Sexual Dimorphism on a Conserved Scaffold: Insights from the Floral Ontogeny of Eurychorda (Restionaceae: Poales)
Abstract
1. Introduction
2. Results
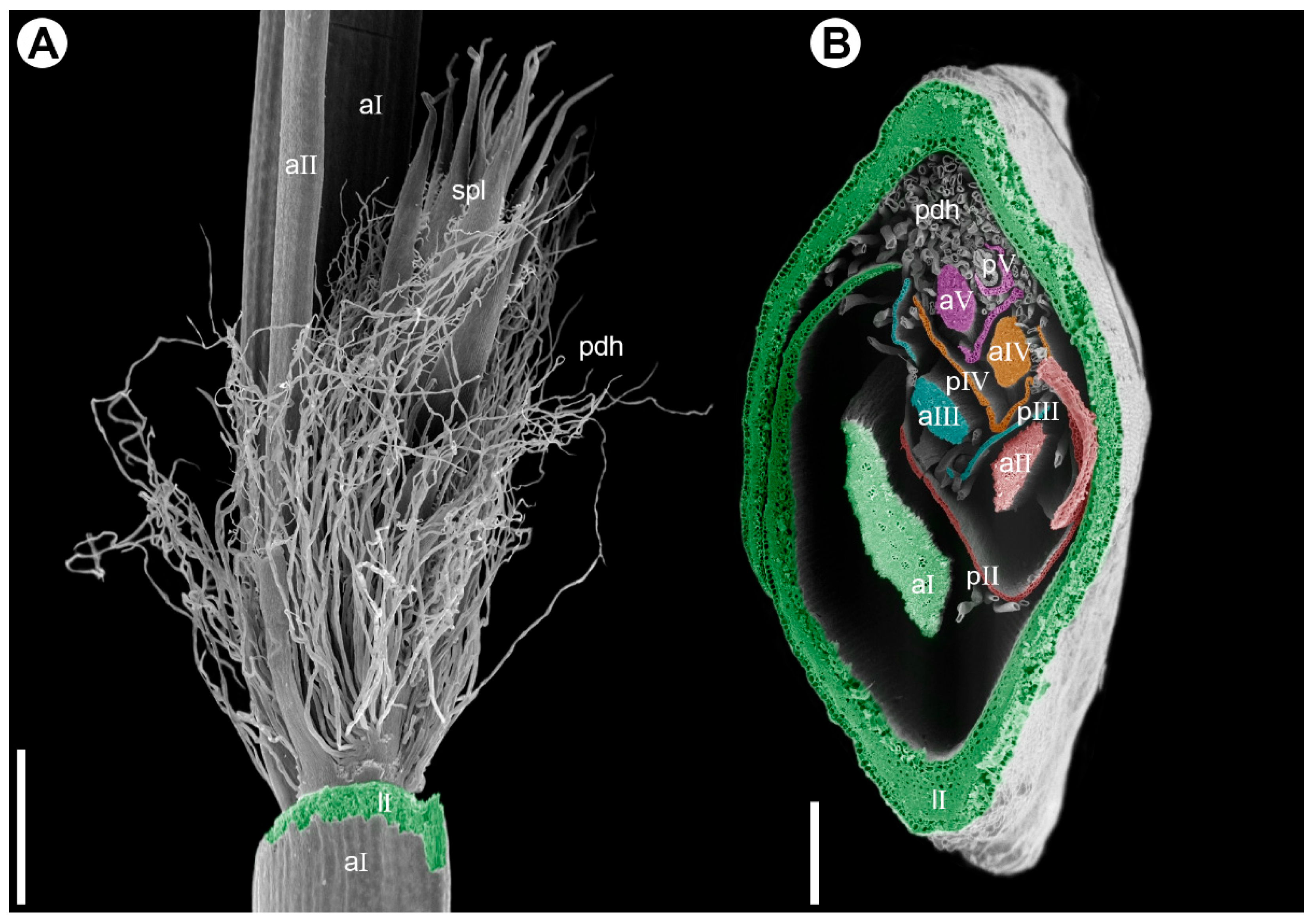
2.1. Inflorescence Morphology
2.2. Floral Organography
2.3. Flower Development
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jabbour, F.; Espinosa, F.; Dejonghe, Q.; Le Péchon, T. Development and Evolution of Unisexual Flowers: A Review. Plants 2022, 11, 155. [Google Scholar] [CrossRef]
- Guerrero-Méndez, C.; Abraham-Juárez, M.J. Factors Specifying Sex Determination in Maize. Plant Reprod. 2024, 37, 171–178. [Google Scholar] [CrossRef]
- Hartwig, T.; Chuck, G.S.; Fujioka, S.; Klempien, A.; Weizbauer, R.; Potluri, D.P.V.; Choe, S.; Johal, G.S.; Schulz, B. Brassinosteroid Control of Sex Determination in Maize. Proc. Natl. Acad. Sci. USA 2011, 108, 19814–19819. [Google Scholar] [CrossRef]
- Acosta, I.F.; Laparra, H.; Romero, S.P.; Schmelz, E.; Hamberg, M.; Mottinger, J.P.; Moreno, M.A.; Dellaporta, S.L. Tasselseed1 Is a Lipoxygenase Affecting Jasmonic Acid Signaling in Sex Determination of Maize. Science 2009, 323, 262–265. [Google Scholar] [CrossRef]
- Wu, X.; Knapp, S.; Stamp, A.; Stammers, D.K.; Jörnvall, H.; Dellaporta, S.L.; Oppermann, U. Biochemical Characterization of TASSELSEED 2, an Essential Plant Short-chain Dehydrogenase/Reductase with Broad Spectrum Activities. FEBS J. 2007, 274, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- DeLong, A.; Calderon-Urrea, A.; Dellaporta, S.L. Sex Determination Gene TASSELSEED2 of Maize Encodes a Short-Chain Alcohol Dehydrogenase Required for Stage-Specific Floral Organ Abortion. Cell 1993, 74, 757–768. [Google Scholar] [CrossRef]
- APG IV. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [CrossRef]
- Meney, K.A.; Pate, J.S. Australian Rushes: Biology, Identification and Conservation of Restionaceae and Allied Families; University of Western Australia Press: Nedlands, Australia, 1999; ISBN 978-1-876268-01-5. [Google Scholar]
- Linder, H.P.; Briggs, B.G.; Johnson, L.A.S. Restionaceae. In The Families and Genera of Flowering Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 3, pp. 401–425. [Google Scholar]
- Ueberfeld, M. Beiträge zur Kenntnis des sexuellen Dimorphismus der Restionaceen. Bot. Jahrb. Syst. 1926, 60, 175–206. [Google Scholar]
- Kircher, P. Untersuchungen zur Blüten- und Infloreszenzmorphologie, Embryologie und Systematik der Restionaceen im Vergleich mit Gramineen und Verwandten Familien; J. Cramer: Berlin, Germany, 1986; ISBN 978-3-443-64006-4. [Google Scholar]
- Fomichev, C.I.; Briggs, B.G.; Macfarlane, T.D.; Sokoloff, D.D. Structure and Development of Female Flowers in Early-Diverging Restiids, Anarthria, Lyginia and Hopkinsia (Restionaceae s.l.): Further Evidence of Multiple Pathways of Gynoecium Reduction in Wind-Pollinated Lineages of Poales. Bot. J. Linn. Soc. 2019, 190, 117–150. [Google Scholar] [CrossRef]
- Fomichev, C.I.; Macfarlane, T.D.; Briggs, B.G.; Sokoloff, D.D. Hygroscopic Awns and Inflorescence Architecture in a Wind-Pollinated Australian Monocot: Functional Convergence with Grasses. Ann. Bot. 2025, mcaf167. [Google Scholar] [CrossRef]
- Briggs, B.G.; Marchant, A.D.; Perkins, A.J. Phylogeny of the Restiid Clade (Poales) and Implications for the Classification of Anarthriaceae, Centrolepidaceae and Australian Restionaceae. Taxon 2014, 63, 24–46. [Google Scholar] [CrossRef]
- Omland, K.E.; Cook, L.G.; Crisp, M.D. Tree Thinking for All Biology: The Problem with Reading Phylogenies as Ladders of Progress. BioEssays 2008, 30, 854–867. [Google Scholar] [CrossRef]
- Endress, P.K.; Doyle, J.A. Reconstructing the Ancestral Angiosperm Flower and Its Initial Specializations. Am. J. Bot. 2009, 96, 22–66. [Google Scholar] [CrossRef]
- Weberling, F. Morphology of Flowers and Inflorescences; Cambridge University Press: Cambridge, UK, 1989; ISBN 978-0-521-25134-1. [Google Scholar]
- Vegetti, C.; Anton, A.M. The Grass Inflorescence. In Grasses: Systematics and Evolution; Jacobs, S.W.L., Everett, J., Eds.; CSIRO: Melbourne, Australia, 2000; pp. 29–31. [Google Scholar]
- Endress, P.K. Disentangling Confusions in Inflorescence Morphology: Patterns and Diversity of Reproductive Shoot Ramification in Angiosperms. J. Syst. Evol. 2010, 48, 225–239. [Google Scholar] [CrossRef]
- Kuznetzova, T.V.; Timonin, A.C. Inflorescence: Morphology, Evolution, Taxonomic Significance; KMK: Moscow, Russia, 2017; ISBN 978-5-9500591-9-3. [Google Scholar]
- Vegetti, A.C. Synflorescence Typology in Cyperaceae. Ann. Bot. Fenn. 2003, 40, 35–46. [Google Scholar]
- Barrett, S.C.H.; Hough, J. Sexual Dimorphism in Flowering Plants. J. Exp. Bot. 2013, 64, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Barrett, S.C.H. Wind of Change: New Insights on the Ecology and Evolution of Pollination and Mating in Wind-Pollinated Plants. Ann. Bot. 2009, 103, 1515–1527. [Google Scholar] [CrossRef]
- Golonka, A.M.; Sakai, A.K.; Weller, S.G. Wind Pollination, Sexual Dimorphism, and Changes in Floral Traits of Schiedea (Caryophyllaceae). Am. J. Bot. 2005, 92, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Linder, H.P.; Rudall, P.J. Evolutionary History of Poales. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 107–124. [Google Scholar] [CrossRef]
- Jeal, K.; Bartlett, M. Bracts and beyond—Spikelet Diversity in the Wider Poales: A Commentary on ‘Hygroscopic Awns and Inflorescence Architecture in a Wind-Pollinated Australian Monocot: Functional Convergence with Grasses’. Ann. Bot. 2025, mcaf279. [Google Scholar] [CrossRef]
- Prusinkiewicz, P.; Erasmus, Y.; Lane, B.; Harder, L.D.; Coen, E. Evolution and Development of Inflorescence Architectures. Science 2007, 316, 1452–1456. [Google Scholar] [CrossRef]
- Harder, L.D.; Prusinkiewicz, P. The Interplay between Inflorescence Development and Function as the Crucible of Architectural Diversity. Ann. Bot. 2013, 112, 1477–1493. [Google Scholar] [CrossRef]
- Pagel, M. The Maximum Likelihood Approach to Reconstructing Ancestral Character States of Discrete Characters on Phylogenies. Syst. Biol. 1999, 48, 612–622. [Google Scholar] [CrossRef]
- Joy, J.B.; Liang, R.H.; McCloskey, R.M.; Nguyen, T.; Poon, A.F.Y. Ancestral Reconstruction. PLoS Comput. Biol. 2016, 12, e1004763. [Google Scholar] [CrossRef]
- Cooke, D.A. Centrolepidaceae. In The Families and Genera of Flowering Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 3, pp. 106–109. [Google Scholar]
- Sokoloff, D.D.; Remizowa, M.V.; Linder, H.P.; Macfarlane, T.; Rudall, P.J. Arrangement of Reproductive Units in Centrolepis (Poales: Centrolepidaceae): Cincinnus or Spikelet? In Diversity, Phylogeny, and Evolution in the Monocotyledons; Seberg, O., Petersen, G., Barford, A.S., Davis, J.I., Eds.; Aarhus University Press: Aarhus, Denmark, 2010; pp. 425–436. [Google Scholar]
- Sokoloff, D.D.; Remizowa, M.V.; Barrett, M.D.; Conran, J.G.; Rudall, P.J. Morphological Diversity and Evolution of Centrolepidaceae (Poales), a Species-poor Clade with Diverse Body Plans and Developmental Patterns. Am. J. Bot. 2015, 102, 1219–1249. [Google Scholar] [CrossRef] [PubMed]
- Hamann, U. Beitrag zur Embryologie der Centrolepidaceae mit Bemerkungen über den Bau der Blüten und Blütenstände und die Systematische Stellung der Familie. Ber. Dtsch. Bot. Ges. 1962, 75, 153–171. [Google Scholar]
- Cooke, D.A. A Taxonomic Revision of Aphelia (Centrolepidaceae). J. Adel. Bot. Gard. 1995, 16, 95–109. [Google Scholar]
- Ronse De Craene, L.P.; Linder, H.P.; Smets, E.F. Floral Ontogenetic Evidence in Support of the Willdenowia Clade of South African Restionaceae. J. Plant Res. 2001, 114, 329–342. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P.; Linder, H.P.; Smets, E.F. Ontogeny and Evolution of the Flowers of South African Restionaceae with Special Emphasis on the Gynoecium. Plant Syst. Evol. 2002, 231, 225–258. [Google Scholar] [CrossRef]
- Endress, P.K.; Igersheim, A. The Reproductive Structures of the Basal Angiosperm Amborella trichopoda (Amborellaceae). Int. J. Plant Sci. 2000, 161, S237–S248. [Google Scholar] [CrossRef]
- Mitchell, C.H.; Diggle, P.K. The Evolution of Unisexual Flowers: Morphological and Functional Convergence Results from Diverse Developmental Transitions. Am. J. Bot. 2005, 92, 1068–1076. [Google Scholar] [CrossRef]
- Diggle, P.K.; Di Stilio, V.S.; Gschwend, A.R.; Golenberg, E.M.; Moore, R.C.; Russell, J.R.W.; Sinclair, J.P. Multiple Developmental Processes Underlie Sex Differentiation in Angiosperms. Trends Genet. 2011, 27, 368–376. [Google Scholar] [CrossRef]
- Testolin, R.; Pilkington, S.M.; Akagi, T. Dioecy in Fruit Crops: The Gender Rise and Decline and Its Agronomic Impact. Front. Plant Sci. 2021, 12, 719588. [Google Scholar] [CrossRef]
- Barrett, S.C.H. Plant Sex: Best to Be Bisexual When Mates Are Scarce. Curr. Biol. 2021, 31, R298–R300. [Google Scholar] [CrossRef]
- Box, M.S.; Glover, B.J. A Plant Developmentalist’s Guide to Paedomorphosis: Reintroducing a Classic Concept to a New Generation. Trends Plant. Sci. 2010, 15, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Heterochrony and Allometry: The Analysis of Evolutionary Change in Ontogeny. Biol. Rev. 1998, 73, 79–123. [Google Scholar] [CrossRef] [PubMed]
- Geuten, K.; Coenen, H. Heterochronic Genes in Plant Evolution and Development. Front. Plant Sci. 2013, 4, 381. [Google Scholar] [CrossRef]
- Linder, H.P. The Evolution of Flowering Phenology: An Example from the Wind-Pollinated African Restionaceae. Ann. Bot. 2020, 126, 1141–1153. [Google Scholar] [CrossRef]
- Honig, M.A.; Linder, H.P.; Bond, W.J. Efficacy of Wind Pollination: Pollen Load Size and Natural Microgametophyte Populations in Wind-pollinated Staberoha banksii (Restionaceae). Am. J. Bot. 1992, 79, 443–448. [Google Scholar] [CrossRef]
- Briggs, B.G.; Johnson, L.A.S. New Genera and Species of Australian Restionaceae (Poales). Telopea 1998, 7, 345–373. [Google Scholar] [CrossRef]
- Timerman, D.; Barrett, S.C.H. The Biomechanics of Pollen Release: New Perspectives on the Evolution of Wind Pollination in Angiosperms. Biol. Rev. 2021, 96, 2146–2163. [Google Scholar] [CrossRef] [PubMed]
- Timerman, D.; Greene, D.F.; Urzay, J.; Ackerman, J.D. Turbulence-Induced Resonance Vibrations Cause Pollen Release in Wind-Pollinated Plantago lanceolata L. (Plantaginaceae). J. R. Soc. Interface 2014, 11, 20140866. [Google Scholar] [CrossRef] [PubMed]
- Timerman, D.; Barrett, S.C.H. Comparative Analysis of Pollen Release Biomechanics in Thalictrum: Implications for Evolutionary Transitions between Animal and Wind Pollination. New Phytol. 2019, 224, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Briggs, B.G. Leptocarpus (Restionaceae) Enlarged to Include Meeboldina and Stenotalis, with New Western Australian Species and Subgenera. Telopea 2014, 16, 19–41. [Google Scholar] [CrossRef]
- Briggs, B.G.; Connelly, C.L.; Krauss, S.L. Leptocarpus denmarkicus. Available online: https://profiles.ala.org.au/opus/foa/profile/Leptocarpus%20denmarkicus (accessed on 7 November 2025).
- Igersheim, A.; Cichocki, O. A Simple Method for Microtome Sectioning of Prehistoric Charcoal Specimens, Embedded in 2-Hydroxyethyl Methacrylate (HEMA). Rev. Palaeobot. Palynol. 1996, 92, 389–393. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Fomichev, C.I.; Briggs, B.G.; Sokoloff, D.D. Sexual Dimorphism on a Conserved Scaffold: Insights from the Floral Ontogeny of Eurychorda (Restionaceae: Poales). Plants 2026, 15, 97. https://doi.org/10.3390/plants15010097
Fomichev CI, Briggs BG, Sokoloff DD. Sexual Dimorphism on a Conserved Scaffold: Insights from the Floral Ontogeny of Eurychorda (Restionaceae: Poales). Plants. 2026; 15(1):97. https://doi.org/10.3390/plants15010097
Chicago/Turabian StyleFomichev, Constantin I., Barbara G. Briggs, and Dmitry D. Sokoloff. 2026. "Sexual Dimorphism on a Conserved Scaffold: Insights from the Floral Ontogeny of Eurychorda (Restionaceae: Poales)" Plants 15, no. 1: 97. https://doi.org/10.3390/plants15010097
APA StyleFomichev, C. I., Briggs, B. G., & Sokoloff, D. D. (2026). Sexual Dimorphism on a Conserved Scaffold: Insights from the Floral Ontogeny of Eurychorda (Restionaceae: Poales). Plants, 15(1), 97. https://doi.org/10.3390/plants15010097





