Short-Term Responses in Maximum Quantum Yield of PSII (Fv/Fm) to ex situ Temperature Treatment of Populations of Bryophytes Originating from Different Sites in Hokkaido, Northern Japan
Abstract
:1. Introduction
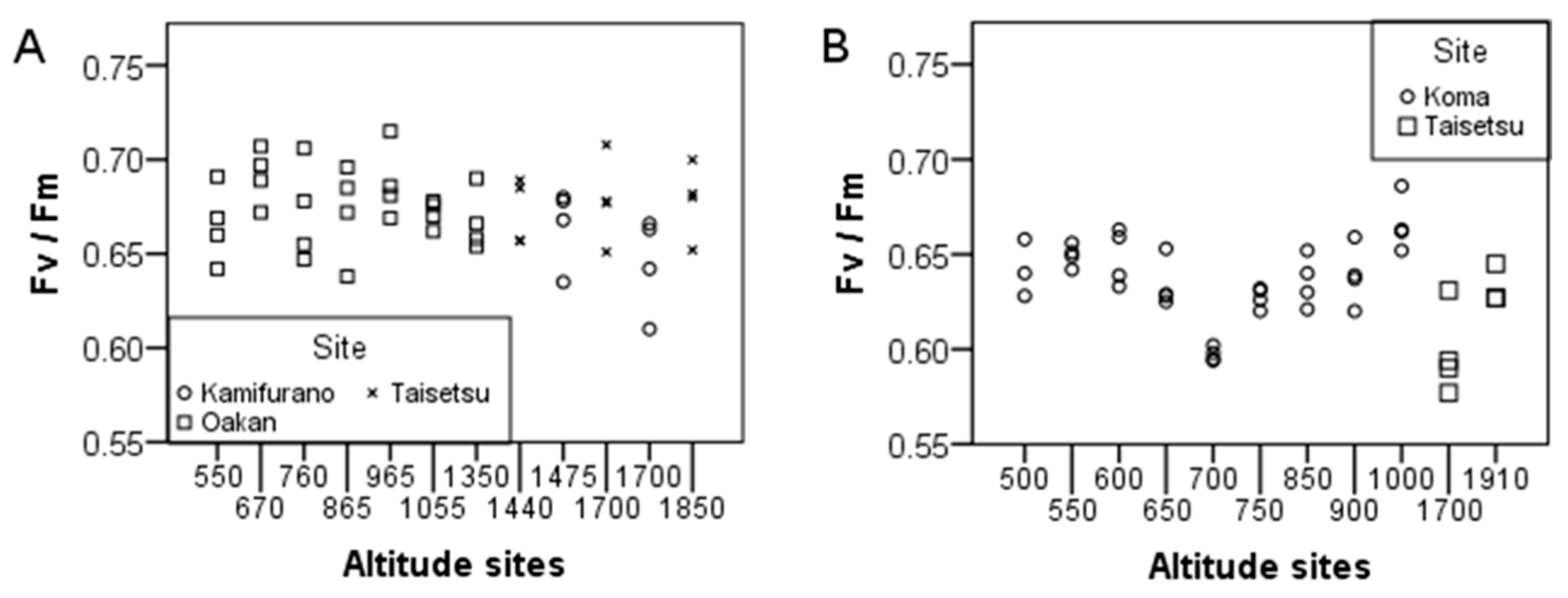
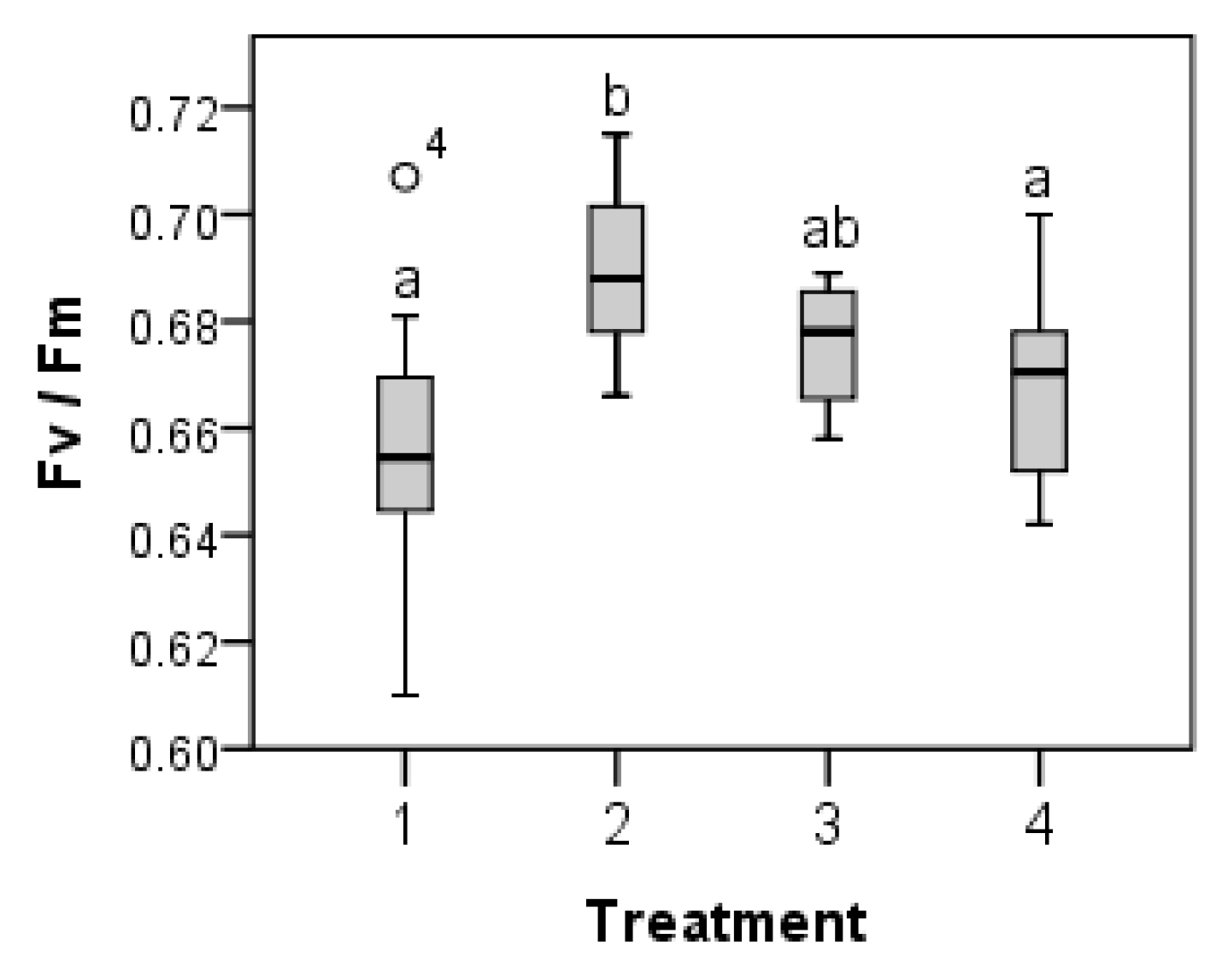
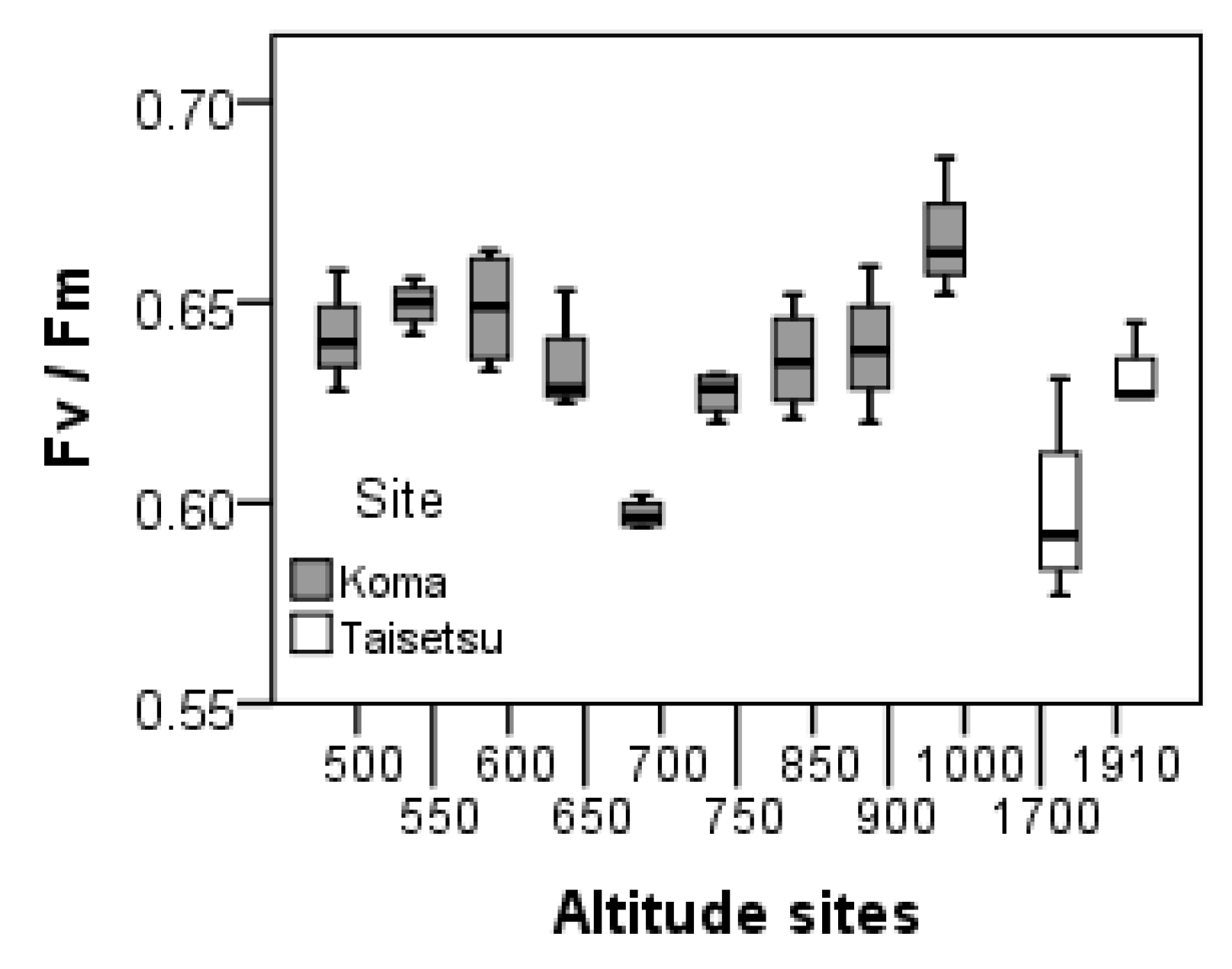
2. Results
3. Discussion
4. Experimental Section
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wagner, S.; Zotz, G.; Salazar Allen, N.; Bader, M.Y. Altitudinal changes in temperature responses of net photosynthesis and dark respiration in tropical bryophytes. Ann. Bot. 2013, 111, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Jägerbrand, A.K.; Alatalo, J.M.; Kudo, G. Variation in responses to temperature treatments ex situ of the moss Pleurozium schreberi (Willd. Ex Brid.) Mitt. originating from eight altitude sites in Hokkaido, Japan. J. Bryol. 2014, 36, 209–216. [Google Scholar] [CrossRef]
- Wagner, S.; Zotz, G.; Bader, M.Y. The temperature acclimation potential of tropical bryophytes. Plant Biol. 2014, 16, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K.; Ishikawa, K.; Borjigidai, A.; Muller, O.; Onoda, Y. Temperature acclimation of photosynthesis: Mechanisms involved in the changes in temperature dependence of photosynthetic rate. J. Exp. Bot. 2006, 57, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Kallio, P.; Saarnio, E. The effect on mosses of transplantation to different altitudes. J. Bryol. 1986, 14, 159–178. [Google Scholar] [CrossRef]
- Furness, S.B.; Grime, J.P. Growth rate and temperature responses in bryophytes. II. A comparative study of species of contrasted ecology. J. Ecol. 1982, 70, 525–536. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence––A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Larcher, W. Photosynthesis as a tool for indicating temperature stress events. In Ecophysiology of Photosynthesis. Ecological Studies 100; Schulze, E.D., Caldwell, M.M., Eds.; Springer-Verlag: Berlin, Germany, 1994; pp. 261–277. [Google Scholar]
- Csintalan, Z.; Proctor, M.C.F.; Tuba, Z. Chlorophyll fluorescence during drying and rehydration in the mosses Rhytidiadelphus loreus (Hedw.) Warnst., Anomodon viticulosus (Hedw.) Hook. and Tayl. and Grimmia pulvinata (Hedw.) Sm. Ann. Bot. 1999, 84, 235–244. [Google Scholar] [CrossRef]
- Proctor, M.C.F. Experiments on the effect of different intensities of desiccation on bryophyte survival, using chlorophyll fluorescence as an index of recovery. J. Bryol. 2003, 25, 201–210. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.C.; Branquinho, C.; da Silva, J.M. Physiological consequences of desiccation in the aquatic bryophyte Fontinalis antipyretica. Planta 2011, 234, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Kallio, P.; Heinonen, S. CO2 exchange and growth of Rhacomitrium lanuginosum and Dicranum elongatum. In Fennoscandian Tundra Ecosystems. Part 1. Plants and Microorganisms; Wielgolaski, F.E., Ed.; Springer: New York, NY, USA, 1975. [Google Scholar]
- Glime, J.M. Bryophyte Ecology. Available online: http://www.bryoecol.mtu.edu (accessed on 21 April 2016).
- Glime, J.M. Response of Fontinalis hypnoides to seasonal temperature variations. J. Hattori Bot. Lab. 1982, 53, 181–193. [Google Scholar]
- Korpelainen, H.; Jägerbrand, A.K.; Cräutlein, M.V. Genetic structure of mosses Pleurozium schreberi (Willd. ex Brid.) Mitt. and Racomitrium lanuginosum (Hedw.) Brid. along altitude gradients in Hokkaido, Japan. J. Bryol. 2012, 34, 309–312. [Google Scholar] [CrossRef]
- Kallio, P.; Heinonen, S. Ecology of Rhacomitrium lanuginosum (Hedw.) Brid. Rept. Kevo Subarct. Res. Stat. 1973, 10, 43–54. [Google Scholar]
- Gabriel, R.; Bates, J.W. Responses of photosynthesis to irradiance in bryophytes of the Azores laurel forest. J. Bryol. 2003, 25, 101–105. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1989, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
| Variable | Pleurozium schreberi | Racomitrium lanuginosum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SS | df | MS | F | p | SS | df | MS | F | p | |
| Between sites | 0.43 | 11 | 0.04 | 1.26 | n.s. | 1.19 | 10 | 0.12 | 7.56 | <0.0001 |
| Within sites | 1.11 | 36 | 0.03 | 0.50 | 32 | 0.02 | ||||
| Total | 1.54 | 47 | 1.69 | 42 | ||||||
| Variable | Pleurozium schreberi | Racomitrium lanuginosum | ||||
|---|---|---|---|---|---|---|
| df | F | p | df | F | p | |
| Treatment | 3 | 6.56 | 0.001 | 3 | 1.03 | n.s. |
| Site | 11 | 1.30 | n.s. | 10 | 7.50 | <0.0001 |
| Variable | Pleurozium schreberi | Racomitrium lanuginosum | ||||
|---|---|---|---|---|---|---|
| df | t | p | df | t | p | |
| Fv/Fm | 47 | 0.74 | n.s. | 43 | −1.6 | n.s. |
| Pleurozium schreberi | Racomitrium lanuginosum | ||
|---|---|---|---|
| Site | Altitude (m.a.s.l.) | Site | Altitude (m.a.s.l.) |
| Mt. Oakan (43°45′N, 144°16′E) | 550 | Mt. Koma (42°07′N, 140°68′E) | 500 |
| 29–30 June 2007 | 670 | 21–22 June 2007 | 550 |
| 760 | 600 | ||
| 865 | 650 | ||
| 965 | 700 | ||
| 1055 | 750 | ||
| 1350 | 850 | ||
| Taisetsu (43°42′N, 142°86′E) | 1440 | 900 | |
| 21 June 2007 | 1700 | 1000 | |
| 1850 | Taisetsu (43°42′N, 142°86′E) | 1700 | |
| Mt. Kamifurano (43°40′N, 142°67′E) | 1475 | 21 June 2007 | 1910 |
| 16 June 2007 | 1700 | ||
| Experimental treatment | Day (12 h) | Night (12 h) |
|---|---|---|
| 1 | 10 °C | 5 °C |
| 2 | 20 °C | 10 °C |
| 3 | 25 °C | 15 °C |
| 4 | 30 °C | 20 °C |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jägerbrand, A.K.; Kudo, G. Short-Term Responses in Maximum Quantum Yield of PSII (Fv/Fm) to ex situ Temperature Treatment of Populations of Bryophytes Originating from Different Sites in Hokkaido, Northern Japan. Plants 2016, 5, 22. https://doi.org/10.3390/plants5020022
Jägerbrand AK, Kudo G. Short-Term Responses in Maximum Quantum Yield of PSII (Fv/Fm) to ex situ Temperature Treatment of Populations of Bryophytes Originating from Different Sites in Hokkaido, Northern Japan. Plants. 2016; 5(2):22. https://doi.org/10.3390/plants5020022
Chicago/Turabian StyleJägerbrand, Annika K., and Gaku Kudo. 2016. "Short-Term Responses in Maximum Quantum Yield of PSII (Fv/Fm) to ex situ Temperature Treatment of Populations of Bryophytes Originating from Different Sites in Hokkaido, Northern Japan" Plants 5, no. 2: 22. https://doi.org/10.3390/plants5020022
APA StyleJägerbrand, A. K., & Kudo, G. (2016). Short-Term Responses in Maximum Quantum Yield of PSII (Fv/Fm) to ex situ Temperature Treatment of Populations of Bryophytes Originating from Different Sites in Hokkaido, Northern Japan. Plants, 5(2), 22. https://doi.org/10.3390/plants5020022






