Cell Structure and Dynamics of Galactomannan Secretion in Caesalpinia pulcherrima (Leguminosae) Endosperm
Abstract
1. Introduction
2. Results
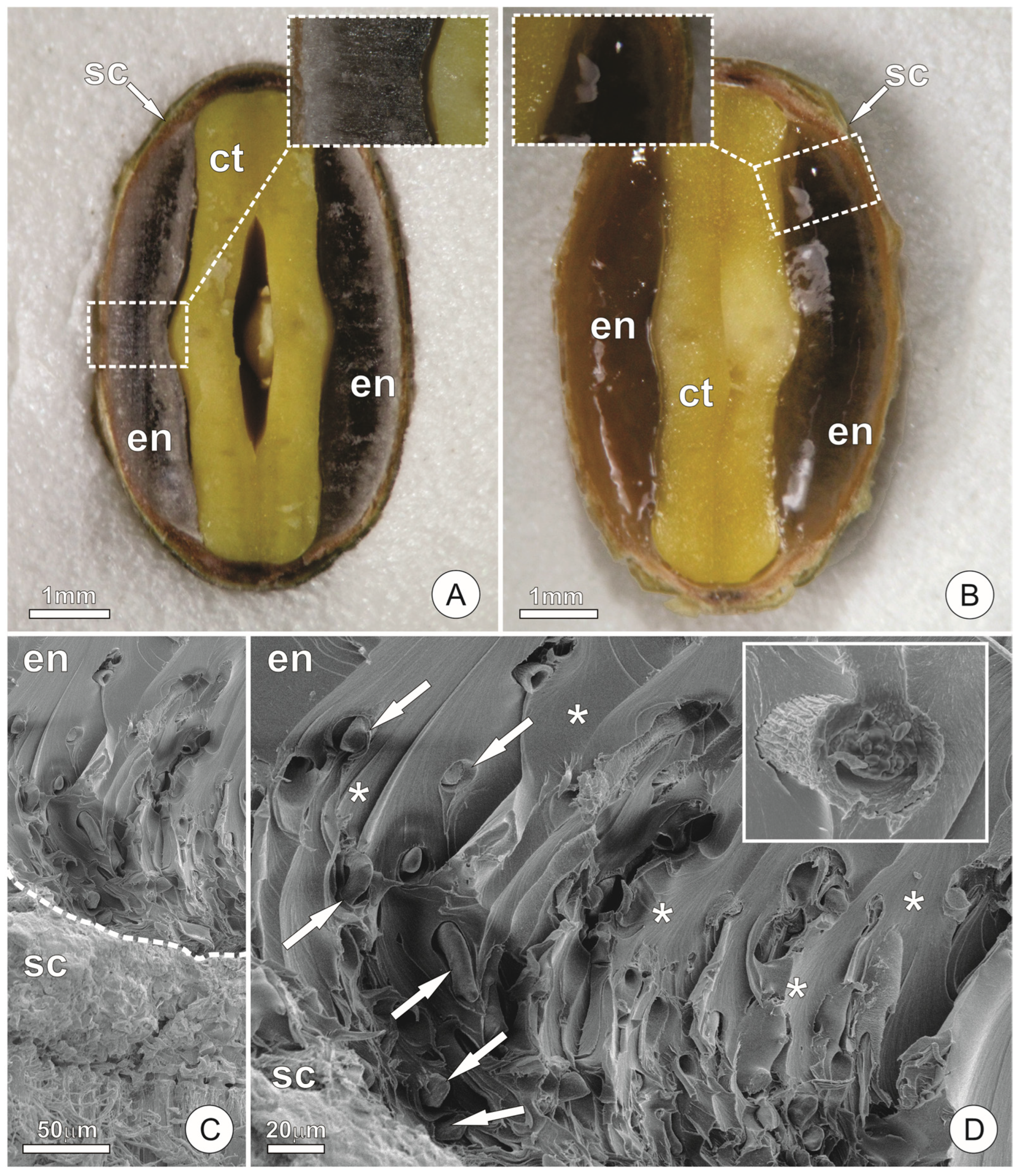
2.1. Endosperm Structure
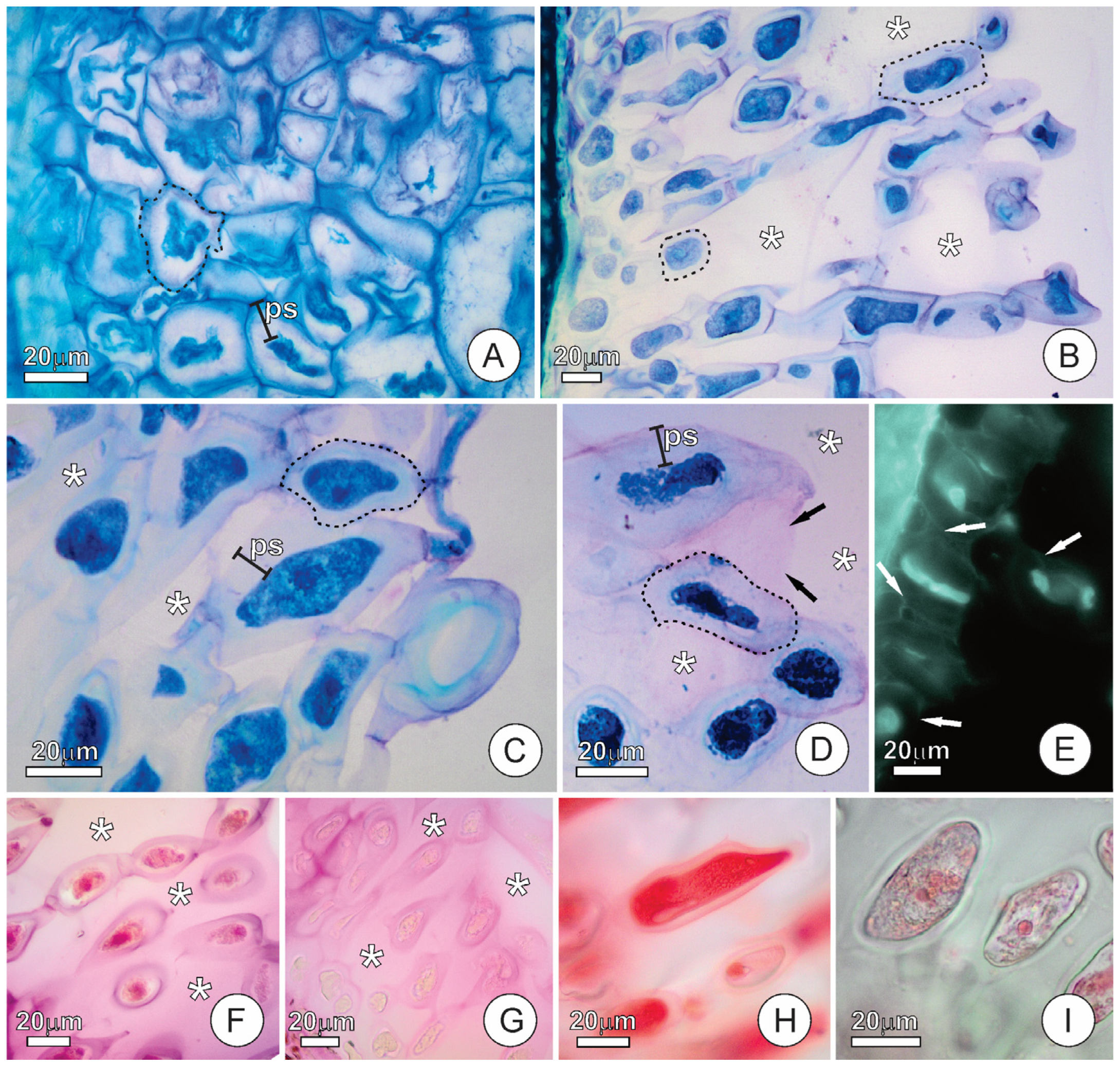
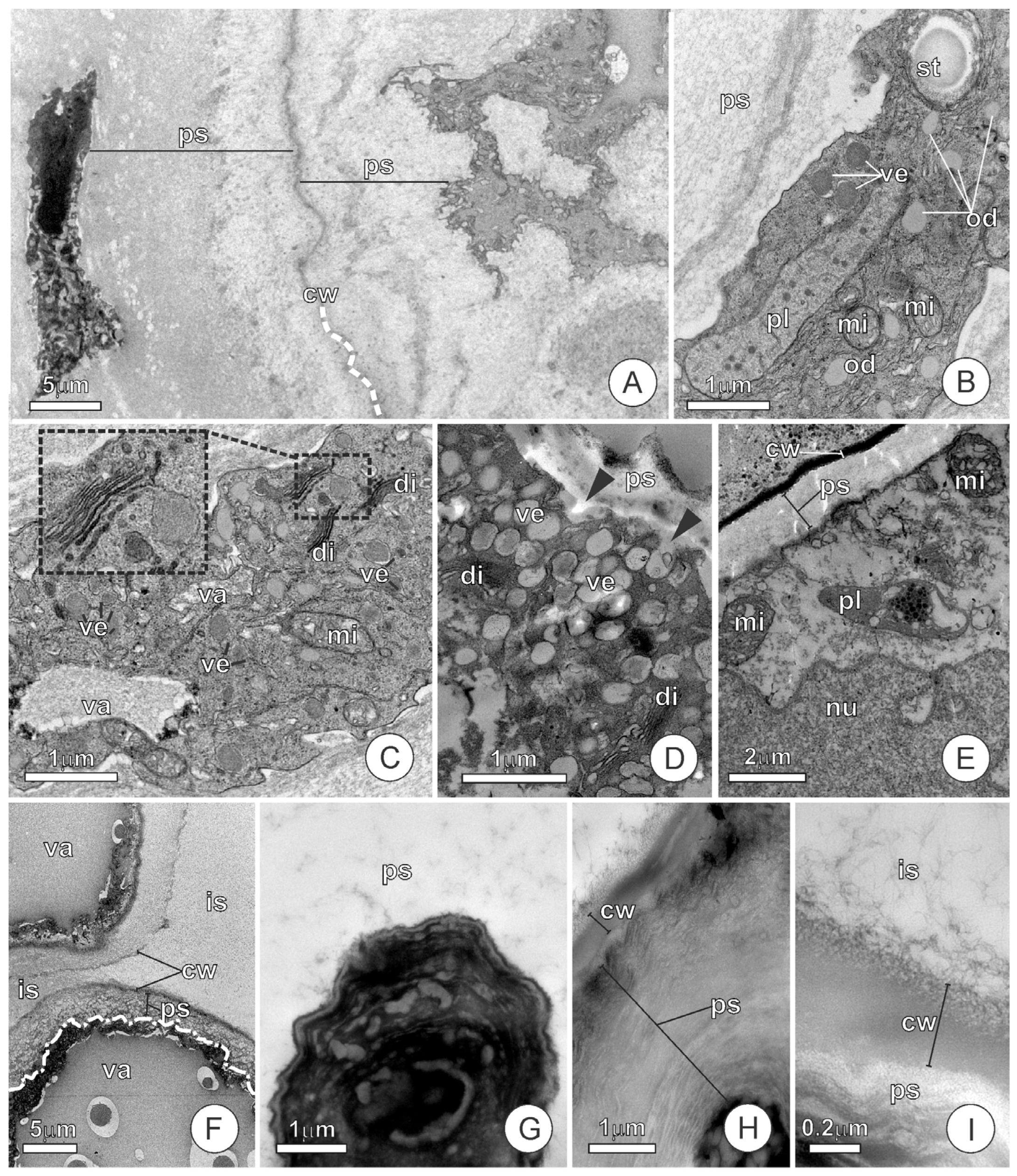
2.2. Endosperm Ultrastructure and Polysaccharides Exudation
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sampling
4.2. Light Microscopy and Histochemistry
4.3. Electron Microscopy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CWSP | Cell wall storage polysaccharides |
| TEM | Transmission electron microscopy |
References
- Gunn, C.R. Fruits and Seeds of Genera in the Subfamily Caesalpinioideae (Fabaceae); U.S. Department of Agriculture: Washington, DC, USA, 1991; 408p.
- Meier, H.; Reid, J.S.G. Reserve polysaccharides other than starch in higher plants. In Plant Carbohydrates I: Encyclopedia of Plant Physiology; Loewus, F.A., Tanner, W., Eds.; Springer: Berlin, Germany, 1982; Volume 13/A, pp. 418–471. [Google Scholar] [CrossRef]
- Lorenzi, H.; Souza, H.M.; Cerqueira, L.S.C.; Costa, J.T.M.; Ferreira, E. (Eds.) Árvores Exóticas No Brasil: Madeireiras, Ornamentais e Aromáticas; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brazil, 2003. [Google Scholar]
- Thombre, N.A.; Gide, P.S. Rheological characterization of galactomannans extracted from seeds of Caesalpinia pulcherrima. Carbohydr. Polym. 2013, 94, 547–554. [Google Scholar] [CrossRef]
- Gagnon, E.; Bruneau, A.; Hughes, C.E.; Queiroz, L.; Lewis, G.P. A new generic system for the pantropical Caesalpinia group (Leguminosae). PhytoKeys 2016, 71, 1–160. [Google Scholar] [CrossRef]
- Jing, W.; Zhang, X.; Zhou, H.; Wang, Y.; Yang, M.; Long, L.; Gao, H. Naturally occurring cassane diterpenoids (CAs) of Caesalpinia: A systematic review of its biosynthesis, chemistry and pharmacology. Fitoterapia 2019, 134, 226–249. [Google Scholar] [CrossRef]
- Andrade, C.T.; Azero, E.G.; Luciano, L.; Gonçalves, M.P. Solution properties of the galactomannans extracted from the seeds of Caesalpinia pulcherrima and Cassia javanica: Comparison with locust bean gum. Int. J. Biol. Macromol. 1999, 26, 181–185. [Google Scholar] [CrossRef]
- Dea, I.C.M.; Morrison, A. Chemistry and interactions of seed galactomannans. In Advances in Carbohydrate Chemistry and Biochemistry; Tipson, R.S., Horton, D., Eds.; Academic Press: New York, NY, USA, 1975; Volume 31, pp. 241–312. [Google Scholar] [CrossRef]
- Buckeridge, M.S.; Dietrich, S.M.C.; Lima, D.U. Galactomannans as the reserve carbohydrate in legume seeds. In Developments in Crop Science; Gupta, A.K., Kaur, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 26, pp. 283–316. [Google Scholar] [CrossRef]
- Pollard, M.A.; Eder, B.; Fischer, P.; Windhab, E.J. Characterization of galactomannans isolated from legume endosperms of Caesalpinioideae and Faboideae subfamilies by multidetection aqueous SEC. Carbohydr. Polym. 2010, 79, 70–84. [Google Scholar] [CrossRef]
- Unrau, A.M.; Choy, Y.M. Identification of linkages of a galactomannan isolated from seed of Caesalpinia pulcherrima. Carbohydr. Res. 1970, 14, 151–158. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Simões, J.; Teixeira, J.A.; Domingues, M.R.M.; Coimbra, M.A.; Vincente, A.A. Structural and thermal characterization of galactomannans from non-conventional sources. Carbohydr. Polym. 2011, 83, 179–185. [Google Scholar] [CrossRef]
- Bento, J.F.; Mazzaro, I.; Silva, L.M.D.A.; Moreira, R.A.; Ferreira, M.L.C.; Reicher, F.; Petkowicz, C.L.D.O. Diverse patterns of cell wall mannan/galactomannan occurrence in seeds of the Leguminosae. Carbohydr. Polym. 2013, 92, 192–199. [Google Scholar] [CrossRef]
- Otegui, M.S. Endosperm cell walls: Formation, composition, and functions. In Endosperm Plant Cell Monographs; Olsen, O.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 8, pp. 159–177. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Gallardo, K.; Thompson, R.; Burstin, J. Reserve accumulation in legume seeds. Comptes Rendus Biol. 2008, 331, 755–762. [Google Scholar] [CrossRef]
- Reid, J.S.G. Cell wall storage carbohydrates in seeds—Biochemistry of the seed “gums” and “hemicelluloses”. In Advances in Botanical Research; Callow, J.A., Woolhouse, H.W., Eds.; Academic Press: London, UK, 1985; Volume 11, pp. 125–155. [Google Scholar] [CrossRef]
- Rodríguez-Gacio, M.D.C.; Iglesias-Fernández, R.; Carbonero, P.; Matilla, Á.J. Softening-up mannan-rich cell walls. J. Exp. Bot. 2012, 63, 3976–3988. [Google Scholar] [CrossRef]
- Baldan, B.; Bonaldo, A.; Rascio, N.; Mariani, P. Cercis siliquastrum L.: A comparative study of endosperm and embryo development and reserve accumulation. Int. J. Plant Sci. 1995, 156, 181–187. [Google Scholar] [CrossRef]
- Rascio, N.; Mariani, P.; Perin, V.; Vecchia, F.D.; Castaldo, P. Seed of Cercis siliquastrum L. (Leguminosae, Caesalpinioideae): Reserves and their utilization after germination. Bot. Acta 1997, 110, 224–233. [Google Scholar] [CrossRef]
- Izydorczyk, M.; Cui, S.; Wang, Q. Polysaccharide gums: Structures, functional properties, and applications. In Food Carbohydrates: Chemistry, Physical Properties and Applications; Cui, S.W., Ed.; CRC Press: Boca Raton, FL, USA, 2005; 46p. [Google Scholar]
- Reid, J.S.G. Reserve carbohydrate metabolism in germinating seeds of Trigonella foenum-graecum L. (Leguminosae). Planta 1971, 100, 131–142. [Google Scholar] [CrossRef]
- Buckeridge, M.S.; Dietrich, S.M.C. Mobilisation of the raffinose family oligosaccharides and galactomannan in germinating seeds of Sesbania marginata Benth. (Leguminosae–Faboideae). Plant Sci. 1996, 117, 33–43. [Google Scholar] [CrossRef]
- Voiniciuc, C. Modern mannan: A hemicellulose’s journey. New Phytol. 2022, 234, 1175–1184. [Google Scholar] [CrossRef]
- Buckeridge, M.S. Seed cell wall storage polysaccharides: Models to understand cell wall biosynthesis and degradation. Plant Physiol. 2010, 154, 1017–1023. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, S.; Ramakrishna, G.; Srivastava, H.; Gaikwad, K. A comprehensive review on leguminous galactomannans: Structural analysis, functional properties, biosynthesis process and industrial applications. Crit. Rev. Food Sci. Nutr. 2020, 62, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.S.G.; Bewley, J.D. A dual role for the endosperm and its galactomannan reserves in the germinative physiology of fenugreek (Trigonella foenum-graecum L.), an endospermic leguminous seed. Planta 1979, 147, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.M. Biochemistry of plant galactomannans. In Advances in Carbohydrate Chemistry and Biochemistry; Tipson, R.S., Horton, D., Eds.; Academic Press: New York, NY, USA, 1978; Volume 35, pp. 341–376. [Google Scholar] [CrossRef]
- Reid, J.S.G.; Meier, H. The function of the aleurone layer during galactomannan mobilisation in germinating seeds of fenugreek (Trigonella foenum-graecum L.), crimson clover (Trifolium incarnatum L.) and lucerne (Medicago sativa L.): A correlative biochemical and ultrastructural study. Planta 1972, 106, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.S.G.; Meier, H. Formation of the endosperm galactomannan in leguminous seeds: Preliminary communications. Caryologia 1973, 25, 219–222. [Google Scholar] [CrossRef]
- Seiler, A. Galaktomannanabbau in keimenden Johannesbrotsamen (Ceratonia siliqua L.). Planta 1977, 134, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Azani, N.; Babineau, M.; Bailey, C.D.; Banks, H.; Barbosa, A.R.; Pinto, R.B.; Boatwright, J.S.; Borges, L.M.; Brown, G.K.; Bruneau, A.; et al. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny: The Legume Phylogeny Working Group (LPWG). Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef]
- McClendon, J.H.; Nolan, W.G.; Wenzler, H.F. The role of the endosperm in the germination of legumes: Galactomannan, nitrogen, and phosphorus changes in the germination of guar (Cyamopsis tetragonoloba; Leguminosae). Am. J. Bot. 1976, 63, 790–797. [Google Scholar] [CrossRef]
- Reid, J.S.G.; Davies, C.; Meier, H. Endo-β-mannanase, the leguminous aleurone layer and the storage galactomannan in germinating seeds of Trigonella foenum-graecum L. Planta 1977, 133, 219–222. [Google Scholar] [CrossRef]
- Hughes, S.G.; Overbeeke, N.; Robinson, S.; Pollock, K.; Smeets, F.L.M. Messenger RNA from isolated aleurone cells directs the synthesis of an alpha-galactosidase found in the endosperm during germination of guar (Cyamopsis tetragonaloba) seed. Plant Mol. Biol. 1988, 11, 783–789. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Matheson, N.K.; Small, D.M. Galactomannans and a galactoglucomannan in legume seed endosperms: Structural requirements for β-mannanase hydrolysis. Phytochemistry 1976, 15, 1111–1117. [Google Scholar] [CrossRef]
- Meier, H.; Reid, J.S.G. Morphological aspects of the galactomannan formation in the endosperm of Trigonella foenum-graecum L. (Leguminosae). Planta 1977, 133, 243–248. [Google Scholar] [CrossRef]
- Gagnon, E.; Lewis, G.P.; Solange Sotuyo, J.; Hughes, C.E.; Bruneau, A. A molecular phylogeny of Caesalpinia sensu lato: Increased sampling reveals new insights and more genera than expected. S. Afr. J. Bot. 2013, 89, 111–127. [Google Scholar] [CrossRef]
- Paiva, E.A.S. Colleters in Cariniana estrellensis (Lecythidaceae): Structure, secretion and evidences for young leaf protection. J. Torrey Bot. Soc. 2012, 139, 1–8. [Google Scholar] [CrossRef]
- Ballego-Campos, I.; Paiva, E.A.S. Colleters in the vegetative axis of Aechmea blanchetiana (Bromeliaceae): Anatomical, ultrastructural, and functional aspects. Aust. J. Bot. 2018, 66, 379. [Google Scholar] [CrossRef]
- Ballego-Campos, I.; Paiva, E.A.S. Mucilage secretion in the inflorescences of Aechmea blanchetiana: Evidence of new functions of scales in Bromeliaceae. Flora 2018, 246–247, 1–9. [Google Scholar] [CrossRef]
- Fahn, A. Secretory tissues in vascular plants. New Phytol. 1988, 108, 229–257. [Google Scholar] [CrossRef] [PubMed]
- Paiva, E.A.S. Occurrence, structure and functional aspects of the colleters of Copaifera langsdorffii Desf. (Fabaceae, Caesalpinioideae). Comptes Rendus Biol. 2009, 332, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.L.; Paiva, E.A.S. Colleters in Mabea fistulifera Mart. (Euphorbiaceae): Anatomy and biology of the secretory process. Flora 2019, 258, 151439. [Google Scholar] [CrossRef]
- Trachtenberg, S.; Fahn, A. The mucilage cells of Opuntia ficus-indica (L.) Mill.—Development, ultrastructure, and mucilage secretion. Bot. Gaz. 1981, 142, 206–213. [Google Scholar] [CrossRef]
- Barker, M.E.; Gerritsen, A.F. The Development of mucilage cells in Hibiscus schizopetalus. Acta Bot. Neerl. 1992, 41, 31–42. [Google Scholar] [CrossRef]
- Mastroberti, A.A.; Mariath, J.E.A. Development of mucilage cells of Araucaria angustifolia (Araucariaceae). Protoplasma 2008, 232, 233–245. [Google Scholar] [CrossRef]
- Paiva, E.A.S. How do secretory products cross the plant cell wall to be released? A new hypothesis involving cyclic mechanical actions of the protoplast. Ann. Bot. 2016, 117, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Ballego-Campos, I.; Forzza, R.C.; Paiva, E.A.S. More than scales: Evidence for the production and exudation of mucilage by the peltate trichomes of Tillandsia cyanea (Bromeliaceae: Tillandsioideae). Plants 2020, 9, 763. [Google Scholar] [CrossRef]
- DeMason, D.A. Endosperm structure and storage reserve histochemistry in the palm Washingtonia filifera. Am. J. Bot. 1986, 73, 1332–1340. [Google Scholar] [CrossRef]
- DeMason, D.A.; Sexton, R.; Gorman, M.; Reid, J.S.G. Structure and biochemistry of endosperm breakdown in date palm seeds. Protoplasma 1985, 126, 159–167. [Google Scholar] [CrossRef]
- Dias, G.P.; Mazzottini-dos-Santos, H.C.; Ribeiro, L.M.; Mercadante-Simões, M.O. Reserve mobilization dynamics and degradation pattern of mannan-rich cell walls in the recalcitrant seed of Mauritia flexuosa (Arecaceae). Plant Physiol. Biochem. 2020, 156, 445–460. [Google Scholar] [CrossRef]
- Buckeridge, M.S.; Rocha, D.C.; Reid, J.S.G.; Dietrich, S.M.C. Xyloglucan structure and post-germinative metabolism in seeds of Copaifera langsdorfii from Savanna and Forest populations. Physiol. Plant. 1992, 86, 145–151. [Google Scholar] [CrossRef]
- Tiné, M.A.S.; Cortelazzo, A.L.; Buckeridge, M.S. Xyloglucan mobilisation in cotyledons of developing plantlets of Hymenaea courbaril L. (Leguminosae-Caesalpinioideae). Plant Sci. 2000, 154, 117–126. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Co.: New York, NY, USA; London, UK, 1940. [Google Scholar]
- Paiva, E.A.S.; Pinho, S.Z.; Oliveira, D.M.T. Large plant samples: How to process for GMA embedding? In Light Microscopy: Methods and Protocols; Chiarini-Garcia, H., Melo, R.C.N., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 37–49. [Google Scholar] [CrossRef]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- O’Brien, T.P.; McCully, M.E. The Study of Plant Structure: Principles and Selected Methods; Termarcarphi Pty. Ltd.: Melbourne, Australia, 1981. [Google Scholar]
- Brundrett, M.C.; Kendrick, B.; Peterson, C.A. Efficient lipid staining in plant material with Sudan Red 7B or Fluoral Yellow 088 in Polyethylene Glycol–Glycerol. Biotech. Histochem. 1991, 66, 111–116. [Google Scholar] [CrossRef]
- Vidal, B.C. Dichroism in collagen bundles stained with Xylidine-Ponceau 2R. Ann. d’ Histochim. 1970, 15, 289–296. [Google Scholar]
- Soukup, A. Selected simple methods of plant cell wall histochemistry and staining for light microscopy. In Plant Cell Morphogenesis: Methods and Protocols; Žárský, V., Cvrčková, F., Eds.; Humana Press: Totowa, NJ, USA, 2014; pp. 25–40. [Google Scholar] [CrossRef]
- Hughes, J.; McCully, M.E. The Use of an Optical Brightener in the Study of Plant Structure. Stain Technol. 1975, 50, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Karnovsky, M.J. A formaldehyde–glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137A–138A. [Google Scholar]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Watson, M.L. Staining of tissue sections for electron microscopy with heavy metals. J. Cell Biol. 1958, 4, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bonifácio-Leite, V.; Paiva, É.A.S.; Oliveira, D.M.T. Cell Structure and Dynamics of Galactomannan Secretion in Caesalpinia pulcherrima (Leguminosae) Endosperm. Plants 2026, 15, 76. https://doi.org/10.3390/plants15010076
Bonifácio-Leite V, Paiva ÉAS, Oliveira DMT. Cell Structure and Dynamics of Galactomannan Secretion in Caesalpinia pulcherrima (Leguminosae) Endosperm. Plants. 2026; 15(1):76. https://doi.org/10.3390/plants15010076
Chicago/Turabian StyleBonifácio-Leite, Victor, Élder Antônio Sousa Paiva, and Denise M. T. Oliveira. 2026. "Cell Structure and Dynamics of Galactomannan Secretion in Caesalpinia pulcherrima (Leguminosae) Endosperm" Plants 15, no. 1: 76. https://doi.org/10.3390/plants15010076
APA StyleBonifácio-Leite, V., Paiva, É. A. S., & Oliveira, D. M. T. (2026). Cell Structure and Dynamics of Galactomannan Secretion in Caesalpinia pulcherrima (Leguminosae) Endosperm. Plants, 15(1), 76. https://doi.org/10.3390/plants15010076







