Biotic and Abiotic Drivers of Phenotypic Diversity in the Genus Lupinus (Fabaceae)
Abstract
1. Introduction
2. Results
2.1. Selection of Floral Traits in Lupinus Mediated by Pollinators
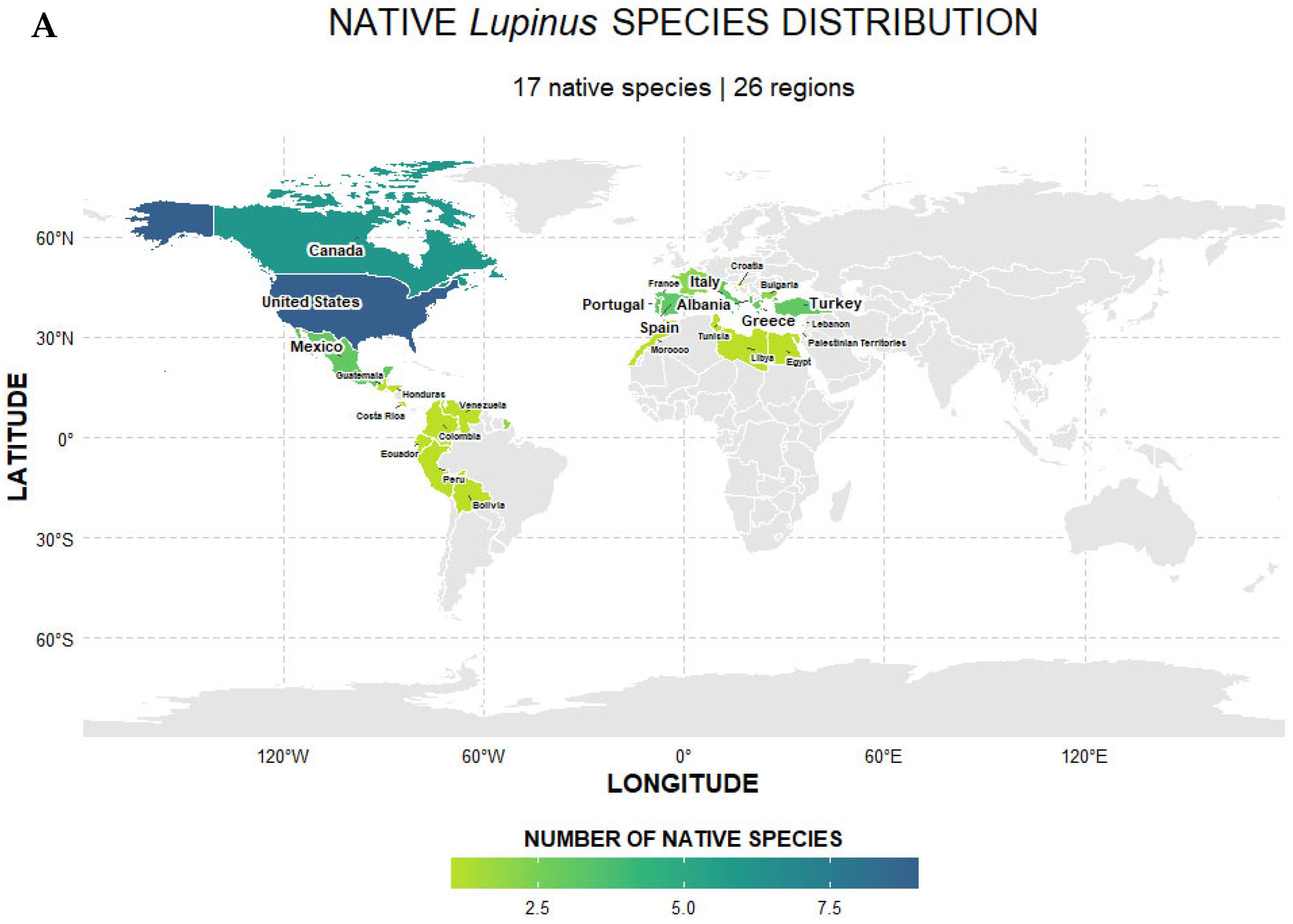
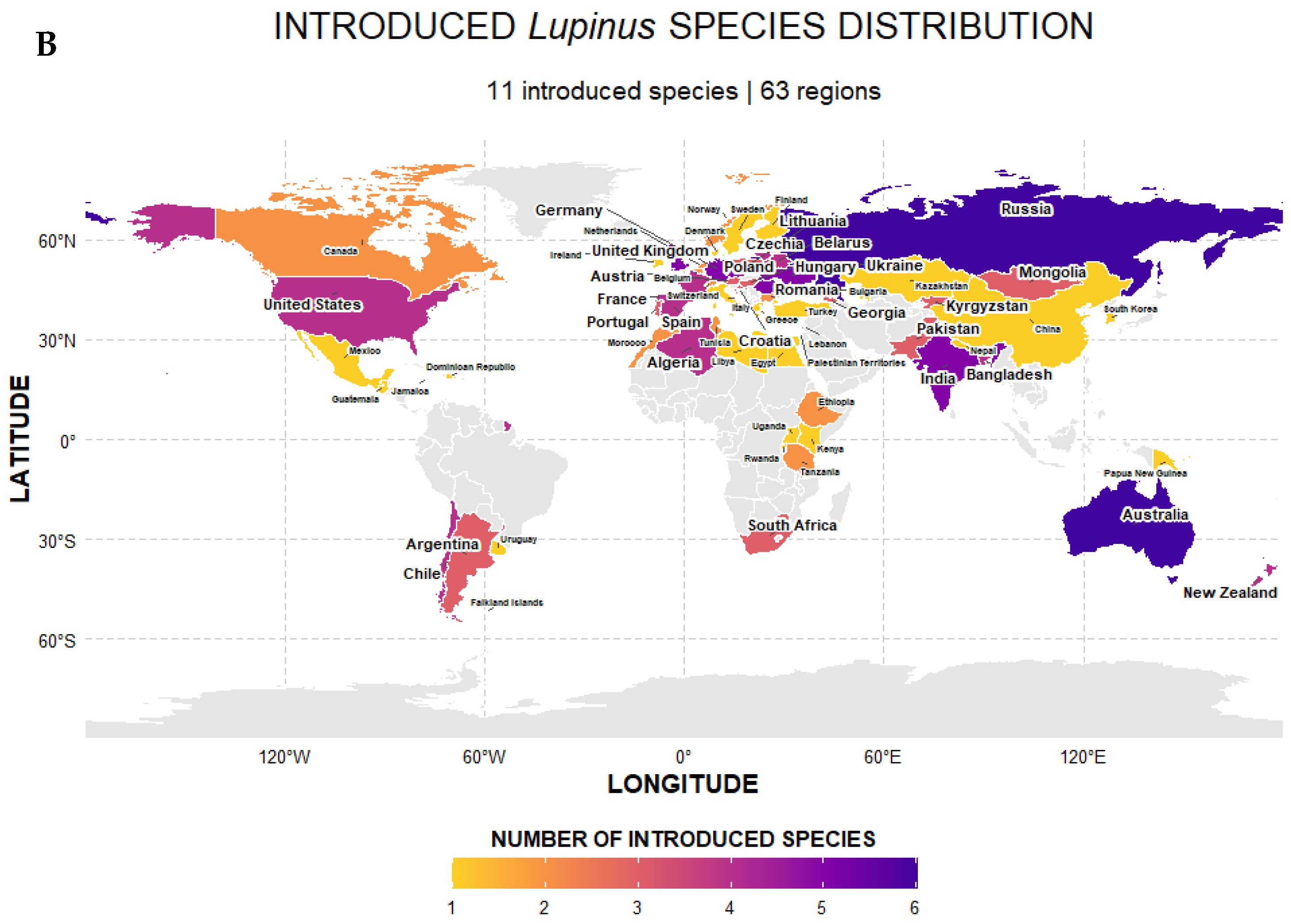
2.2. Selection of Floral Traits in Lupinus Mediated by Abiotic Factors
3. Discussion
3.1. Drivers of Phenotypic Diversity
3.2. Evolutionary Mechanisms and Plasticity
3.3. Knowledge Gaps and Research Bias
3.4. Ecological and Evolutionary Implications
3.5. Methodological Considerations and Future Directions
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ollerton, J. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Tong, Z.-Y.; Wu, L.-Y.; Feng, H.-H.; Zhang, M.; Armbruster, W.S.; Renner, S.S.; Huang, S.-Q. New Calculations Indicate That 90% of Flowering Plant Species Are Animal-Pollinated. Natl. Sci. Rev. 2023, 10, nwad219. [Google Scholar] [CrossRef] [PubMed]
- Landry, C. Mighty Mutualisms: The Nature of Plant-Pollinator Interactions. Nat. Educ. Knowl. 2010, 3, 37. [Google Scholar]
- Ellstrand, C. Gene Flow among Seed Plant Populations. New For. 1992, 6, 241–256. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Wright, G.A. Plant–Pollinator Interactions and Threats to Pollination: Perspectives from the Flower to the Landscape. Funct. Ecol. 2017, 31, 22–25. [Google Scholar] [CrossRef]
- Jirgal, N.; Ohashi, K. Effects of Floral Symmetry and Orientation on the Consistency of Pollinator Entry Angle. Sci. Nat. 2023, 110, 19. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, A.; Lyu, T.; Dimitrov, D.; Liu, Y.; Li, Y.; Xu, X.; Freckleton, R.P.; Hao, Z.; Wang, Z. Global Distribution and Evolutionary Transitions of Floral Symmetry in Angiosperms. Sci. Adv. 2023, 9, eadg2555. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, A.; Dohzono, I.; Takami, Y.; Hyodo, F. Flower Orientation Enhances Pollen Transfer in Bilaterally Symmetrical Flowers. Oecologia 2009, 160, 667–674. [Google Scholar] [CrossRef]
- Krishna, S.; Keasar, T. Morphological Complexity as a Floral Signal: From Perception by Insect Pollinators to Co-Evolutionary Implications. Int. J. Mol. Sci. 2018, 19, 1681. [Google Scholar] [CrossRef]
- Alemán, M.M.; Hoc, P.; Etcheverry, Á.V.; Ortega-Baes, P.; Sühring, S.; López-Spahr, D. Morphological Traits in Keel Flowers of Papilionoideae (Fabaceae) and Their Relationships with the Pollination Mechanisms. Plant Syst. Evol. 2022, 308, 43. [Google Scholar] [CrossRef]
- Ashman, T.-L.; Majetic, C.J. Genetic Constraints on Floral Evolution: A Review and Evaluation of Patterns. Heredity 2006, 96, 343–352. [Google Scholar] [CrossRef]
- Auge, G.A.; Penfield, S.; Donohue, K. Pleiotropy in Developmental Regulation by Flowering-pathway Genes: Is It an Evolutionary Constraint? New Phytol. 2019, 224, 55–70. [Google Scholar] [CrossRef]
- Wessinger, C.A.; Hileman, L.C.; Rausher, M.D. Identification of Major Quantitative Trait Loci Underlying Floral Pollination Syndrome Divergence in Penstemon. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130349. [Google Scholar] [CrossRef]
- Bukhari, G.; Zhang, J.; Stevens, P.F.; Zhang, W. Evolution of the Process Underlying Floral Zygomorphy Development in Pentapetalous Angiosperms. Am. J. Bot. 2017, 104, 1846–1856. [Google Scholar] [CrossRef]
- Fatiukha, A.; Deblieck, M.; Klymiuk, V.; Merchuk-Ovnat, L.; Peleg, Z.; Ordon, F.; Fahima, T.; Korol, A.; Saranga, Y.; Krugman, T. Genomic Architecture of Phenotypic Plasticity in Response to Water Stress in Tetraploid Wheat. Int. J. Mol. Sci. 2021, 22, 1723. [Google Scholar] [CrossRef]
- Murren, C.J.; Auld, J.R.; Callahan, H.; Ghalambor, C.K.; Handelsman, C.A.; Heskel, M.A.; Kingsolver, J.G.; Maclean, H.J.; Masel, J.; Maughan, H.; et al. Constraints on the Evolution of Phenotypic Plasticity: Limits and Costs of Phenotype and Plasticity. Heredity 2015, 115, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Jogesh, T.; Overson, R.P.; Raguso, R.A.; Skogen, K.A. Herbivory as an Important Selective Force in the Evolution of Floral Traits and Pollinator Shifts. AoB Plants 2016, 9, plw088. [Google Scholar] [CrossRef]
- Kessler, D.; Kallenbach, M.; Diezel, C.; Rothe, E.; Murdock, M.; Baldwin, I.T. How Scent and Nectar Influence Floral Antagonists and Mutualists. eLife 2015, 4, e07641. [Google Scholar] [CrossRef] [PubMed]
- Wolko, B.; Clements, J.C.; Rocha, B.; Nelson, M.N.; Yang, H. Wild Crop Relatives: Genomic and Breeding Resources: Legume Crops and Forages; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-14386-1. [Google Scholar]
- Ishaq, A.R.; El-Nashar, H.A.S.; Younis, T.; Mangat, M.A.; Shahzadi, M.; Ul Haq, A.S.; El-Shazly, M. Genus Lupinus (Fabaceae): A Review of Ethnobotanical, Phytochemical and Biological Studies. J. Pharm. Pharmacol. 2022, 74, 1700–1717. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.H. The Pollination of Lupins. Bee World 1987, 68, 10–16. [Google Scholar] [CrossRef]
- Barda, M.S.; Chatzigeorgiou, T.; Papadopoulos, G.K.; Bebeli, P.J. Agro-Morphological Evaluation of Lupinus mutabilis in Two Locations in Greece and Association with Insect Pollinators. Agriculture 2021, 11, 236. [Google Scholar] [CrossRef]
- Uluer, D.A.; Forest, F.; Armbruster, S.; Hawkins, J.A. Reconstructing an Historical Pollination Syndrome: Keel Flowers. BMC Ecol. Evol. 2022, 22, 45. [Google Scholar] [CrossRef]
- Córdoba, S.A.; Benitez-Vieyra, S.; Cocucci, A.A. Functional Modularity in a Forcible Flower Mechanism: Relationships among Morphology, Biomechanical Features and Fitness. Evol. Ecol. 2015, 29, 719–732. [Google Scholar] [CrossRef]
- Ne’eman, G.; Nesher, R. Pollination ecology and the significance of floral color change in Lupinus pilosos L. (Fabaceae). Isr. J. Plant Sci. 1995, 43, 135–145. [Google Scholar] [CrossRef]
- Fontaine, C.; Dajoz, I.; Meriguet, J.; Loreau, M. Functional Diversity of Plant–Pollinator Interaction Webs Enhances the Persistence of Plant Communities. PLoS Biol. 2005, 4, e1. [Google Scholar] [CrossRef]
- Karoly, K. Pollinator Limitation in the Facultatively Autogamous Annual, Lupinus nanus (Leguminosae). Am. J. Bot. 1992, 79, 49–56. [Google Scholar] [CrossRef]
- Reid, C. Floral Longevity and Attraction of Arctic Lupine, Lupinus arcticus: Implications for Pollination Efficiency. Arbutus Rev. 2019, 10, 83–99. [Google Scholar] [CrossRef]
- Matesanz, S.; Ramos-Muñoz, M.; Rubio Teso, M.L.; Iriondo, J.M. Effects of Parental Drought on Offspring Fitness Vary among Populations of a Crop Wild Relative. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220065. [Google Scholar] [CrossRef]
- Gavini, S.S.; Sáez, A.; Tur, C.; Aizen, M.A. Pollination Success Increases with Plant Diversity in High-Andean Communities. Sci. Rep. 2021, 11, 22107. [Google Scholar] [CrossRef]
- Fijen, T.P.M.; Morra, E.; Kleijn, D. Pollination Increases White and Narrow-Leaved Lupin Protein Yields but Not All Crop Visitors Contribute to Pollination. Agric. Ecosyst. Environ. 2021, 313, 107386. [Google Scholar] [CrossRef]
- Williams, I.H.; Martin, A.P.; Ferguson, A.W.; Clark, S.J. Effect of Pollination on Flower, Pod and Seed Production in White Lupin (Lupinus albus). J. Agric. Sci. 1990, 115, 67–73. [Google Scholar] [CrossRef]
- Poyatos, C.; Sacristán-Bajo, S.; Tabarés, P.; Prieto-Benítez, S.; Teso, M.L.R.; Torres, E.; Morente-López, J.; Lara-Romero, C.; Iriondo, J.M.; Fernández, A.G. Differential Patterns of Within- and between-Population Genetically Based Trait Variation in Lupinus angustifolius. Ann. Bot. 2023, 132, 541–552. [Google Scholar] [CrossRef]
- Stout, J.C.; Kells, A.R.; Goulson, D. Pollination of the Invasive Exotic Shrub Lupinus arboreus (Fabaceae) by Introduced Bees in Tasmania. Biol. Conserv. 2002, 106, 425–434. [Google Scholar] [CrossRef]
- Gori, D.F. Floral color change in Lupinus argenteus (fabaceae): Why should plants advertise the location of unrewarding flowers to pollinators? Evolution 1989, 43, 870–881. [Google Scholar] [CrossRef]
- Rivest, S.; Lee, S.T.; Cook, D.; Forrest, J.R.K. Consequences of Pollen Defense Compounds for Pollinators and Antagonists in a Pollen-rewarding Plant. Ecology 2024, 105, e4306. [Google Scholar] [CrossRef]
- Pfitsch, W.A.; Williams, E.H. Habitat Restoration for Lupine and Specialist Butterflies. Restor. Ecol. 2009, 17, 226–233. [Google Scholar] [CrossRef]
- Halinski, R.; Tavares, L.; Santos, J.; Loose, D. Biologia Floral de Lupinus Sp. (Fabaceae) e Espectro de Visitantes, Com Ênfase Em Apis Mellifera (Hymenoptera, Apidae) Nos Campos de Altitude No Sul Do Brasil. In Biologia e Ecologia da Polinização; Universidade Federal da Bahia: Bahia, Brasil, 2014; Volume 3, p. 134. [Google Scholar]
- Annicchiarico, P.; Romani, M.; Pecetti, L. White Lupin (Lupinus albus) Variation for Adaptation to Severe Drought Stress. Plant Breed. 2018, 137, 782–789. [Google Scholar] [CrossRef]
- Atnaf, M.; Yao, N.; Martina, K.; Dagne, K.; Wegary, D.; Tesfaye, K. Molecular Genetic Diversity and Population Structure of Ethiopian White Lupin Landraces: Implications for Breeding and Conservation. PLoS ONE 2017, 12, e0188696. [Google Scholar] [CrossRef] [PubMed]
- Rychel-Bielska, S.; Bielski, W.; Surma, A.; Annicchiarico, P.; Belter, J.; Kozak, B.; Galek, R.; Harzic, N.; Książkiewicz, M. A GWAS Study Highlights Significant Associations between a Series of Indels in a FLOWERING LOCUS T Gene Promoter and Flowering Time in White Lupin (Lupinus albus L.). BMC Plant Biol. 2024, 24, 722. [Google Scholar] [CrossRef]
- Keeve, R.; Loubser, H.L.; Krüger, G.H.J. Effects of Temperature and Photoperiod on Days to Flowering, Yield and Yield Components of Lupinus albus (L.) under Field Conditions. J. Agron. Crop Sci. 2000, 184, 187–196. [Google Scholar] [CrossRef]
- Vallejos Arnéz, J.; Mamani Rojas, P.; Huiza Nina, J.; Gabriel-Ortega, J. Adaptabilidad de dos especies de Lupinus en diferentes ambientes de los valles interandinos de Bolivia. J. Selva Andin. Biosph. 2021, 9, 69–80. [Google Scholar] [CrossRef]
- Ortúzar, M.; Riesco, R.; Criado, M.; Alonso, M.D.P.; Trujillo, M.E. Unraveling the Dynamic Interplay of Microbial Communities Associated to Lupinus angustifolius in Response to Environmental and Cultivation Conditions. Sci. Total Environ. 2024, 946, 174277. [Google Scholar] [CrossRef]
- Chen, Y.L.; Dunbabin, V.M.; Postma, J.A.; Diggle, A.J.; Palta, J.A.; Lynch, J.P.; Siddique, K.H.M.; Rengel, Z. Phenotypic Variability and Modelling of Root Structure of Wild Lupinus angustifolius Genotypes. Plant Soil 2011, 348, 345–364. [Google Scholar] [CrossRef]
- Heiling, J.M.; Cook, D.; Lee, S.T.; Irwin, R.E. Pollen and Vegetative Secondary Chemistry of Three Pollen-rewarding Lupines. Am. J. Bot. 2019, 106, 643–655. [Google Scholar] [CrossRef]
- Soto-Correa, J.C.; Sáenz-Romero, C.; Lindig-Cisneros, R.; De La Barrera, E. The Neotropical Shrub Lupinus elegans, from Temperate Forests, May Not Adapt to Climate Change. Plant Biol. 2013, 15, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Bielski, W.; Surma, A.; Belter, J.; Kozak, B.; Książkiewicz, M.; Rychel-Bielska, S. Molecular Dissection of the Genetic Architecture of Phenology Underlying Lupinus hispanicus Early Flowering and Adaptation to Winter- or Spring Sowing. Sci. Rep. 2025, 15, 15324. [Google Scholar] [CrossRef]
- Jurkonienė, S.; Jankauskienė, J.; Mockevičiūtė, R.; Gavelienė, V.; Jankovska-Bortkevič, E.; Sergiev, I.; Todorova, D.; Anisimovienė, N. Elevated Temperature Induced Adaptive Responses of Two Lupine Species at Early Seedling Phase. Plants 2021, 10, 1091. [Google Scholar] [CrossRef] [PubMed]
- Simioniuc, D.P.; Simioniuc, V.; Topa, D.; Van Den Berg, M.; Prins, U.; Bebeli, P.J.; Gabur, I. Assessment of Andean Lupin (Lupinus mutabilis) Genotypes for Improved Frost Tolerance. Agriculture 2021, 11, 155. [Google Scholar] [CrossRef]
- Motta, C.I.; Luong, J.C.; Seltmann, K.C. Plant–Arthropod Interactions of an Endangered California Lupine. Ecol. Evol. 2022, 12, e8688. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Luong, J.C.; Wishingrad, V.; Stratton, L.; Loik, M.E.; Meyer, R.S. Soil Biome Variation of Lupinus nipomensis in Wet-cool vs. Dry-warm Microhabitats and Greenhouse. Am. J. Bot. 2025, 112, e70020. [Google Scholar] [CrossRef]
- Eckstein, R.L.; Welk, E.; Klinger, Y.P.; Lennartsson, T.; Wissman, J.; Ludewig, K.; Hansen, W.; Ramula, S. Biological Flora of Central Europe—Lupinus polyphyllus Lindley. Perspect. Plant Ecol. Evol. Syst. 2023, 58, 125715. [Google Scholar] [CrossRef]
- Jakobsson, A.; Padrón, B.; Ågren, J. Distance-Dependent Effects of Invasive Lupinus polyphyllus on Pollination and Reproductive Success of Two Native Herbs. Basic Appl. Ecol. 2015, 16, 120–127. [Google Scholar] [CrossRef]
- Sõber, V.; Ramula, S. Seed Number and Environmental Conditions Do Not Explain Seed Size Variability for the Invasive Herb Lupinus polyphyllus. Plant Ecol. 2013, 214, 883–892. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Erskine, W.; Berger, J.D.; Nelson, M.N. Phenotypic Characterisation and Linkage Mapping of Domestication Syndrome Traits in Yellow Lupin (Lupinus luteus L.). Theor. Appl. Genet. 2020, 133, 2975–2987. [Google Scholar] [CrossRef]
- Chalampuente-Flores, D.; Mosquera-Losada, M.R.; Ron, A.M.D.; Tapia Bastidas, C.; Sørensen, M. Morphological and Ecogeographical Diversity of the Andean Lupine (Lupinus mutabilis Sweet) in the High Andean Region of Ecuador. Agronomy 2023, 13, 2064. [Google Scholar] [CrossRef]
- Bernhardt, C.E.; Mitchell, R.J.; Michaels, H.J. Effects of Population Size and Density on Pollinator Visitation, Pollinator Behavior, and Pollen Tube Abundance in Lupinus perennis. Int. J. Plant Sci. 2008, 169, 944–953. [Google Scholar] [CrossRef]
- Helenurm, K.; Schaal, B.A. Genetic and Maternal Effects on Offspring Fitness in Lupinus texensis (Fabaceae). Am. J. Bot. 1996, 83, 1596–1608. [Google Scholar] [CrossRef]
- Gulisano, A.; Alves, S.; Rodriguez, D.; Murillo, A.; Van Dinter, B.-J.; Torres, A.F.; Gordillo-Romero, M.; Torres, M.D.L.; Neves-Martins, J.; Paulo, M.-J.; et al. Diversity and Agronomic Performance of Lupinus mutabilis Germplasm in European and Andean Environments. Front. Plant Sci. 2022, 13, 903661. [Google Scholar] [CrossRef] [PubMed]
- Plants of the World Online. 2025. Available online: https://powo.science.kew.org/ (accessed on 10 December 2025).
- Kurlovich, B.S. Lupins: Geography, Classification, Genetic Resources and Breeding; Intan: St. Petersbourg, Russia, 2002; ISBN 978-5-86741-034-6. [Google Scholar]
- Wang, H.; Ran, N.; Jiang, H.-Q.; Wang, Q.-Q.; Ye, M.; Bowler, P.A.; Jin, X.-F.; Ye, Z.-M. Complex Floral Traits Shape Pollinator Attraction to Flowering Plants in Urban Greenspaces. Urban For. Urban Green. 2024, 91, 128165. [Google Scholar] [CrossRef]
- Miño Gallardo, O.; López, G.; Valencia Yaguana, D.; Yupa Ortiz, A. Polinizadores del cultivo de chocho (Lupinus mutabilis Sweet) en sistemas de producción orgánica y convencional. Rev. Alfa 2023, 7, 309–324. [Google Scholar] [CrossRef]
- Ortiz Barbosa, G.S.O. Biología Reproductiva de Lupinus sp. en el Páramo El Verjón. Bachelor’s Thesis, Universidad de Los Andes, Bogotá, Colombia, 2015. [Google Scholar]
- Gulisano, A.; Lippolis, A.; Van Loo, E.N.; Paulo, M.-J.; Trindade, L.M. A Genome Wide Association Study to Dissect the Genetic Architecture of Agronomic Traits in Andean Lupin (Lupinus mutabilis). Front. Plant Sci. 2023, 13, 1099293. [Google Scholar] [CrossRef]
- Burnette, T.E.; Eckhart, V.M. Evolutionary Divergence of Potential Drought Adaptations between Two Subspecies of an Annual Plant: Are Trait Combinations Facilitated, Independent, or Constrained? Am. J. Bot. 2021, 108, 309–319. [Google Scholar] [CrossRef]
- Moreira, X.; Castagneyrol, B.; Abdala-Roberts, L.; Traveset, A. A Meta-analysis of Herbivore Effects on Plant Attractiveness to Pollinators. Ecology 2019, 100, e02707. [Google Scholar] [CrossRef]
- Rusman, Q.; Lucas-Barbosa, D.; Poelman, E.H.; Dicke, M. Ecology of Plastic Flowers. Trends Plant Sci. 2019, 24, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Josephs, E.B.; Stinchcombe, J.R.; Wright, S.I. What Can Genome-wide Association Studies Tell Us about the Evolutionary Forces Maintaining Genetic Variation for Quantitative Traits? New Phytol. 2017, 214, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Pancaldi, F.; Gulisano, A.; Severing, E.I.; Van Kaauwen, M.; Finkers, R.; Kodde, L.; Trindade, L.M. The Genome of Lupinus mutabilis: Evolution and Genetics of an Emerging Bio-based Crop. Plant J. 2024, 120, 881–900. [Google Scholar] [CrossRef]
- Prass, M.; Ramula, S.; Jauni, M.; Setälä, H.; Kotze, D.J. The Invasive Herb Lupinus polyphyllus Can Reduce Plant Species Richness Independently of Local Invasion Age. Biol. Invasions 2022, 24, 425–436. [Google Scholar] [CrossRef]
- Ludewig, K.; Klinger, Y.P.; Donath, T.W.; Bärmann, L.; Eichberg, C.; Thomsen, J.G.; Görzen, E.; Hansen, W.; Hasselquist, E.M.; Helminger, T.; et al. Phenology and Morphology of the Invasive Legume Lupinus polyphyllus along a Latitudinal Gradient in Europe. NeoBiota 2022, 78, 185–206. [Google Scholar] [CrossRef]
- Moyroud, E.; Glover, B.J. The Evolution of Diverse Floral Morphologies. Curr. Biol. 2017, 27, R941–R951. [Google Scholar] [CrossRef]
- Li, S.-L.; Vasemägi, A.; Ramula, S. Genetic Variation and Population Structure of the Garden Escaper Lupinus polyphyllus in Finland. Plant Syst. Evol. 2016, 302, 399–407. [Google Scholar] [CrossRef]
- Australian Government the Biology of Lupinus L. 2013. Available online: https://www.ogtr.gov.au/sites/default/files/files/2021-07/the_biology_of_lupins.pdf (accessed on 10 December 2025).
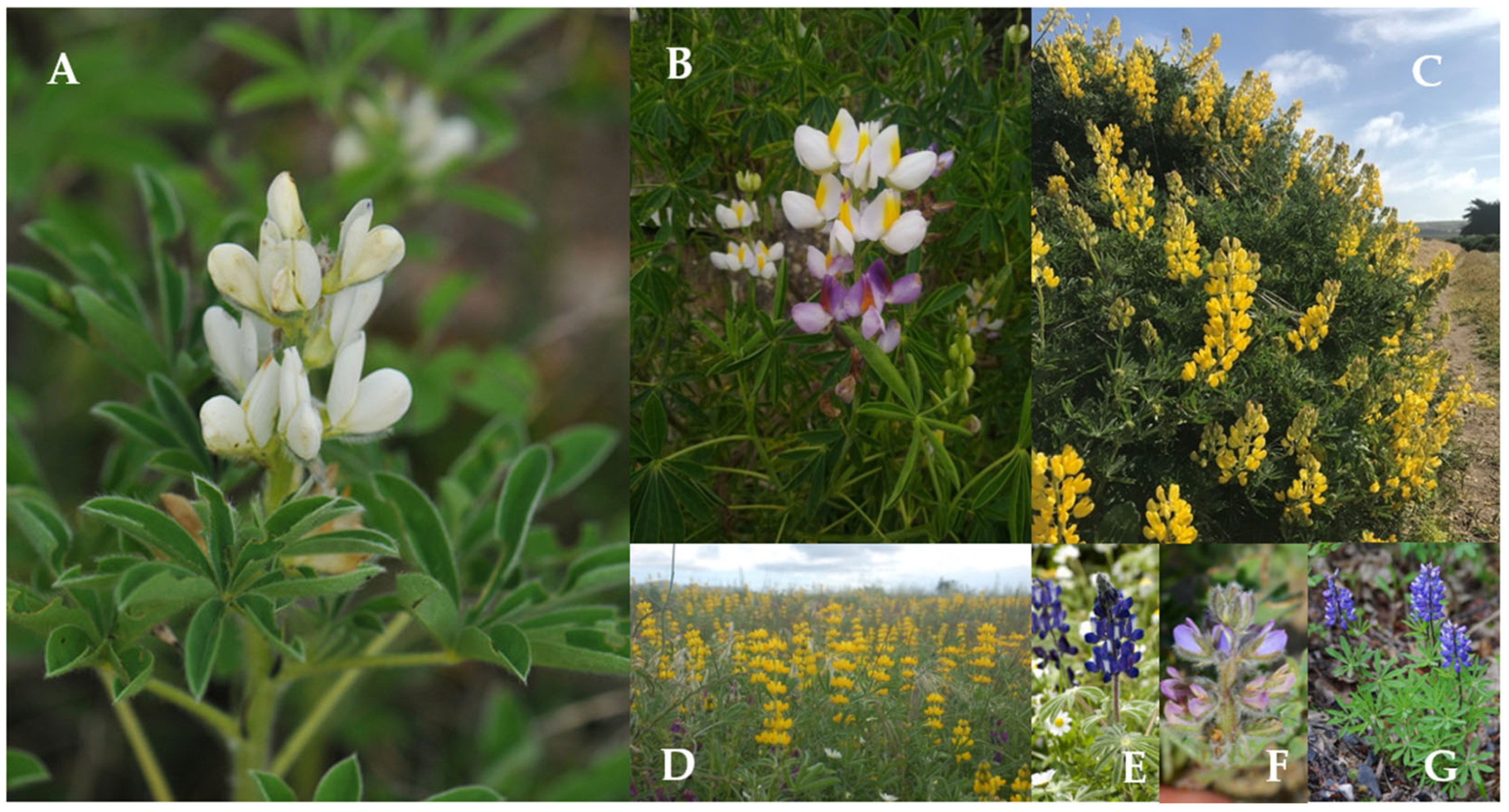
| Biotic Factors | ||
| Species | Trait | Reference |
| L. albus L. | Pollen is the only resource, benefitting specialist pollinators | [31] |
| Shortening of flowering duration due to visitor activity | [32] | |
| L. angustifolius L. | Pollen is the only resource which benefits specialist pollinators | [31] |
| Synchronization between floral phenology and pollinator activity | [33] | |
| L. arboreus Sims. | Cross-pollination increases seed viability | [34] |
| L. argenteus Pursh | Yellow color maximizes flower attraction | [35] |
| Produces metabolites that filter pollinators | [36] | |
| L. nanus Douglas ex Benth. | Self-pollination increases when pollinator numbers decline | [27] |
| L. perennis L. | Distribution depends largely on its pollinator | [37] |
| L. pilosus L. | Presence of deceptive and honest signals under low pollinator numbers | [25] |
| L. sp. | Shape and size of the corolla affect the number of visitors | [38] |
| Abiotic Factors | ||
| Species | Trait | Reference |
| L. albus L. | High drought tolerance | [39] |
| Possesses private alleles for water stress | [40] | |
| Vernalization is an adaptation to cold | [41] | |
| Flowering time is altered by temperature and light exposure | [42] | |
| Environmental factors increase genetic and phenotypic variability | [43] | |
| L. angustifolius L. | Production of smaller seeds under drought | [29] |
| Regulation of microbiota enables resistance to poor soils and droughts | [44] | |
| Environmental factors increase genetic and phenotypic variability | [43] | |
| Soil resource availability (nutrients, water, texture) and cultivation medium significantly influence root architecture (length, branching, biomass) and phenotypic plasticity | [45] | |
| L. arcticus S. Watson | Scent production regulates pollination in cold climates | [28] |
| L. bakeri Greene | Alkaloids in pollen deter pollen thieves | [46] |
| L. elegans Kunth | Climatic factors regulate its phenotypic variability | [47] |
| L. hispanicus Boiss. & Reut. | Altered phenology and vernalization response under elevated temperatures, potentially leading to flowering desynchronization and reduced frost resistance | [48] |
| L. luteus L. | Hormonal regulation to withstand climatic factors | [49] |
| L. mutabilis Sweet | Elevation affects reproductive success and inflorescence morphology | [22] |
| Frost resistance down to −10 °C | [50] | |
| L. nipomensis Eastw | Mutualistic interaction enabling growth under climatic stress | [51] |
| Adjustment of rhizospheric microbiota in extreme habitats | [52] | |
| L. polyphyllus Lindl. | Tolerance of temperatures down to −5 °C | [53] |
| Physiological adjustment to tolerate poor soils | [54] | |
| Hormonal regulation to withstand climatic factors | [49] | |
| Increase in seed size in extreme environments | [55] | |
| L. sulphureus Douglas | Alkaloids in pollen deter pollen thieves | [46] |
| Combined Factors | ||
| Species | Trait | Reference |
| L. argenteus Pursh | Alkaloids in pollen deter pollen thieves; nectar production ceases in arid sites | [46] |
| L. luteus L. | Flower plasticity is a response to pollinator preferences and climatic factors | [56] |
| L. mutabilis Sweet | Color and shape are traits that co-evolve with pollinators; Seed adaptation to higher radiation and lower competition at higher elevations | [57] |
| L. perennis L. | Pollinator behavior, especially in small/dense populations, and abiotic conditions, such as weather, light, temperature, interact to determine pollination efficiency, seed set, and genetic diversity. | [58] |
| L. texensis Hook. | Pollinator-mediated outcrossing and soil resources (nutrients, water, light) interact to influence early-life survival, reproductive success, and inbreeding depression across populations. | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Burke Irazoque, M.; Moraes R., M.; Lozada-Gobilard, S. Biotic and Abiotic Drivers of Phenotypic Diversity in the Genus Lupinus (Fabaceae). Plants 2026, 15, 456. https://doi.org/10.3390/plants15030456
Burke Irazoque M, Moraes R. M, Lozada-Gobilard S. Biotic and Abiotic Drivers of Phenotypic Diversity in the Genus Lupinus (Fabaceae). Plants. 2026; 15(3):456. https://doi.org/10.3390/plants15030456
Chicago/Turabian StyleBurke Irazoque, Mateo, Mónica Moraes R., and Sissi Lozada-Gobilard. 2026. "Biotic and Abiotic Drivers of Phenotypic Diversity in the Genus Lupinus (Fabaceae)" Plants 15, no. 3: 456. https://doi.org/10.3390/plants15030456
APA StyleBurke Irazoque, M., Moraes R., M., & Lozada-Gobilard, S. (2026). Biotic and Abiotic Drivers of Phenotypic Diversity in the Genus Lupinus (Fabaceae). Plants, 15(3), 456. https://doi.org/10.3390/plants15030456






