | i) Work-up and Conditions ii) Purification/Clean-up 1 | Reference |
|---|---|---|---|---|---|---|
| i) Mentha pulegium; Nepeta nuda ii) Aerial parts | Reflux condensation | Phenolic acids; Flavonoids | i) methanol (MeOH) ii)1:10 weight/volume (w/v) | i) 30 min ii) not available (n/a) | i) Exhaustive-extraction (two times); Filtration ii) n/a | [46] |
| i) Thymus vulgaris ii) Aerial parts | Reflux (hot) extraction | Flavonoids (flavones) | i) MeOH ii) 1:6 (w/v) | i) n/a ii) n/a | i) 3 Extraction Repetitions; Drying (rotary evaporator); Reconstitution of residue (1.5 g residue: 5 mL MeOH); Filtration; Dilution (1:2) with 0.5 mL borax buffer (20 mM, pH 10.0) ii) n/a | [47] |
| i) 11 species of Mentha; 2 Mixtures of Mentha species ii) Plant material; Pharmaceutical products | Soxhlet extraction of residue after chlorophyll removal | Hydroxycinnamic acids; Flavonoids | i) MeOH ii) 1:10 (w/v) | i) 8 h ii) n/a | i) Evaporation (water bath, 0.9 atm); Dissolution of residue to 25 mL with MeOH ii) Isolation of Chlorophylls: Soxhlet extraction with chloroform, 8 h, 20 g of solute | [48] |
| i) Melissa officinalis ii) Fresh herbs or leaves | Sonication | Hydroxybenzoic, Hydroxycinnamic acids | i) 80% aqueous MeOH ii) 1:8 (w/v) | i) 30 min ii) ambient | i) Centrifugation (20,000 rpm, 10 min); Two process repetitions; Combination of extracts; Dilution (Final volume: 25 mL, with 80% aqueous MeOH); Filtration ii) n/a | [49] |
| i) Origanum vulgare ssp. hirtum; Thymus capitatus; Satureja thymbra; Melissa officinalis; Rosmarinus officinalis ii) Aerial parts, dried, grounded leaves and flowers | Sonication | Phenolic acids and their derivatives; Flavonoids; Phenolic monoterpenes | i) 70% aqueous MeOH or water (H2O) ii) 1:8 (w/v) | i) 20 min ii) ≤30 °C | i) Centrifugation (12,500 rpm, 15 min, 4 °C); Filtration ii) n/a | [50] |
| i) Rosmarinus officinalis; Origanum vulgare; Thymus vulgaris; Origanum majorana ii) Dried, grounded | Sonication | Flavonoids; Phenolic acids; Phenolic terpenes | i) 0.1% formic acid in 50% aqueous ethanol (EtOH) ii) 1:5 (w/v) | i) 5 min ii) n/a | i) Centrifugation (3000 g, 10 min, 4 °C); Two repetitions (residue); Combination of extracts; Evaporation with N2; Reconstitution of extracts to 5 mL with 0.1% aqueous formic acid ii) Solid-Phase Extraction (SPE: Dilution (1 mL extract, 1 mL H2O, 34 µL 35% hydrochloric acid (HCl)); Equilibration (1 mL MeOH, 1 mL sodium acetate 50 mmol/L, pH 7); Rinsing (sodium acetate 50 mmol/L, pH 7.5% MeOH); Elution of polyphenols (1800 µL 2% formic acid in MeOH); Evaporation (N2); Residue dilution to 250 µL with 1% formic acid in H2O); Filtration | [11,51] |
| i) Mentha pulegium; Origanum majorana ii) Aerial parts | Sonication | Flavonoids; Hydroxybenzoic, Hydroxycinnamic acids and their derivatives | i) MeOH ii) 1:10 (w/v) | i) 30 min ii) ambient | i) Centrifugation (3500 rpm, 10 min); four repetitions; Collection of supernatants; Evaporation (reduced pressure, 35 °C); Residue re-constitution to 2 mL with MeOH; Filtration ii) n/a | [52] |
| i) Rosmarinus officinalis ii) Branded extract rich in carnosic acid | Sonication | Flavonoids (mainly flavones); Phenolic terpenes (diterpenoids and derivatives); Phenolic acids | i) 2% formic acid in acetonitrile (MeCN) ii) 1:6.7 volume/volume (v/v) | i) 10 min ii) n/a | i) Centrifugation (10,480 g, 5 min, Ambient T); Direct injection after centrifugation ii) n/a | [53] |
| i) 6 Ocimum spp. ii) Leaves, dried, grounded | Sonication (53 kHz) | Phenolic acids; Flavonoids; Propenyl phenols; Terpenoids | i) 80% aqueous MeOH ii) 1:10 (w/v) | i) 30 min ii) ambient | i) Maintenance 24 h (22–24 °C); Filtration; Evaporation (reduced pressure, 40 °C) ii) Sonication of the residue (1 mg) in MeCN (1 mL) Filtration (0.22 µm filter); Dilution to 30 ng/mL (MeCN); Spiking (andrographolide). | [54] |
| i) Satureja montana ssp. kitaibelii ii) Aerial parts of wild plant, air-dried, milled | Solid-liquid extraction, Sonication | Hydroxybenzoic, Hydroxycinnamic acids; Phenyl acetic acids; Flavonoids (flavones, flavonols) | i) 60%, 70% and 80% aqueous MeOH, EtOH and acetone ii) 1:10 (w/v) | i) 10 min ii) n/a | i) Centrifugation (1000 g, 15 min); Removal of supernatant and exhaustive extractions (three repetitions); Evaporation of supernatants; Reconstitution in MeOH: H2O 50:50 (v/v) (1 mL); Filtration ii) n/a | [55] |
| i) Mentha spicata ii) Commercial extract | Solid-liquid extraction, Sonication | Hydroxybenzoic, hydroxycinnamic acids; Flavonoids (flavones, flavonols) | i) 80% aqueous MeOH with 1% formic acid ii) 1:5 (w/v) | i) 25 min ii) ambient | i) Centrifugation (10,480 g, 5 min, ambient T); Exhaustive extraction (three repetitions: on the same sample) ii) n/a | [56] |
| i) 3 Mentha spp. ii) Dried and powdered leaves | Solid-liquid extraction of defatted residues | Phenolic acids; Flavonoids | i) EtOH ii) 1:40 (w/v) | i) 24 h ii) ambient | i) Filtration (on cellulose); Concentration (vacuum evaporator, 40 °C) ii) Defatting: Stirring (130 rpm); 25 g of sample in n-hexane (600 mL); Ambient T; 3 h | [57] |
| i) Origanum vulgare; Ocimum basilicum; Rosmarinus officinalis; Origanum majorana; Thymus vulgaris; Satureja hortensis ii) Commercial, dried, grounded leaves | Shaking, Solid-liquid extraction | Phenolic acids | i) 70% aqueous EtOH ii) 1:10 (w/v) | i) 2 h ii) ambient | i) Filtration; Vacuum evaporation (40 °C); Freeze-drying; Analysis concentration: 0.1% (w/v) ii) n/a | [2] |
| i) Thymus vulgaris; Salvia officinalis ii) Aerial parts | Maceration (herbal tinctures) | Phenolic acids (hydroxycinnamic acids); Flavonoids (flavonols, flavones) | i) 70% aqueous EtOH ii) n/a | i) 7 days ii) n/a | i) (According to the Polish Pharmacopoeia VI protocol) ii) n/a | [58] |
| i) Thymus x citriodorus ii) Mixture of leaves and stems, dried | Maceration of residue (defatted) | Phenolic acid derivatives; Flavonoids (flavones, flavonols, flavanones) | i) 80% aqueous EtOH ii) 1:60 (w/v) | i) 30 min ii) ambient | i) Filtration; Four re-extractions of residue; Combination of extracts; Lyophilization ii) Defatting: Maceration with n-hexane (150 mL); 5 g of sample; 30 min; Ambient (T); three repetitions | [59] |
| i) Origanum majorana ii) Commercially produced, dried, grounded | Solid-liquid extraction | Flavonoids; Phenolic acids | i) 80% MeOH ii) 1:10 (w/v) | i) 6 h, 16 h ii) 23 °C | i) Filtration; Combination of extracts; Drying (rotary evaporator, 50 °C); Dissolution in H2O (16.5 g/500 mL) ii) Liquid-liquid partitioning for flash chromatography (FC): ethyl acetate (AcOEt) (500 mL) in H2O (500 mL) with 16.5 g of extract; Dissolution of polar part (14.7 g) in H2O (50 mL) and non-polar part (1.7 g) in AcOEt (50 mL) | [60] |
| i) Rosmarinus officinalis, Origanum majorana, Origanum vulgare Ocimum basilicum, Mentha spicata, Thymus vulgaris Mentha x piperita, Thymus x citriodorus ii) Fresh; Dried; Organic dried | Solid-liquid extraction aided by shaking | Hydroxybenzoic, hydroxycinnamic acids; Flavonoids; Phenolic terpenes | i) MeOH ii) 1:100 (dried) (w/v)/1:12.5 (fresh) (w/v) | i) 10 min ii) n/a | i) Centrifugation (2000 rpm, 10 min); Residue re-extraction (initial conditions); Combination of supernatants; Evaporation (40 °C, Final Volume: 5 mL); Dilution to 10 mL with MeOH ii) n/a | [14] |
| i) Origanum vulgare ii) Herb sample from 2 different sources, dried | Solid-liquid extraction aided by shaking (Soluble, Bound extracts) | Hydroxycinnamic, hydroxybenzoic acids;Phenolic monoterpenes (Soluble extracts) Hydroxycinnamic, hydroxybenzoic acids (Bound extracts) | i) 80% aqueous MeOH (Soluble extracts); 2 M sodium hydroxide (NaOH) (Bound extracts) ii) 1:20 (w/v) (Soluble extracts); n/a (Bound extracts) | i) 24 h (Soluble extracts); 4 h (Bound extracts) ii) ambient | i) Soluble extracts: Centrifugation (2000 g, 30 min, Ambient T); Supernatant and soluble fraction collection Bound extracts: pH 2.0 with 6 M HCl; Centrifugation (2000 g, 30 min, ambient T); Collection of supernatant; Extraction (15 mL 1:1 (v/v) Diethylether: AcOEt-three times); Evaporation of organic layers (30 °C); Dissolution to 10 mL with 80% aqueous MeOH ii) n/a | [13] |
| i) Sicilian Origanum vulgare ssp. hirtum, Rosmarinus officinalis, Thymus capitatus L. ii) Dried-aerial parts, flowering season samples from various sites | Solid-liquid extraction (Nonvolatile fraction); Hydrodistillation (Volatile fraction) | Flavonoids (flavones, flavanones) (Nonvolatile fraction); Phenolic terpenes (Volatile fraction) | i) AcOEt and EtOH (Nonvolatile fraction); n/a (Volatile fraction) ii) 1:6.7 (w/v) (3 times) (Nonvolatile fraction); n/a (Volatile fraction) | i) Overnight in the dark (Nonvolatile fraction); 3 h (Volatile fraction) ii) ambient | i) Nonvolatile fraction: Storage: 4 °C, N2-rich atmosphere; Analysis concentration: Dissolution of 10–20 mg of each sample in MeOH (1.5 mL); Filtration. Volatile fraction: (According to European Pharmacopoeia); Drying with sodium sulfate anhydrous (Na2SO4); Storage: under N2 ii) Nonvolatile fraction: Defatting with n-hexane; 30 g dried, grounded aerial parts/200 mL; 3 times | [61,62,63] |
| i) Thymus serpyllum ii) Whole-dried | Solid-liquid extraction (Phenolic fraction); Purge & Trap (N2, 500 mL N2/min) followed by SPE (Volatile fraction) | Flavonoids; Phenolic acids; Phenolic terpenes (monoterpenes) | i) 75% aqueous MeOH (Phenolic fraction); adsorbent: Lichrolut EN (Volatile fraction) ii) 1:4 (w/v) (Phenolic fraction); 3 g/200 mg (Volatile fraction) | i) 2hr (Phenolic fraction); 90 min (Volatile fraction) ii) n/a | i) Phenolic fraction: Residue washing (5 mL of 75% aqueous MeOH); Combination of extracts; Filtration; Vacuum evaporation (20 °C). Volatile fraction: Elution (Dichloromethane); Dehydration (Anhydrous Sodium Sulphate); Concentration (5 mL, Snyder column, 40 °C); Re-concentration to 0.5 mL (N2); Filtration ii) n/a | [64] |
| i) Mentha australis R. Br ii) Fresh leaves and stems | Solid-liquid extraction following sonication | Phenolic acids; Flavonoids (flavanone glycosides) | i) 80% aqueous MeOH ii) 1:20 (w/v) | i) 10 min, 2 h; overnight ii) 4 °C | i) Extraction 1: Centrifugation (10,000 g, 15 min). Extraction 1, 2, 3: Combination of supernatants; Solvent evaporation (vacuum rotary evaporator, 40 °C) ii) Purification: Glass column (25 × 300 mm i.d.); 50 mL extract; Addition of Amberlite resin; Washing with H2O; Elution with 80% aqueous MeOH; Vacuum evaporation (40 °C); Lyophilization (−109 °C, 0.015 k Pa); Analysis concentration: 1 mg (lyophilized, purified) extract/mL MeOH | [65] |
| i) 3 species of Salvia ii) Aerial parts, dried, pulverized | Solid-liquid extraction of the residue obtained after removal of lipophilic substances | Flavonoids (flavones, flavone glycosides) | i) Hot H2O (~90 °C) ii) 1:40 (w/v) | i) Left to reach ambient (T) ii) n/a | i) Partitioning (3 × 100 mL AcOEt, 3 × 100 mL n-butanol); Combination of organic phases; Drying (anhydrous magnesium sulfate); Drying (rotary evaporator, 40 °C; Dissolution to 3 mL with MeOH ii) Lipophilic content removal: Shaking (5 g of pulverized sample in n-hexane (100 mL), 30 °C, 2 h); Filtration; Stirring overnight (30 °C, 100 mL MeOH: dichloromethane 1:1); Filtration; Drying (rotary evaporator, 40 °C) | [66] |
| i) Rosmarinus officinalis ii) Leaves from 20 geographical zones | Microwave assisted extraction (MAE); two pre-heating steps (160 and 320 W); two extraction cycles (800 W) | Flavonoids; Phenolic diterpenes | i) 70% aqueous MeOH ii) 1:12.5 (w/v) | i) Each pre-heating step:1 min; Heating gaps: 15 s; Each extraction cycle: 5 min ii) n/a | i) Combination of extracts (two extraction cycles); Filtration; Evaporation (rotary evaporator); Analysis concentration: 800 μg/mL in 50% aqueous MeOH; Filtration ii) n/a | [67] |
| i) (a) Origanum majorana; (b) Mentha pulegium; (c) Lavandula officinalis ii) (a) Leaves and aerial parts; (b) Flowers; (c) Leaves, dried, milled | MAE (500 W) | Flavonoids Hydroxycinnamic, hydroxybenzoic acids | i) 60 and 80% aqueous MeOH, EtOH and acetone ii) 1:15 (w/v) | i) 15 min ii) 80 °C | i) Irradiation process: 3 min heating for reaching 80 °C, 3 min for balancing at 80 °C, 5 min for cooling; Filtration ii) n/a | [68] |
| i) Rosmarinus officinalis; Salvia officinalis; Origanum vulgare; Thymus vulgaris ii) Leaves, or herbalmix, or as ingredients in chimichurri sauce | Supercritical fluid extraction—carbondioxide (SFE-CO2); Soxhlet Low Pressure Solvent Extraction (LPSE) (17.3 g/min); Ultrasound assisted extraction (UAE) (40 kHz;1 bar; 20 g of CO2/g raw material solvent) | Phenolic terpenes (diterpenes) | i) CO2 for SFE; EtOH for Soxhlet LPSE and UAE; ii) n/a for SFE and UAE; 1:30 for Soxhlet and UAE. | i) 6 h ii) 40 °C for SFE and S; n/a for Soxhlet; 50 °C for UAE | i) n/a for SFE; Vacuum evaporation (40 °C) for Soxhlet and UAE ii) n/a | [69] |
| i) 10 Salvia species ii) Plant material, dried | SFE-CO2 (45 MPa, CO2: 2 L/min) Accelerated solvent extraction (ASE) (10.3 MPa) | Flavonoids; Phenolic terpenes; Hydroxybenzoic, hydroxycinnamic acids; Phenolic acids (caffeic acid derivatives) | i) CO2 (99.9%) for SFE; 96% EtOH, followed by H2O for ASE ii) n/a for SFE; 3:1 in diatomaceous earth for ASE | i) 60 min. (SFE-CO2); 30 min. (ASE) ii) 60°C for SFE; 140 °C for ASE | i) ASE: EtOH evaporation; Lyophilization of H2O extracts ii) n/a | [3] |
| i) Salvia officinalis, Thymus serpyllum, Origanum vulgare, Melissa officinalis ii) Plant raw material, grounded | Heating; MAE; Sonication; Subcritical extraction | Phenol carboxylic; Cinnamic acids; Flavonoids; Phenolic terpenes (diterpenes) | i) 70% aqueous EtOH ii) 1:50 (w/v) | i) n/a ii) n/a | i) (According to the Russian State Pharmacopoeia, FS.2.5.0051.15). Centrifugation; Filtration ii) n/a | [70] |
| i) Lamiaceae Species ii) Plant Part | Polyphenols Analysed 1 | Chromatography | Detection System | Chromatographic Conditions and Method Validation Results | Reference(s) |
|---|---|---|---|---|---|
| i) Thymus vulgaris ii) Aerial parts | C17, C21 | Capillary electrophoresis (CE) | UV-diode array detector (DAD) | Capillary: Fused silica (66 cm length, 58 cm effective length, 75 mm internal diameter (i.d.)) Capillary (T): 23 °C Background electrolyte solution: borax buffer (20 mM, pH 10.0): 90% MeOH Driving voltage: 23 kV limit of detection (LOD) for C17: 0.53 μg/mL, LOD for C21: 1.05 μg/mL limit of quantification (LOQ) for C17: 1.41 μg/mL, LOQ for C21: 2.98 μg/mL correlation/determination coefficient (R2) for C17: 0.9990, (R2) for C21: 0.9999 | [47] |
| i) Melissa officinalis ii) Fresh herbs or leaves from 12 manufacturers | C57, C59, C63, C64, C66, C67 | High performance liquid chromatography (HPLC) | UV/Vis | Column: Hypersil GOLD C18 (250 mm × 4.6 mm i.d., 5.0 µm particle size (p.s.)) (T): 30 °C Eluents: (A) 0.05% trifluoroacetic acid (TFA) in MeOH; (B) 0.05% TFA in H2O Run (t): 35 min LOD: 0.16–0.51 µg/mL, LOQ: 0.42–1.54 µg/mL, (R2): ≥0.9089 | [49] |
| i) Mentha pulegium, Nepeta nuda ii) Aerial parts | C17, C19, C21, C22, C33, C41, C44, C45, C46, C57, C59, C64 | HPLC | UV-photodiode array (PDA) detector | Column: LiChrospher 100 RP C18 endcapped (250 mm × 4.6 mm i.d., 5.0 µm p.s.) Eluents: (A) H2O containing 0.02% phosphoric acid and (B) MeCN Run (t): 70 min | [46] |
| i) Origanum vulgare ii) Herb sample from different sources, dried | C15, C16, C34, C36, C38, C55, C56, C57, C58, C59, C61, C63, C66, C69, C71, C75 | HPLC | DAD | Column: Zorbax SB-Aq (250 mm × 4.6 mm i.d., 5.0 µm p.s.) Eluents: (A) 0.5% formic acid in H2O; (B) MeOH Run (t): 95 min | [13] |
| i) Salvia officinalis, Thymus serpyllum, Origanum vulgare, Melissa officinalis ii) Plant (raw) material | C22, C23, C46, C57, C58, C59, C61, C63, C64, C65, C66, C68, C69, C70, C78 | HPLC | DAD | Column: Phenomenex Luna C18 (250 mm × 4.6 mm i.d., 5.0 µm p.s.) (T): 40 °C Eluents: (A) MeCN; (B) 1% acetic acid in H2O Run (t): 35 min LOD: 0.10–0.30 µg/mL, (R2): ≥ 0.999 | [70] |
| i) Origanum vulgare ssp. hirtum, Thymus capitatus, Satureja thymbra, Melissa officinalis, Rosmarinus officinalis ii)Aerial parts, dried, grounded leaves and flowers | C1, C17, C21, C34, C36, C37, C40, C46, C48, C50, C57, C58, C59, C60, C61, C63, C64, C66, C67, C68, C69, C70, C74, C75 | RP-HPLC | DAD | Column: Nucleosil 100 C18 (250 mm × 4.6 mm i.d., 5.0 µm p.s.) (T): 30 °C Eluents: (A) 1% acetic acid in H2O; (B) MeCN; (C) MeOH Run (t): 55 min LOD: 0.002–0.16 µg/mL, LOQ: 0.01–0.48 µg/mL, (R2): ≥ 0.9961 | [50] |
| i) (a) Rosmarinus officinalis, Origanum majorana, Origanum vulgare; (b) Ocimum basilicum, Mentha spicata, Thymus vulgaris; (c) Mentha x piperita, Thymus x citriodorus ii) (a) Fresh; (b) Dried; (c) Organic-dried | C5, C16, C36, C40, C50, C55, C58, C59, C61, C62, C63, C64, C66, C69, C74, C75, C78, C79 | ultra-high-performance liquid chromatography (UHPLC) | DAD | Column: Acquity ‘ethylen e bridged hybrid (BEH C18 (50 mm × 2.1 mm i.d., 1.7 μm p.s.) with an Acquity UHPLC BEH C18 VanGuard pre-column (5 mm × 2.1 mm i.d., 1.7 μm p.s.) (T): 20 °C Eluents: (A) 0.1% acetic acid in H2O; (B) 0.1% Acetic acid in MeCN Run (t): 30 min LOD: 0.01–0.38 µg/mL, LOQ: 0.04–1.14 µg/mL, (R2): ≥0.9990 | [14] |
| i) 3Mentha ssp. ii) Dried and powdered leaves | C1, C4, C7, C21, C28, C46, C48, C57, C58, C59, C63, C64, C66, C70 | HPLC | DAD | Column: GraceTM AlltechTM AlltimaTM C18 (250 mm × 4.6 mm i.d., 5.0 µm p.s.) (T): 40 °C Eluents: (A) MeCN: H2O: formic acid (19:80:1); (B) MeCN: MeOH: formic acid (59:40:1) Run (t): 45 min | [57] |
| i) (a) 11 species of Mentha, (b) 2 Mixtures of Mentha species ii) (a) Plant material; (b) Finished Pharmaceutical products (2 Manufactures) | C1, C3, C10, C17, C21, C22, C28, C32, C46, C57, C63 | two-dimensional micro-thin layer chromatography (2D-mTLC) | UV | Plate: HPTLC CNF 254 (10 cm × 10 cm, in 5 cm × 5 cm squares) Derivatization reagent: Naturstoff reagent 1st Condition: Non-aqueous eluent: 40% propan-2-ol in n-heptane; Aqueous eluent: 30% MeCN 2nd Condition: Non-aqueous eluent: 80% AcOEt in n-heptane; Aqueous eluent: 50% aqueous MeOH Sample quantity: 5 µL Conditioning: 20–30 min | [48] |
| i) Thymus vulgaris; Salvia officinalis ii) Aerial parts | C17, C19, C21, C22, C40, C45, C46, C57, C63, C64, C74 | TLC | UV | Plate: Pre-coated silica gel TLC plates Si60 F254 Derivatization reagent: natural products-polyethylene glycol reagent (NP/PEG); 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•_ in 0.2% in MeOH; Wavelength: 366 nm Eluents: For flavonoid aglycones: toluene: diethyl ether: acetic acid (60:40:10); For flavonoid glycosides: AcOEt: acetic acid: formic acid: H2O (100:11:11:26); For phenolic acids: chloroform: ethyl acetate: acetone: formic acid (40:30:20:10) | [58] |
| HPLC | DAD; MS in positive ion mode | Column: Zorbax Eclipse Plus PAH C18 (100 mm × 2.1 mm i.d. × 1.8 µm p.s.) Eluents: (A) 0.1% formic acid in H2O; (B) 0.1% formic acid in MeCN Run (t): 30 min | |||
| i) Origanum vulgare, Ocimum basilicum, Rosmarinus officinalis, Origanum majorana, Thymus vulgaris, Satureja hortensis ii) Commercial, dried, grounded leaves | C63, C81 | HPLC | UV-DPPH•; electrospray ionization (ESI)-MS in negative and positive ion mode | Column: Synergi Max-RP C12 (250 mm × 4.6 mm i.d., 4.0 µm p.s.) (T): 25 °C Eluents: (A) 0.05% TFA in H2O; (B) 60% MeCN in MeOH Run (t): 60 min | [2] |
| i) Rosmarinus officinalis; Origanum vulgare; Salvia officinalis; Thymus vulgaris; Origanum vulgare ii) Leaves, or herbal mix, or as ingredients in chimichurri sauce | C63, C76 *, C78, C79 *, C80 * | HPLC | PDA | Column: Kinetex Polar C18 (250 mm × 4.6 mm i.d., 2.6 µm p.s.) (T): 55 °C Eluents: (A) 0.1% acetic acid in H2O; (B) 0.1% acetic acid in MeCN Run (t): 10 min LOD: 0.25 μg/mL, LOQ: 1.0 μg/mL, (R2): ≥0.9998 | [69] |
| UHPLC | MS in negative ion mode | Column: Acquity UHPLC BEH C18 (50 mm × 2.1 mm i.d., 1.7 µm p.s.) (T): 55 °C Eluents: (A) 0.1% acetic acid in H2O; (B) 0.1% acetic acid in MeCN Run (t): 10 min | |||
| i) 3 species of Savlia ii) Aerial parts, dried | Tentative identification only | LC | DAD-ESI-MS in positive ion mode | Column: Phenomenex Superspher 100 RP C18 (125 mm × 4.6 mm i.d. × 4.0 µm p.s.) (T): 40°C Eluents: (A) 2.5% acetic acid in H2O; (B) MeOH Run (t): 30 min | [66] |
| i) Satureja montana ssp. kitaibelii ii) Aerial parts of wild plant, air-dried | C17, C40, C46, C57, C59, C64, C69, C73 | HPLC | DAD–ESI-time-of flight (TOF)–MS | Column: Agilent Poroshell 120 C18 endcapped (100 mm × 4.6 mm i.d., 2.7 µm p.s.) (T): 25 °C Eluents: (A) 1% acetic acid in H2O; (B) MeCN Run (t): 36 min LOD: 0.187–2.471 μg/mL, LOQ: 0.623–8.238 μg/mL, (R2): ≥0.9983 | [55] |
| i) Sicilian Origanum vulgare ssp. hirtum, Rosmarinus officinalis, Thymus capitatus L. ii) Dried-aerial parts, flowering season, samples from various sites | C1, C9, C13, C14 *, C17, C21, C57, C63, C74 *, C75 *, C78 *, C79 *, C80 * | HPLC | PDA/ESI-MS in positive and negative ion mode | Column: Phenomenex Luna C18 endcapped (250 mm × 4.6 mm i.d., 5.0 µm p.s.) (T): 25 °C Eluents: (A) 1% formic acid in H2O; (B) MeCN Run (t): 64 min | [61,62,63] |
| GC | flame ionization detector (FID)/MS | Column: SPB-5 capillary (15 m length × 0.1 mm i.d. × 0.15 μm thickness) Injection: Split ratio (1:200)Oven (T): 60 °C for 1 min, linearly rising from 60 to 280 °C with a rate of 10 °C/min, 280 °C for 1 min | |||
| i) (a) Origanum majorana; (b) Mentha pulegium; (c) Lavandula officinalis ii) (a) Leaves and aerial parts; (b) Flowers; (c) Leaves, dried, milled | C1, C17, C34, C40, C46, C48, C51, C52, C57, C58, C59, C60, C63, C66, C67, C68, C69 | UHPLC | DAD; ESI-tandem mass spectrometry (MS/MS) in negative ion and multiple reaction monitoring (MRM) mode | Column: Acquity UHPLC BEH C18 (100 mm × 2.1 mm i.d., 1.7 µm p.s.) (T): 30 °C Eluents: (A) 1% formic acid in H2O; (B) 1% formic acid in MeOH Run (t): 12 min LOD: 0.02–5.52 ng/mL, LOQ: 0.06–18.20 ng/mL, linear regression (r): ≥0.9988 | [68] |
| i) Thymus x citriodorus ii) Mixture of leaves and stems, dried | C2, C8, C19, C20, C20 *, C22, C23 *, C24, C63 | RP-HPLC | DAD; ESI–MS and multi-stage mass spectrometry (MSn) in negative ion mode; nuclear magnetic resonance (NMR) | Column: Nucleosil C18 endcapped (250 mm × 4.0 mm i.d., 5.0 µm p.s.) (T): 30 °C Eluents: (A) 0.1% formic acid in H2O; (B) MeCN Run (t): 30 min LOD: 1.0–12.4 µg/mL, LOQ: 3.0–37.7 µg/mL, (R2): ≥0.9984 | [59] |
| i) Origanum majorana ii)Commercially produced, dried/grounded | C17, C22, C37, C40, C62 *, C63, C66 | LC | ESI-MS/MS in negative ion mode; 1H NMR | Column: Atlantis T3 C18 (100 mm × 2.1 mm i.d. × 3 µm p.s.) (T): 40 °C Eluents: (A) 0.5% formic acid in H2O; (B) 0.5% formic acid in (MeCN: MeOH, 50:50) Run (t): 26 min | [60] |
| i) Rosmarinus officinalis; Origanum vulgare; Origanum majorana; Thymus vulgaris ii) Dried, grounded | C34, C36, C40, C57, C58, C59, C63, C67, C64, C69, C70 | LC | PDA; ESI-linear ion trap quadrupole (LTQ)-Orbitrap-MS in negative ion mode | Column: Atlantis T3 RP C18 (100 mm × 2.1 mm i.d., 3 µm p.s.) (T): 25 °C Eluents: (A) 0.1% formic acid in H2O; (B) 0.1% formic acid in MeCN Run (t): 36 min LOD: 1.7 × 10−3–8.9 × 10−3 µg/g DW | [11,51] |
| i) Rosmarinus officinalis ii) Leaves from 20 differentgeographical zones | C6, C22, C25, C26, C27 *, C35 *, C37, C63, C67, C78, C79 | HPLC | ESI-QTOF-MS and MS/MS in negative ion mode | Column: Zorbax Eclipse Plus C18 (150 mm × 4.6 mm i.d., 1.8 µm p.s.) (T): ≈20–25 °C Eluents: (A) 0.1% formic acid in H2O; (B) MeCN Run (t): 30 min LOD: 0.014–0.24 µg/mL, LOQ: 0.04–0.8 µg/mL, (R2): ≥0.9803 | [67] |
| i) Mentha pulegium, Origanum majorana ii) Aerial parts | C13, C17, C21, C37, C54 | RP-UHPLC | ESI-QTOF-MS and MS/MS in negative ion mode | Column: Zorbax Eclipse Plus C18 (150 mm × 4.6 mm i.d., 1.8 µm p.s.) (T): 25 °C Eluents: (A) 0.5% acetic acid in H2O; (B) MeCN Run (t): 33 min | [52] |
| i) Mentha spicata ii) Commercial extract | C3, C31, C46, C63, C64, C65, C82, C83, C84 | UHPLC | ESI-MSn in negative ion mode | Column: BlueOrchid C18 (50 mm × 2.0 mm i.d., 1.8 µm p.s.) (T): 30 °C Eluents: (A) 0.1% formic acid in H2O; (B) 0.1% formic acid in MeCN Run (t): 20 min | [56] |
| i) Thymus serpyllum ii) Whole-dried | C7, C21, C22 *, C39, C46, C57, C63, C64, C66, C69, C74, C75 | RP-LC | DAD-ESI-MS/MS FID; mass selective detector (MSD); | Column: Phenomenex RP C18 (250 mm × 4.6 mm i.d. × 5.0 µm p.s.) (T): 25 °C Flow rate: 0.7 mL/min Eluents: (A) 1% formic acid in H2O; (B) (MeCN/Solvent A) (60:40) Run (t): 106 min | [64] |
| GC | mass spectrometry-olfactometry (MS-O) | Column: DB-Wax column (30 m length × 0.25 mm i.d. × 0.5 μm thickness) Injection: Pulsed splitless (40 psi; 0.5 min) Injector (T): 270 °C FID (T): 280 °C Oven (T): 250 °C for 10 min (50–250 °C with a rate of 4 °C/min) | |||
| i) Rosmarinus officinalis ii) Branded extract rich in carnosic acid | C4, C18, C46, C57, C63, C76, C77, C78, C79 | UHPLC | ESI-MSn in negative ion mode | Column: XSelect HSS T3 C18 (50 mm × 2.1 mm i.d., 2.5 µm p.s.) (T): 30 °C Eluents: (A) 0.1% formic acid in H2O; (B) 0.1% formic acid in MeCN Run (t): 35 min | [53] |
| i) Mentha australis R. Br ii) Fresh leaves and stems | C1, C4, C5, C11 *, C17, C57, C63, C64 | HPLC | PDA | Column: Phenomenex Luna C18 endcapped (250 mm × 4.6 mm i.d., 5.0 µm p.s.) (T): 30 °C Eluents: (A) 2.5% acetic acid in H2O; (B) MeCN Run (t): 34 min Fraction collection (major peaks): Column: Phenomenex Luna 10 μm C18 (250 mm × 15 mm) Eluents: Similar to HPLC Run (t): Similar to HPLC | [65] |
| LC | Heated electrospay ionization (HESI)/atmospheric pressure chemical ionization | Similar conditions with HPLC. LOD: 0.25 ng | |||
| LC | (APCI)-MS/MS positive and negative ion mode; NMR HESI/APCI-high resolution mass spectrometry (HRMS) in positive and negative ion mode | Column: Phenomenex Synergi Hydro-RP C18 (250 mm × 1.0 mm i.d., 4.0 µm p.s.) (T): 45 °C Eluents: (A) 5 mM ammonium formate in H2O (pH 7.4) (B) 5 mM ammonium formate in 90% aqueous MeOH (pH 7.4) Run (t): 19 min LOD: 0.625 ng | |||
| i) 6 Ocimum ssp. ii) Leaves, dried, grounded | C17, C21, C40, C43, C46, C48, C49, C55, C57, C59, C60, C63, C64, C66, C69 | UHPLC | ESI-hybrid linear ion trap (QqQLIT) in negative ion mode | Column: Acquity UHPLC BEH C18 (50 mm × 2.1 mm i.d., 1.7 µm p.s.) (T): 50 °C Eluents: (A) 0.1% formic acid in H2O; (B) 0.1% formic acid in MeCN Run (t): 13 min LOD: 0.041–0.357 ng/mL, LOQ: 0.124–1.082 ng/mL | [54] |
| i) 10 Salvia species ii) Plant material, dried | C17, C20, C21, C23, C29, C30, C42, C45, C47, C53, C57, C63, C71, C78, C79 | HPLC | UV-DPPH•-MS PDA | Column: Discovery HS C18 (250 mm × 4.6 mm i.d., 5.0 µm p.s.) Flow rate: 0.8 mL/min Injection Volume: 20 µL Eluents: (A) 0.1% formic acid in H2O; (B) MeOH Run (t): 60 min | [3] |
| UHPLC | ESI-QTOF, triple quadrupole-spectrometer (TQ-S) in negative mode | Column: Acquity UHPLC BEH C18 (100 mm × 2.1 mm i.d., 1.7 µm p.s.) (T): 40 °C Eluents: (A) 0.1% formic acid in H2O; (B) MeCN Run (t): 11 min LOD: 1.67–13.39 µg/mL, LOQ: 5.56–44.65 µg/mL |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzima, K.; Brunton, N.P.; Rai, D.K. Qualitative and Quantitative Analysis of Polyphenols in Lamiaceae Plants—A Review. Plants 2018, 7, 25. https://doi.org/10.3390/plants7020025
Tzima K, Brunton NP, Rai DK. Qualitative and Quantitative Analysis of Polyphenols in Lamiaceae Plants—A Review. Plants. 2018; 7(2):25. https://doi.org/10.3390/plants7020025
Chicago/Turabian StyleTzima, Katerina, Nigel P. Brunton, and Dilip K. Rai. 2018. "Qualitative and Quantitative Analysis of Polyphenols in Lamiaceae Plants—A Review" Plants 7, no. 2: 25. https://doi.org/10.3390/plants7020025
APA StyleTzima, K., Brunton, N. P., & Rai, D. K. (2018). Qualitative and Quantitative Analysis of Polyphenols in Lamiaceae Plants—A Review. Plants, 7(2), 25. https://doi.org/10.3390/plants7020025






