Exploring Chemical Composition of the Aerial Parts of Vernoniastrum migeodii and Anti-Inflammatory Activity of the Compounds
Abstract
1. Introduction
2. Results
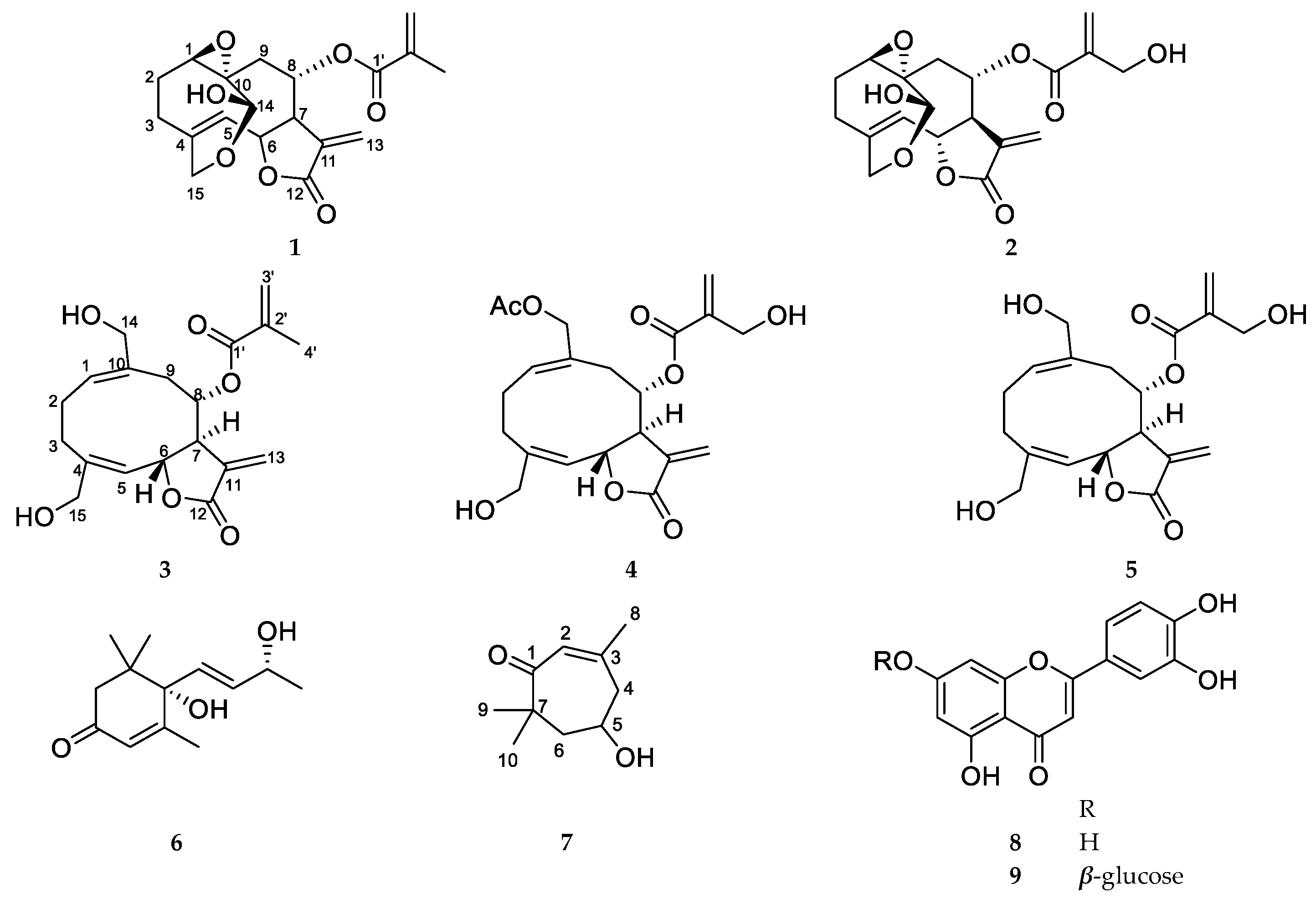
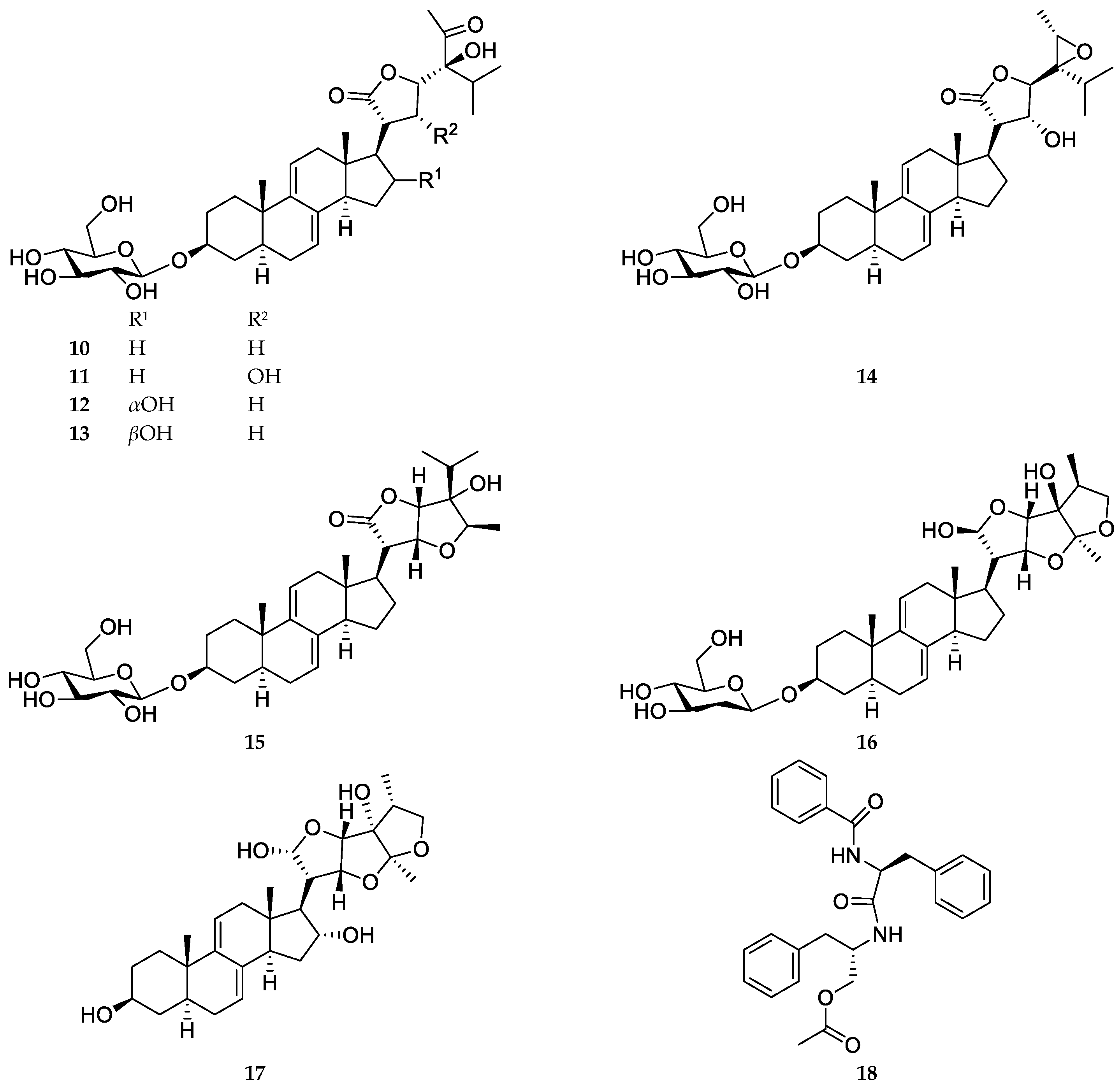
2.1. Compound Identification
2.2. Anti-Inflammatory Assay
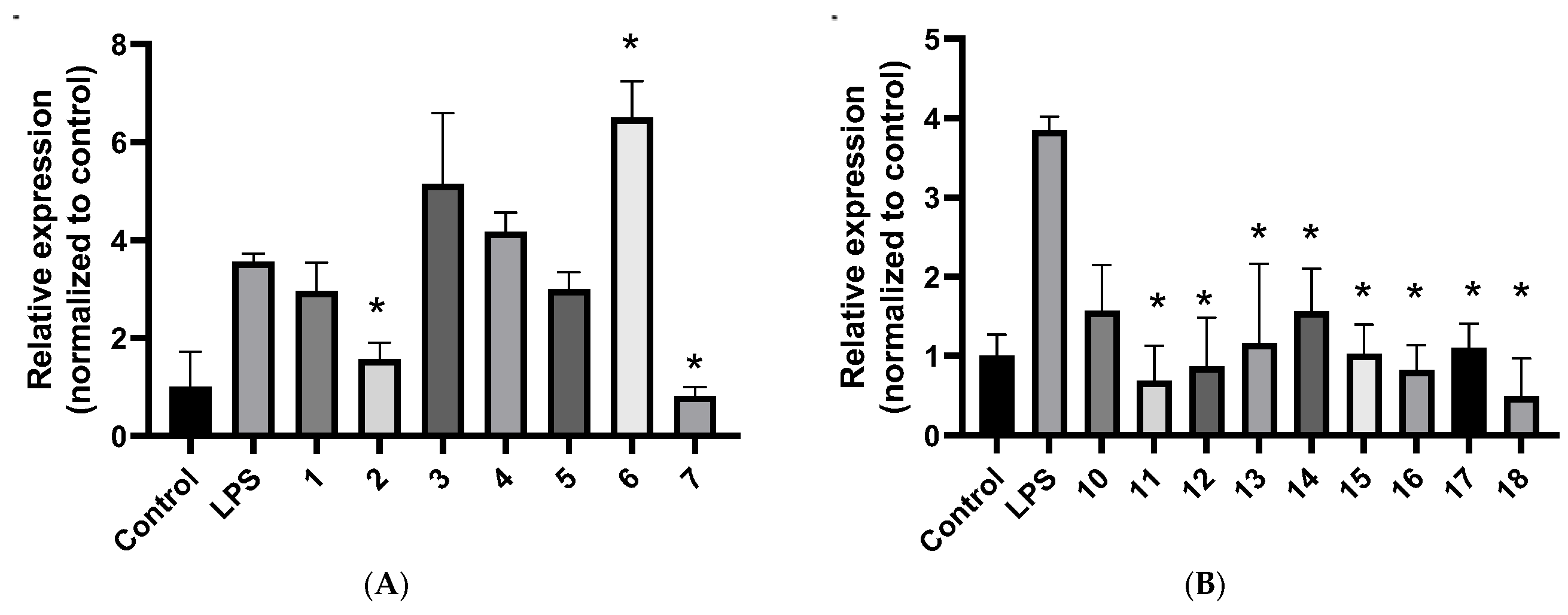
2.3. Gene Expression Analysis by qPCR
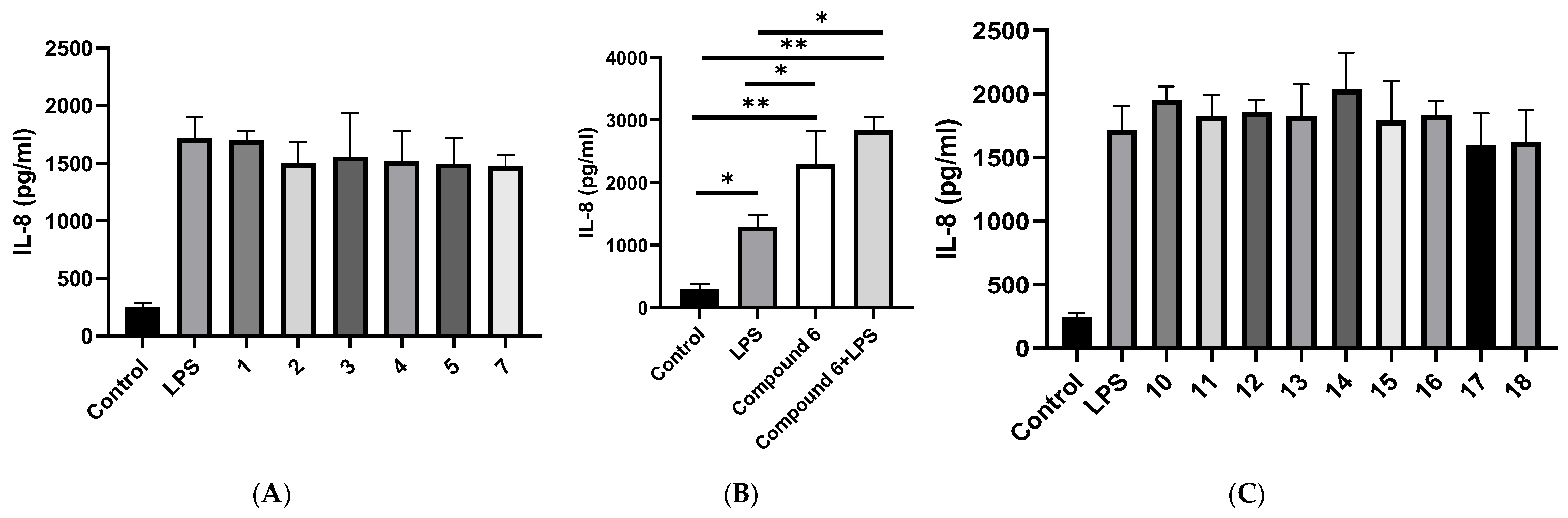
2.4. Cytokine Measurements by ELISA
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Spectroscopic Data of the Isolated Compounds
4.5. Anti-Inflammatory Assay
4.6. mRNA Extraction and cDNA Synthesis
4.7. Quantitative PCR (qPCR) Validation of the Target Genes
4.8. Cytokine Measurements
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| IL6 | interleukin-6 |
| IL1β | interleukin-1β |
| PTGS2 | prostaglandin-endoperoxide synthase 2 |
| ELISA | enzyme-linked immunosorbent assay |
| LPS | lipopolysaccharide |
| TNF-α | tumor necrosis factor alpha |
| SLs | sesquiterpene lactones |
| VLC | vacuum liquid chromatography |
| FC | flash chromatography |
| PDA | photodiode-array |
| TLC | thin-layer chromatography |
| NARICT | National Research Institute for Chemical Technology |
| NP-FC | normal-phase flash chromatography |
| NP-HPLC | normal-phase HPLC |
| GF | gel filtration |
| RP-HPLC | reversed-phase HPLC |
| NP-VLC | normal-phase vacuum-liquid chromatography |
| qPCR | quantitative PCR |
| SD | standard deviation |
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Han, J.; Yang, G.; Song, Z.; Zou, K.; Lv, K.; Lin, Z.; Ma, L.; Liu, M.; Feng, Y.; et al. New sesquiterpenoids with anti-inflammatory effects from phytopathogenic fungus Bipolaris sorokiniana. Nat. Prod. Bioprospect. 2025, 15, 11134. [Google Scholar]
- Wang, J.; Huo, X.; Wang, H.; Dong, A.; Zheng, Q.; Si, J. Undescribed sesquiterpene coumarins from the aerial parts of Ferula sinkiangensis and their anti-inflammatory activities in lipopolysaccharide-stimulated RAW 264.7 macrophages. Phytochemistry 2023, 210, 113664. [Google Scholar]
- Habtemariam, S. Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol. Biomedicines 2023, 11, 545. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, T.; Ma, X.; Guo, S.; Zhou, Q.; Zahoor, A.; Deng, G. Recent Advances in Anti-Inflammatory Active Components and Action Mechanisms of Natural Medicines. Inflammopharmacology 2023, 31, 2901–2937. [Google Scholar] [CrossRef]
- Choo, M.Z.Y.; Chua, J.A.T.; Lee, S.X.Y.; Ang, Y.; Wong, W.S.F.; Chai, C.L.L. Privileged Natural Product Compound Classes for Anti-Inflammatory Drug Development. Nat. Prod. Rep. 2025, 42, 856–875. [Google Scholar] [CrossRef] [PubMed]
- Dantas, L.B.R.; Alcântara, I.S.; Júnior, C.P.S.; De Oliveira, M.R.C.; Martins, A.O.B.P.B.; Dantas, T.M.; Ribeiro-Filho, J.; Coutinho, H.D.M.; Passos, F.R.S.; Quintans-Júnior, L.J.; et al. In Vivo and in Silico Anti-Inflammatory Properties of the Sesquiterpene Valencene. Biomed. Pharmacother. 2022, 153, 113478. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Ke, C.; Yao, S.; Feng, Z.; Tang, C.; Ye, Y. Sesquiterpene Lactones from Artemisia verlotorum and Their Anti-Inflammatory Activities. Fitoterapia 2023, 169, 105560. [Google Scholar] [CrossRef]
- Shen, X.; Chen, H.; Zhang, H.; Luo, L.; Wen, T.; Liu, L.; Hu, Q.; Wang, L. A Natural Sesquiterpene Lactone Isolinderalactone Attenuates Lipopolysaccharide-Induced Inflammatory Response and Acute Lung Injury through Inhibition of NF-κB Pathway and Activation Nrf2 Pathway in Macrophages. Int. Immunopharmacol. 2023, 124, 110965. [Google Scholar]
- Chaturvedi, D.; Dwivedi, P.K. Recent Developments on the Antidiabetic Sesquiterpene Lactones and Their Semisynthetic Analogues. In Discovery and Development of Antidiabetic Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 185–207. [Google Scholar]
- Shulha, O.; Zidorn, C. Sesquiterpene Lactones and Their Precursors as Chemosystematic Markers in the Tribe Cichorieae of the Asteraceae Revisited: An Update (2008–2017). Phytochemistry 2019, 163, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Mangisa, M.; Peter, X.K.; Khosa, M.C.; Fouche, G.; Nthambeleni, R.; Senabe, J.; Tarirai, C.; Tembu, V.J. Ethnomedicinal and Phytochemical Properties of Sesquiterpene Lactones from Dicoma (Asteraceae) and Their Anticancer Pharmacological Activities: A Review. Sci. Afr. 2021, 13, e00919. [Google Scholar] [CrossRef]
- Rabelo, M.B.O.; Freitas, K.M.; Oliveira, L.C.; Frade, A.C.M.; Filho, J.D.S.; Heiden, G.; Fernandes, G.W.; Silva, I.T.; Teixeira, M.M.; Braga, F.C.; et al. Activity of sesquiterpene lactones and umbelliferone from campovassouria cruciata on SARS-CoV-2 replication and on the release of pro-inflammatory cytokines in lung cells. Chem. Biodivers. 2025, 22, e202402824. [Google Scholar] [CrossRef]
- Meng, X.H.; Lv, H.; Ding, X.G.; Jian, T.Y.; Guo, D.L.; Feng, X.J.; Ren, B.R.; Chen, J. Sesquiterpene lactones with anti-inflammatory and cytotoxic activities from the roots of Cichorium intybus. Phytochemistry 2022, 203, 113377. [Google Scholar] [CrossRef]
- Kłeczek, N.; Malarz, J.; Gierlikowska, B.; Skalniak, Ł.; Galanty, A.; Kiss, A.K.; Stojakowska, A. Germacranolides from Carpesium divaricatum: Some new data on cytotoxic and anti-inflammatory activity. Molecules 2021, 26, 4644. [Google Scholar] [CrossRef]
- Zhong, W.; Li, M.; Han, S.; Sun, J.; Cao, L.; Mu, Z.; Du, X.; Cui, Y.; Feng, Y.; Zhong, G. Carpelipines C and D, two anti-inflammatory germacranolides from the flowers of Carpesium lipskyi Winkl. (Asteraceae). Chem. Biodivers. 2022, 19, e202200415. [Google Scholar] [CrossRef]
- McKinnon, R.; Binder, M.; Zupkó, I.; Afonyushkin, T.; Lajter, I.; Vasas, A.; de Martin, R.; Unger, C.; Dolznig, H.; Diaz, R.; et al. Pharmacological insight into the anti-inflammatory activity of sesquiterpene lactones from Neurolaena lobata (L.) R.Br. ex Cass. Phytomedicine 2014, 21, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Orabueze, C.I.; Adesegun, S.A.; Nwafor, F.I.; Coker, H.A. Ethnobotanical survey of medicinal plants and herbal formulations used in management of malaria in Nsukka, South East, Nigeria. Niger. J. Nat. Prod. Med. 2017, 21, 66–81. [Google Scholar]
- Yazdani, M.; Barta, A.; Hetényi, A.; Saidu, M.B.; Gallah, U.S.; Berkecz, R.; Csámpai, A.; Burián, K.; Paróczai, D.; Ahmed, S.H.H.; et al. Vernomigeodiins A–D, highly oxygenated stigmastane-type steroids isolated from Vernoniastrum migeodii with anti-Herpes simplex virus activity. Phytochemistry 2026, 241, 114659. [Google Scholar] [CrossRef] [PubMed]
- Gosslau, A.; Li, S.; Ho, C.T.; Chen, K.Y.; Rawson, N.E. The importance of natural product characterization in studies of their anti-inflammatory activity. Mol. Nutr. Food Res. 2011, 55, 74–82. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug. Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Rabe, T.; Mullholland, D.; van Staden, J. Isolation and identification of antibacterial compounds from Vernonia colorata leaves. J. Ethnopharmacol. 2002, 80, 91–94. [Google Scholar] [CrossRef]
- Mompon, B.; Toubiana, R. Sesquiterpene lactones from Vernonia pectoralis Baker (Compositae). Stereochemistry of pectorolide, and structures of vernopectolides A and B. Tetrahedron 1976, 32, 2545–2548. [Google Scholar] [CrossRef]
- Demir, S.; Karaalp, C.; Bedir, E. Unusual sesquiterpenes from Centaurea athoa DC. Phytochem. Lett. 2016, 15, 245–250. [Google Scholar] [CrossRef]
- Atabaki, V.; Pourahmad, J.; Hosseinabadi, T. Phytochemical compounds from Jurinea macrocephala subsp. elbursensis and their cytotoxicity evaluation. S. Afr. J. Bot. 2021, 137, 399–405. [Google Scholar] [CrossRef]
- Cutillo, F.; Dellagreca, M.; Previtera, L.; Zarrelli, A. C13 Norisoprenoids from Brassica fruticulosa. Nat. Prod. Res. 2005, 19, 99–103. [Google Scholar] [CrossRef]
- César, A.N.; de Heluani, C.C.S.; Kotowicz, C.; Gedris, T.E.; Herz, W. A linear sesterterpene, two squalene derivatives and two peptide derivatives from Croton hieronymi. Phytochemistry 2003, 64, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Pai, Y.-F.; Tsai, T.-H. Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 2015, 63, 7700−7706. [Google Scholar] [CrossRef]
- Wang, M.J. Effect of LPS combined with ATP on the expression of IL-1β of human A549 cells regulated by NLRP3 inflammasome and its mechanism. Med. J. Chin. PLA 2016, 12, 395–400. [Google Scholar]
- Buccitelli, C.; Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Khabar, K.S.A. Post-transcriptional control during chronic inflammation and cancer: A focus on AU-rich elements. Cell. Mol. Life Sci. 2010, 67, 2937–2955. [Google Scholar] [CrossRef] [PubMed]
- Quasie, O.; Zhang, Y.-M.; Zhang, H.-J.; Luo, J.; Kong, L.-Y. Four new steroid saponins with highly oxidized side chains from the leaves of Vernonia amygdalina. Phytochem. Lett. 2016, 15, 16–20. [Google Scholar] [CrossRef]
- Fang, Z.; Fang, J.; Gao, C.; Wu, Y.; Yu, W. Aurantiamide acetate ameliorates lung inflammation in lipopolysaccharide-induced acute lung injury in mice. Biomed. Res. Int. 2022, 2022, 3510423. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.; Wajs-Bonikowska, A.; Magiera, A.; Wosiak, A.; Balcerczak, E.; Czerwińska, M.E.; Olszewska, M.A. Anti-inflammatory and antioxidant effects of (6S,9R)-vomifoliol from Gaultheria procumbens L.: In vitro and ex vivo study in human immune cell models. Int. J. Mol. Sci. 2025, 26, 1571. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yazdani, M.; Paróczai, D.; Barta, A.; Burián, K.; Hohmann, J. Exploring Chemical Composition of the Aerial Parts of Vernoniastrum migeodii and Anti-Inflammatory Activity of the Compounds. Plants 2026, 15, 321. https://doi.org/10.3390/plants15020321
Yazdani M, Paróczai D, Barta A, Burián K, Hohmann J. Exploring Chemical Composition of the Aerial Parts of Vernoniastrum migeodii and Anti-Inflammatory Activity of the Compounds. Plants. 2026; 15(2):321. https://doi.org/10.3390/plants15020321
Chicago/Turabian StyleYazdani, Morteza, Dóra Paróczai, Anita Barta, Katalin Burián, and Judit Hohmann. 2026. "Exploring Chemical Composition of the Aerial Parts of Vernoniastrum migeodii and Anti-Inflammatory Activity of the Compounds" Plants 15, no. 2: 321. https://doi.org/10.3390/plants15020321
APA StyleYazdani, M., Paróczai, D., Barta, A., Burián, K., & Hohmann, J. (2026). Exploring Chemical Composition of the Aerial Parts of Vernoniastrum migeodii and Anti-Inflammatory Activity of the Compounds. Plants, 15(2), 321. https://doi.org/10.3390/plants15020321










