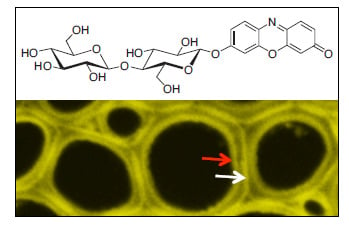
Glycoside Hydrolase Activities in Cell Walls of Sclerenchyma Cells in the Inflorescence Stems of Arabidopsis thaliana Visualized in Situ
Abstract
:1. Introduction
2. Results
2.1. Biosynthesis of Different Fluorogenic Substrates
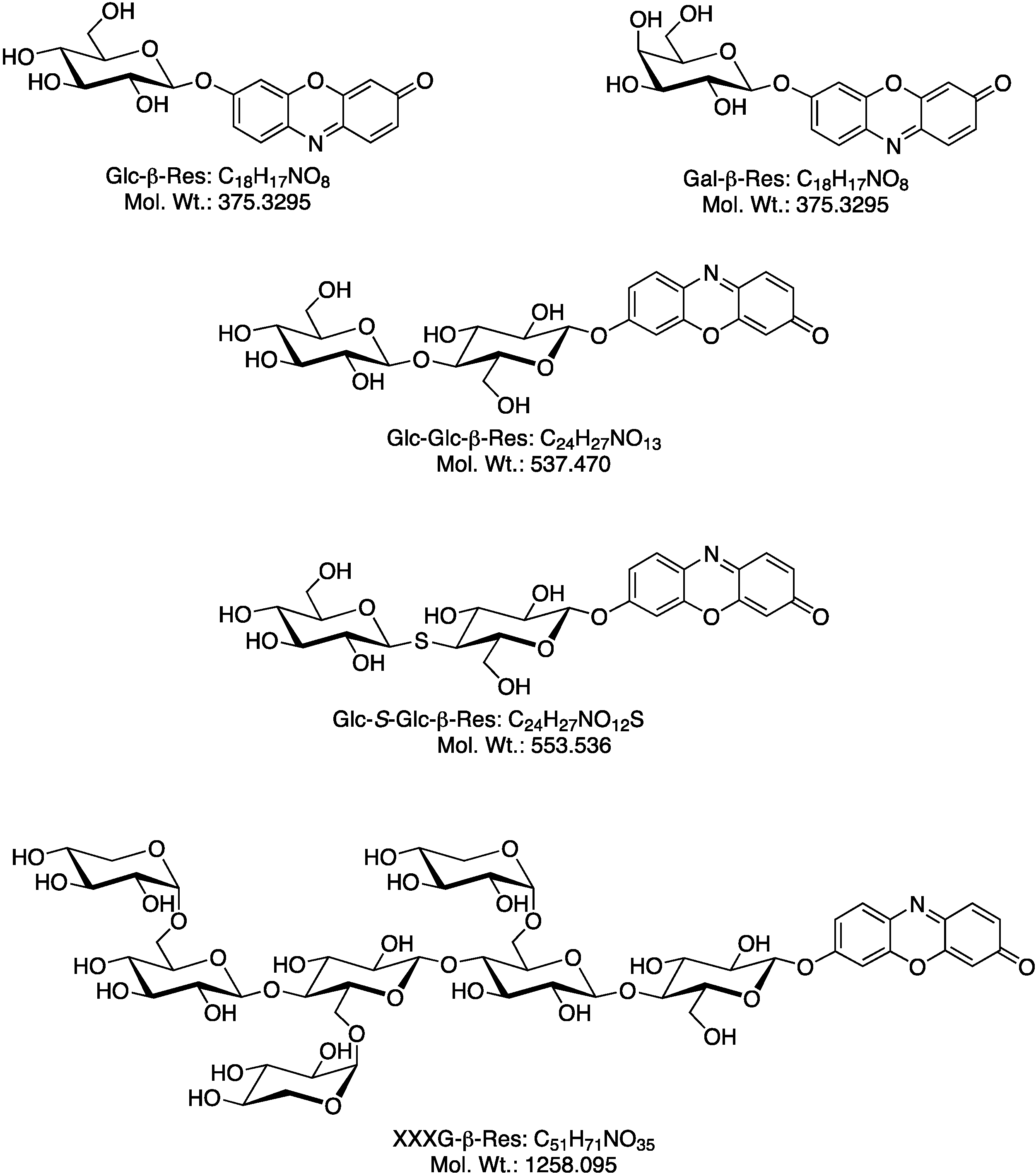
2.2. Optimization of in Situ Reaction Conditions for the Different Fluorogenic Substrates
| Reaction Times | Substrates | Concentration (M) | pH | ||||
|---|---|---|---|---|---|---|---|
| 5.0 | 5.5 | 6.0 | 6.5 | 7.0 | |||
| 20 min | Glc-β-Res | 9.3 × 10−5 | ++ | ++ | +++ | +++ | ++ |
| Glc-Glc-β-Res | 9.3 × 10−5 | ++ | ++ | +++ | +++ | ++ | |
| Gal-β-Res | 4.7 × 10−5 | ++ | +++ | +++ | ++++ | ++ | |
| 60 min | Glc-S-Glc-β-Res | 9.3 × 10−4 | - | - | + | + | ++ |
| XXXG-β-Res | 9.3 × 10−4 | - | - | + | ++ | + | |
2.3. In Situ Enzymatic Activity Distribution Observed in Real Time at the Tissue Level in Inflorescence Stems of Arabidopsis thaliana
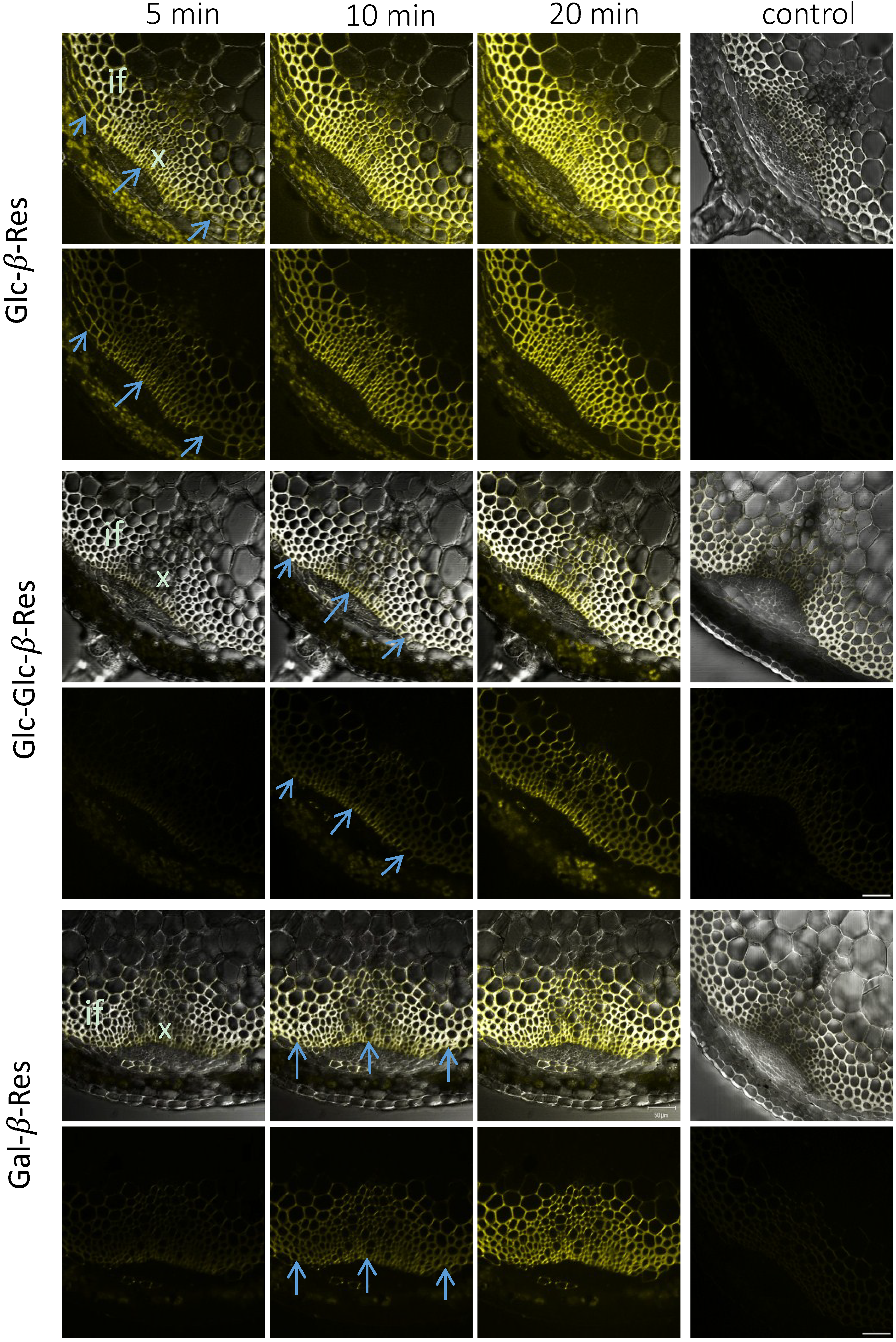
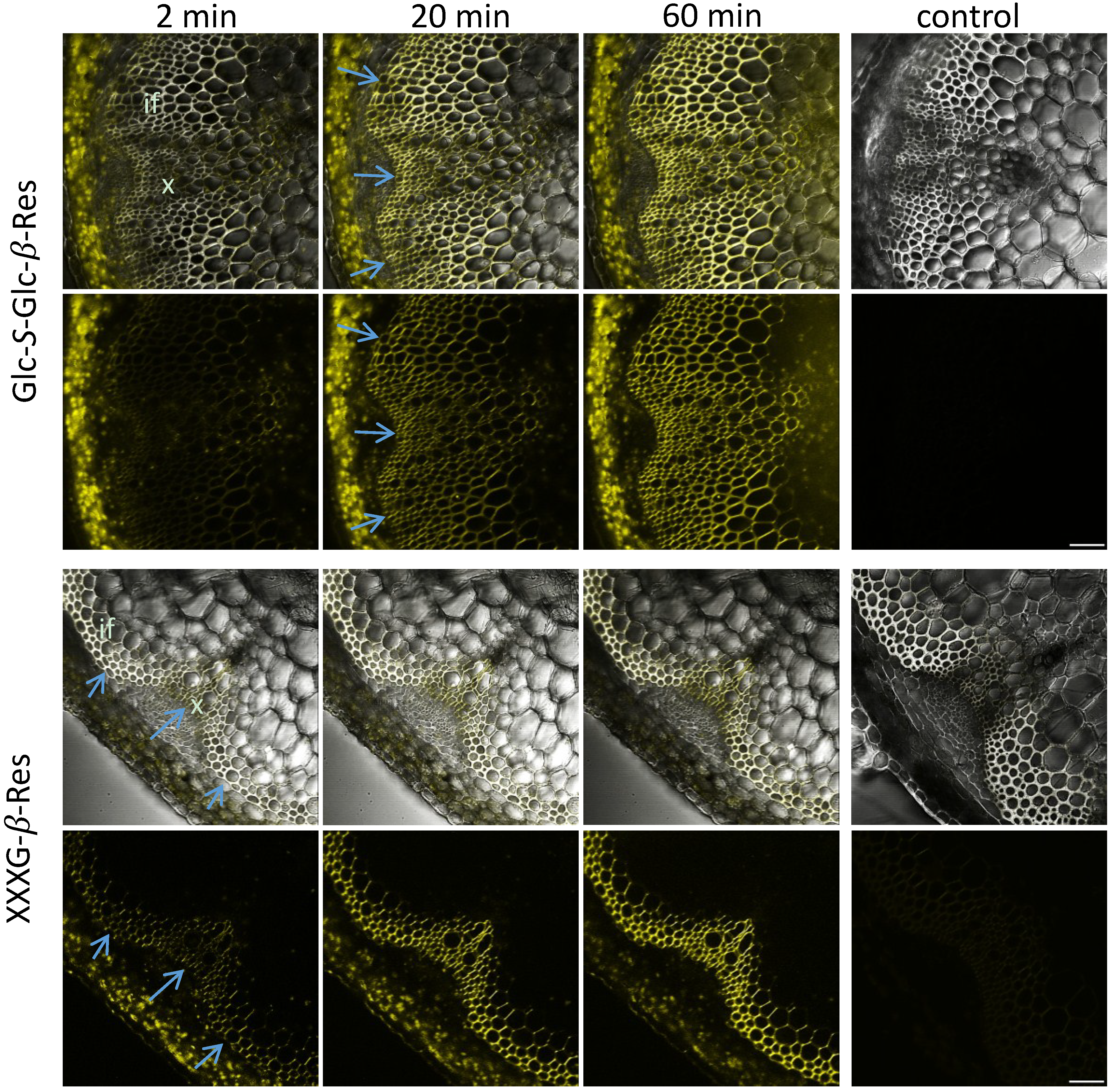
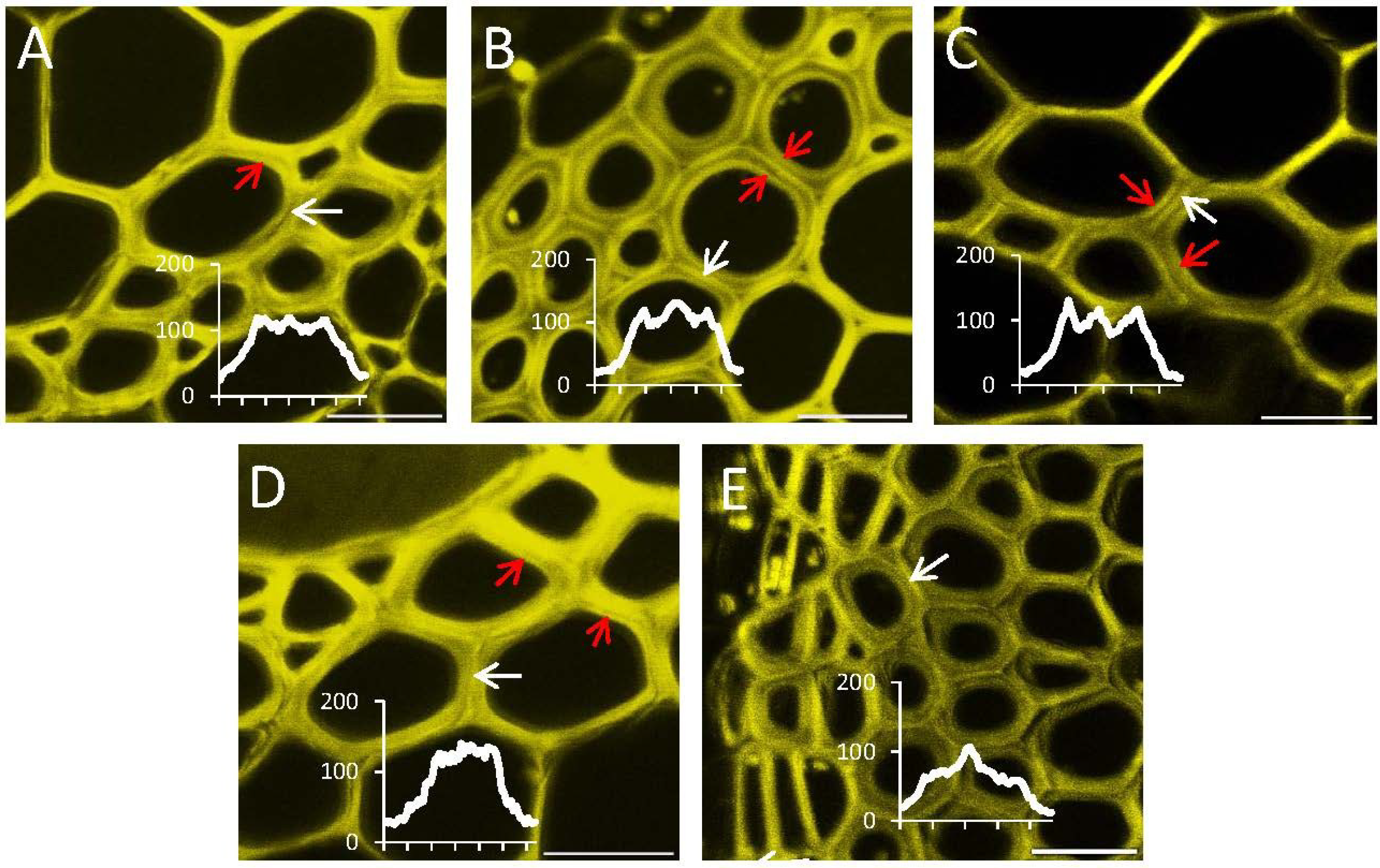
2.4. In Situ Enzymatic Activity Observed in Real Time on the Cell Level in Interfascicular Fibers of Inflorescence Stems in Arabidopsis thaliana
3. Discussion
4. Experimental Section
4.1. Substrates Synthesis
4.2. Material and Growth Conditions
4.3. Optimization of the Enzymatic Reaction Conditions
4.3.1. The Reaction Time Optimization
4.3.2. pH Optimization
4.3.3. Substrate Concentration Optimization
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ibatullin, F.M.; Banasiak, A.; Baumann, M.J.; Greffe, L.; Takahashi, J.; Mellerowicz, E.J.; Brumer, H. A real-time fluorogenic assay for the visualization of glycoside hydrolase activity in planta. Plant Physiol. 2009, 151, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B.; Coutinho, P.M.; Davies, G.J. A census of carbohydrate-active enzymes in the genome of Arabidopsis thaliana. Plant Mol. Biol. 2001, 47, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Geisler-Lee, J.; Geisler, M.; Coutinho, P.M.; Segerman, B.; Nishikubo, N.; Takahashi, J.; Aspeborg, H.; Djerbi, S.; Master, E.; Andersson-Gunnerås, S.; et al. Poplar Carbohydrate-Active Enzymes (CAZymes). Gene identification and expression analyses. Plant Physiol. 2006, 140, 946–962. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.C. Primary cell wall metabolism: Tracking the careers of wall polymers in living plant cells. New Phytol. 2004, 161, 641–675. [Google Scholar] [CrossRef]
- Del Bem, E.L.; Vincentz, M.G. Evolution of xyloglucan-related genes in green Plants. BMC Evol. Biol. 2010, 10, 341–388. [Google Scholar] [CrossRef] [PubMed]
- Eklöf, J.M.; Brumer, H. The XTH gene family: An update on enzyme structure, function, and phylogeny in xyloglucan remodelling. Plant Physiol. 2010, 153, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Franková, L.; Fry, S.C. Biochemistry and physiological roles of enzymes that “cut and paste” plant cell-wall polysaccharides. J. Exp. Bot. 2013, 64, 3519–3550. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Günl, M.; Neumetzler, L.; Kraemer, F.; de Souza, A.; Schultink, A.; Pena, M.; York, W.S.; Pauly, M. AXY8 encodes an α-fucosidase, underscoring the importance of apoplastic metabolism on the fine structure of Arabidopsis cell wall polysaccharides. Plant Cell 2011, 23, 4025–4040. [Google Scholar] [CrossRef] [PubMed]
- Günl, M.; Pauly, M. AXY3 encodes a α-xylosidase that impacts the structure and accessibility of the hemicellulose xyloglucan in Arabidopsis plant cell walls. Planta 2011, 233, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, J.; Pardo, B.; Gianzo, C.; Guitián, E.; Revilla, G.; Zarra, I. Lack of α-xylosidase activity in Arabidopsis alters xyloglucan composition and results in growth defects. Plant Physiol. 2010, 3, 1105–1115. [Google Scholar] [CrossRef]
- Sampedro, J.; Gianzo, C.; Iglesias, N.; Guitián, E.; Revilla, G.; Zarra, I. AtBGAL10 is the main xyloglucan β-galactosidase in Arabidopsis, and its absence results in unusual xyloglucan subunits and growth defects. Plant Physiol. 2012, 3, 1146–1157. [Google Scholar] [CrossRef]
- Brummell, D.A.; Cin, V.D.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2004, 55, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, V.; Nishikubo, N.; Abe, H.; Brumer, H.; Denman, S.; Eklund, M.; Christiernin, M.; Teeri, T.T.; Sundberg, B.; Mellerowicz, E.J. Xyloglucan endotransglycosylases have a function during the formation of secondary cell walls of vascular tissues. Plant Cell 2002, 14, 3073–3088. [Google Scholar] [CrossRef] [PubMed]
- Nishikubo, N.; Awano, T.; Banasiak, A.; Bourquin, V.; Ibatullin, F.; Funada, R.; Brumer, H.; Teeri, T.T.; Hayashi, T.; Sundberg, B.; et al. Xyloglucan endo-transglycosylase (XET) functions in gelatinous layers of tension wood fibers in poplar—A glimpse into the mechanism of the balancing act of trees. Plant Cell Physiol. 2007, 48, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Nishikubo, N.; Takahashi, J.; Roos, A.A.; Derba-Maceluch, M.; Piens, K.; Brumer, H.; Teeri, T.T.; Stålbrand, H.; Mellerowicz, E.J. XET-mediated xyloglucan rearrangements in developing wood of hybrid aspen (Populus tremula x tremuloides). Plant Physiol. 2011, 155, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Derba-Maceluch, M.; Awano, T.; Takahashi, J.; Lucenius, J.; Ratke, C.; Kontro, I.; Busse-Wicher, M.; Kosik, O.; Tanaka, R.; Winzéll, A.; et al. Suppression of xylan transglycosylase PtxtXyn10A affects cellulose microfibril angle in secondary wall in aspen wood. New Phytol. 2014, in press. [Google Scholar]
- Mellerowicz, E.J.; Immerzeel, P.; Hayashi, T. Xyloglucan—The molecular muscle of trees. Ann. Bot. 2008, 101, 659–665. [Google Scholar] [CrossRef]
- Baba, K.; Park, Y.W.; Kaku, T.; Kaida, R.; Takeuchi, M.; Yoshida, M.; Hosoo, Y.; Ojio, Y.; Okuyama, T.; Taniguchi, T.; et al. Xyloglucan for generating tensile stress to bend tree stem. Mol. Plant 2009, 2, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Mellerowicz, E.J.; Gorshkova, T. Tensional stress generation in gelatinous fibres: A review and possible mechanism based on cell wall structure and composition. J. Exp. Bot. 2012, 63, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Minic, Z.; Jamet, E.; Négroni, L.; der Garabedian, P.A.; Zivy, M.; Jouanin, L. A sub-proteome of Arabidopsis thaliana mature stems trapped on Concanavalin A is enriched in cell wall glycoside hydrolases. J. Exp. Bot. 2007, 58, 2503–2512. [Google Scholar] [PubMed]
- Day, A.; Fénart, S.; Neutelings, G.; Hawkins, S.; Rolando, C.; Tokarski, C. Identification of cell wall proteins in the flax (Linum usitatissimum) stem. Proteomics 2013, 13, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.J.; Studler, M.J.; Naleway, J.J. A long-wavelength fluorescent substrate for continuous fluorometric determination of cellulase activity: Resorufin-β-d-cellobioside. Anal. Biochem. 2007, 371, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Tuomivaara, S.T.; Yaoi, K.; O’Neill, M.A.; York, W.S. Generation and structural validation of a library of diverse xyloglucan-derived oligosaccharides, including an update on xyloglucan nomenclature. Carbohydr. Res. 2014. [Google Scholar] [CrossRef]
- Miyamoto, K.; Schopfer, P. Sugar release from maize coleoptiles during auxin-, fusicoccin- and acid-mediated elongation growth. J. Plant Physiol. 1997, 150, 309–316. [Google Scholar] [CrossRef]
- Mikshina, P.V.; Chemikosova, S.B.; Mokshina, N.E.; Ibragimova, N.N.; Gorshkova, T.A. Free galactose and galactosidase activity in the course of flax fiber development. Russ. J. Plant Physiol. 2009, 56, 58–67. [Google Scholar] [CrossRef]
- Roach, M.J.; Mokshina, N.Y.; Badhan, A.; Snegireva, A.V.; Hobson, N.; Deyholos, M.K.; Gorshkova, T.A. Development of cellulosic secondary walls in flax fibers requires b-galactosidase. Plant Physiol. 2011, 156, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Rudsander, U.J.; Hedenström, M.; Banasiak, A.; Harholt, J.; Amelot, N.; Immerzeel, P.; Ryden, P.; Endo, S.; Ibatullin, F.M.; et al. KORRIGAN1 and its aspen homolog PttCel9A1 decrease cellulose crystallinity in Arabidopsis stems. Plant Cell Physiol. 2009, 50, 1099–1115. [Google Scholar] [CrossRef] [PubMed]
- Maloney, V.J.; Mansfield, S.D. Characterization and varied expression of a membrane-bound endo-β-1,4-glucanase in hybrid poplar. Plant Biotechnol. J. 2010, 8, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.J.; Eklöf, J.M.; Michel, G.; Kallas, Å.M.; Teeri, T.T.; Czjzek, M.; Brumer, H. Structural evidence for the evolution of xyloglucanase activity from xyloglucan endo-transglycosylases: Biological implications for cell wall metabolism. Plant Cell 2007, 19, 1947–1963. [Google Scholar] [CrossRef] [PubMed]
- Kaewthai, N.; Gendre, D.; Eklöf, J.M.; Ibatullin, F.M.; Ezcurra, I.; Bhalerao, R.P.; Brumer, H. Group III a XTH genes of Arabidopsis encode predominant xyloglucan endohydrolases that are dispensable for normal growth. Plant Physiol. 2013, 161, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Eklöf, J.M.; Shojania, S.; Okon, M.; McIntosh, L.P.; Brumer, H. Structure-function analysis of a broad-specificity Populus trichocarpa endo-beta-glucanase reveals an evolutionary link between bacterial licheninases and plant XTH gene products. J. Biol. Chem. 2013, 288, 15786–15799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Shi, Y.Z.; Lei, G.J.; Fry, S.C.; Zhang, B.C.; Zhou, Y.H.; Braam, J.; Jiang, T.; Xu, X.Y.; Mao, C.Z.; et al. XTH31, Encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell 2012, 24, 4731–4747. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Wan, J.X.; Sun, Y.; Shi, Y.Z.; Braam, J.; Li, G.X.; Zheng, S.J. Xyloglucan ENDOTRANSGLUCOSYLASE-Hydrolase17 interacts with Xyloglucan Endotransglucosylase-Hydrolase31 to confer xyloglucan endotransglucosylase action and affect aluminum sensitivity in Arabidopsis. Plant Physiol. 2014, 165, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Shoichi Tokutake, S.; Kasai, K.; Tomikura, T.; Yamaji, N.; Kato, M. Glycosides having chromophores as substrates for sensitive enzyme analysis. II. Synthesis of phenolindophenyl-β-d-glucopyranosides having an electron-withdrawing substituent as substrates for β-glucosidase. Chem. Pharm. Bull. 1990, 38, 3466–3470. [Google Scholar] [CrossRef]
- Orgeret, C.; Seillier, E.; Gautier, C.; Defaye, J.; Driguez, H. 4-Thiocellooligosaccharides. Their synthesis and use as ligands for the separation of cellobiohydrolases of Trichoderma reesei by affinity chromatography. Carbohydr. Res. 1992, 224, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Görlach, J. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banasiak, A.; Ibatullin, F.M.; Brumer, H.; Mellerowicz, E.J. Glycoside Hydrolase Activities in Cell Walls of Sclerenchyma Cells in the Inflorescence Stems of Arabidopsis thaliana Visualized in Situ. Plants 2014, 3, 513-525. https://doi.org/10.3390/plants3040513
Banasiak A, Ibatullin FM, Brumer H, Mellerowicz EJ. Glycoside Hydrolase Activities in Cell Walls of Sclerenchyma Cells in the Inflorescence Stems of Arabidopsis thaliana Visualized in Situ. Plants. 2014; 3(4):513-525. https://doi.org/10.3390/plants3040513
Chicago/Turabian StyleBanasiak, Alicja, Farid M. Ibatullin, Harry Brumer, and Ewa J. Mellerowicz. 2014. "Glycoside Hydrolase Activities in Cell Walls of Sclerenchyma Cells in the Inflorescence Stems of Arabidopsis thaliana Visualized in Situ" Plants 3, no. 4: 513-525. https://doi.org/10.3390/plants3040513
APA StyleBanasiak, A., Ibatullin, F. M., Brumer, H., & Mellerowicz, E. J. (2014). Glycoside Hydrolase Activities in Cell Walls of Sclerenchyma Cells in the Inflorescence Stems of Arabidopsis thaliana Visualized in Situ. Plants, 3(4), 513-525. https://doi.org/10.3390/plants3040513