Physiological and Morphological Response Mechanisms of Theobroma cacao L. Rootstocks Under Flooding and Evaluation of Their Adaptability
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Conduction and Seedling Production
2.2. Gas Exchanges and Chlorophyll Relative Content
2.3. Stem and Leaf Anatomical Evaluations
2.4. Photosynthetic Pigments
2.5. Carbohydrate Extraction and Quantification
2.6. Statistical Analysis
3. Results
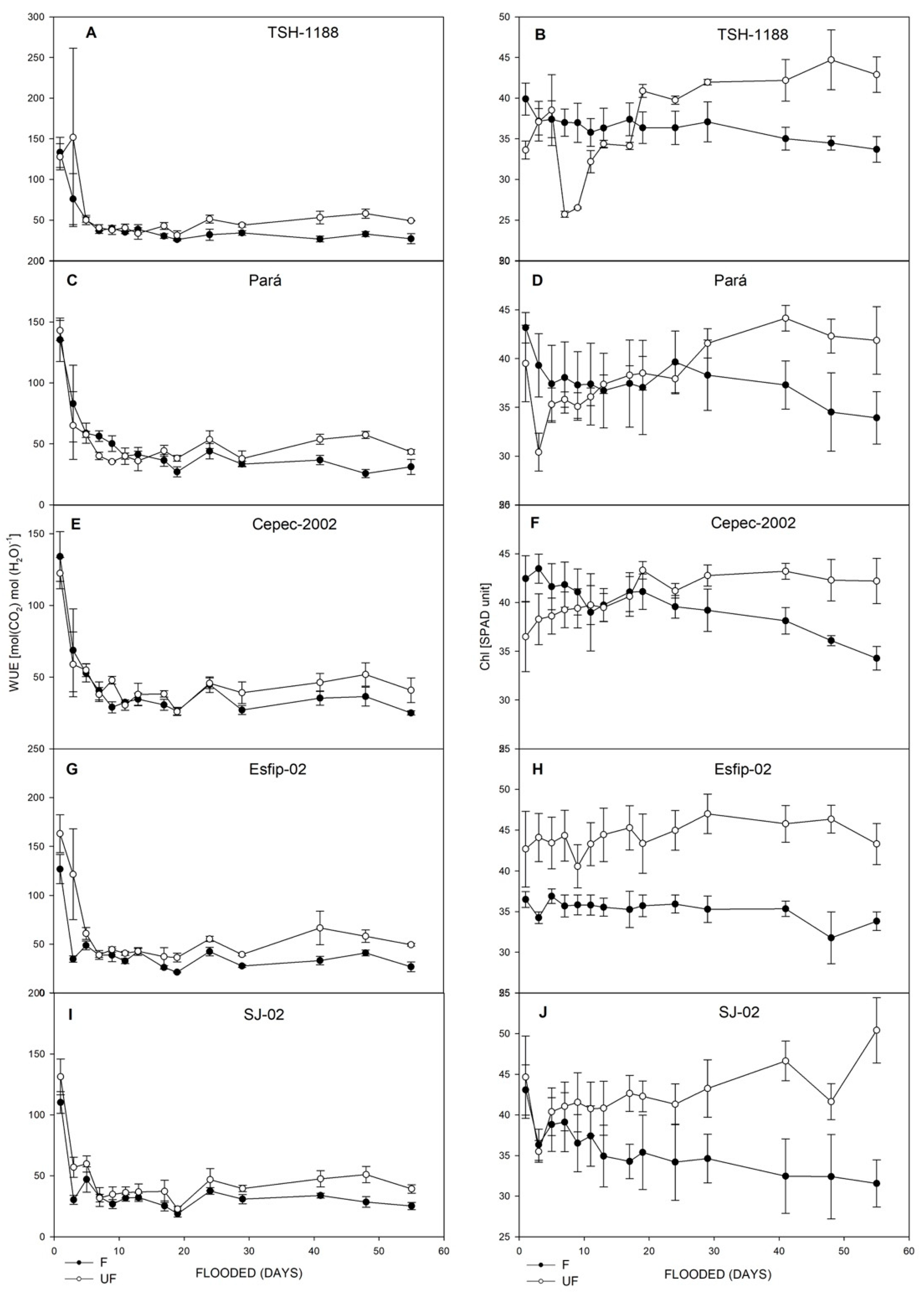
3.1. The Recovery of Gas Exchanges and SPAD
3.2. Stem and Leaf Anatomy
3.3. Flooding on Pigment Contents
3.4. Effect of Flooding and Recovery on the Carbohydrates
4. Discussion
4.1. Gas Exchange and SPAD
4.2. Adaptations of the Anatomy of the Stem and Leaf
4.3. Pigment Contents
4.4. The Carbohydrates
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ci/Ca | ratio between intra/extracellular CO2 concentrations |
| Chl a | chlorophyll a |
| Chl b | chlorophyll b |
| DNS | dinitrosalicylic acid method |
| E | transpiration |
| FAA 70% | formaldehyde, acetic acid, and 70% ethanol |
| gs | stomatal conductance |
| INCAPER | Capixaba Institute for Research, Technical Assistance and Rural Extension |
| PD/DE | polar diameter/equatorial diameter ratio |
| PN | net photosynthetic rate |
| RS | reducing sugars |
| SPAD | Soil–Plant Analysis Development |
| TSS | total soluble sugars |
| WUE | water-use efficiency |
References
- Lahive, F.; Hadley, P.; Daymond, A.J. The physiological responses of cacao to the environment and the implications for climate change resilience. A review. Agron. Sustain. Dev. 2019, 39, 5. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations—FAO: Faostat. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 21 June 2025).
- Oliveira, V.S.; Pinheiro, A.P.B.; Cerri Neto, B.; Arantes, S.D.; Silva, C.A.; Crasque, J.; Pinto, M.L.P.B.; Santos, G.P.; Pagoto, A.L.R.; Nascimento, A.L.; et al. Effect of Flooding Under the Gas Exchange of Cocoa Seedlings. J. Agric. Sci. 2019, 11, 233. [Google Scholar] [CrossRef]
- Souza, C.A.S.; Aguilar, M.A.G.; Dias, L.A.S.; Siqueira, P.R. Relações Hídrica e Irrigação. In Cacau: Do Plantio à Colheita; Souza, C.A.S., Dias, L.A.S., Aguilar, M.A.G., Borém, A., Eds.; UFV: Viçosa, Brazil, 2016; p. 287. [Google Scholar]
- Vidal, D.B.; Andrade, I.L.M.M.; Dalmolin, Â.; Mielke, M. Photosynthesis and growth of copaiba seedlings subjected to soil flooding. Floresta Ambiente 2019, 26, e20160596. [Google Scholar] [CrossRef]
- Sairam, R.K.; Kumutha, D.; Ezhilmathi, K.; Deshmukh, P.S.; Srivastava, G.C. Physiology and biochemistry of waterlogging tolerance in plants. Biol. Plant. 2008, 52, 401–412. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Aclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Yeung, E.; Bailey-Serres, J.; Sasidharan, R. After The Deluge: Plant Revival Post-Flooding. Trends Plant Sci. 2019, 24, 443–454. [Google Scholar] [CrossRef]
- Rosa, D.B.C.J.; Scalon, S.D.P.Q.; Cremon, T.; Dresch, D.M. Gas exchanges and antioxidant activity in Copaifera langsdorffii desf. seedlings after flooding. Am. J. Plant Sci. 2018, 9, 979–994. [Google Scholar] [CrossRef]
- Jackson, M.B.; Colmer, T.D. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef]
- Chen, X.; Visser, E.J.; De Kroon, H.; Pierik, R. Fitness consequences of natural variation in flooding induced shoot elongation in Rumex palustris. New Phytol. 2011, 190, 409–420. [Google Scholar] [CrossRef]
- Herrera, A.; Tezara, W.; Marin, O.; Rengifo, E. Estomatal and non estomatal limitations of photosynthesis in trees of a tropical seasonally flooded forest. Physiol. Plant. 2008, 134, 41–48. [Google Scholar] [CrossRef]
- Bertolde, F.Z.; Almeida, A.A.F.; Pirovani, C.P.; Gomes, F.P.; Ahnert, D.; Baligar, V.C.; Valle, R.R. Physiological and biochemical responses of Theobroma cacao L. genotypes to flooding. Photosynthetica 2012, 50, 447–457. [Google Scholar] [CrossRef]
- Pinto, M.L.P.B.; Crasque, J.; Cerri Neto, B.; Ferreira, T.R.; Souza, C.A.S.; Falqueto, A.R.; Souza, T.C.; Machado Filho, J.A.; Arantes, L.O.; Dousseau-Arantes, S. Morphophysiological responses of Theobroma cacao L. rootstocks to flooding and post-flooding conditions. Photosynthetica 2023, 61, 377–389. [Google Scholar] [CrossRef]
- Sena Gomes, A.R.; Kozlowski, T.T. The effects of flooding on water relations and growth of Theobroma cacao var. catongo seedlings. J. Hortic. Sci. 1986, 61, 265–276. [Google Scholar] [CrossRef]
- Almeida, A.-A.F.; Valle, R.R. Ecophysiology of the cacao tree. Braz. J. Plant Physiol. 2007, 19, 425–448. [Google Scholar] [CrossRef]
- Sodré, G.A. Formação de Mudas de Cacaueiro, Onde Nasce a Boa Cacauicultura; Boletim Técnico; CEPLAC/CEPEC: Itabuna, Bazil, 2013. [Google Scholar]
- Dousseau, S.; Alvarenga, A.A.; Castro, E.M.; Soares, R.P.; Emrich, E.B.; Melo, L.A. Anatomia foliar de Tabebuia serratifolia (Vahl) Nich. (Bignoniaceae) propagadas in vitro, in vivo e durante a aclimatização. Cienc. Agrotecnol. 2008, 32, 1694–1700. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolates choroplasts. Polyphenoloxidade in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Duke, S.O.; Kenyon, W.H. Effects of dimethazone (FMC 57020) on chloroplast development II. Pigment synthesis and photosynthetic function in cowpea (Vigna unguiculata L.) primary leaves. Pestic. Biochem. Physiol. 1986, 25, 11–18. [Google Scholar] [CrossRef]
- Sandmann, G.; Böger, P. Comporison of the bleaching activity of Norflurazon and Oxyfluorfen. Weed Sci. 1983, 31, 338–341. [Google Scholar] [CrossRef]
- Zanandrea, I.; Alves, J.D.; Deuner, S.; Goulart, P.F.P.; Henrique, P.D.C.; Silveira, N.M. Tolerance of Sesbania virgata plants to flooding. Aust. J. Bot. 2010, 57, 661–669. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.I. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. J. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar 4.3 Sistema de Análises Estatísticas; UFLA: Lavras, Brazil, 1999. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 21 June 2025).
- Dalmolin, A.C.; Dalmagro, H.J.; Lobo, F.; Antunes, M.Z.; Ortíz, C.E.R.; Vourlitis, G.L. Photosynthetic light and carbon dioxide response of the invasive tree Vochysia divergens Pohl to experimental flooding and shading. Photosynthetica 2013, 51, 379–386. [Google Scholar] [CrossRef]
- Branco, M.C.S.; Almeida, A.A.F.; Dalmolin, Â.C.; Ahnert, D.; Baligar, V.C. Influence of low light intensity and soil flooding on cacao physiology. Sci. Hortic. 2017, 217, 243–257. [Google Scholar] [CrossRef]
- Bertolde, F.Z.; Almeida, A.A.F.; Corrêa, R.X.; Gomes, F.P.; Gaiotto, F.A.; Baligar, V.C.; Loguércio, L.L. Molecular, physiological and morphological analysis of waterlogging tolerance in clonal genotypes of Theobroma cacao L. Tree Physiol. 2010, 30, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Klumb, E.K.; Braga, E.J.B.; Bianchi, V.J. Differential expression of genes involved in the response of Prunus spp. rootstocks under soil flooding. Sci. Hortic. 2019, 261, 109038. [Google Scholar] [CrossRef]
- Mielke, M.S.; De Almeida, A.A.F.; Gomes, F.P.; Aguilar, M.A.G.; Mangabeira, P.A.O. Leaf gas exchange, chlorophyll fluorescence and growth responses of Genipa americana seedlings to soil flooding. Environ. Exp. Bot. 2003, 50, 221–231. [Google Scholar] [CrossRef]
- Li, F.; Bao, W.; Wu, N.; You, C. Growth, biomass partitioning, and water-use efficiency of a leguminous shrub (Bauhinia faberi var. Microphylla) in response to various water availabilities. New For. 2008, 36, 53–65. [Google Scholar] [CrossRef]
- Silva, C.E.M.; Gonçalves, J.F.C.; Feldpausch, T.R. Water-use efficiency of tree species following calcium and phosphorus application on an abandoned pasture, central amazonia, Brazil. Environ. Exp. Bot. 2008, 64, 189–195. [Google Scholar] [CrossRef]
- Mielke, M.S.; De Almeida, A.A.F.; Gomes, F.P.; Mangabeira, P.A.O.; Silva, D.D.C. Effects of soil flooding on leaf gas exchange and growth of two neotropical pioneer tree species. New For. 2005, 29, 161–168. [Google Scholar] [CrossRef]
- Camison, Á.; Martín, M.Á.; Dorado, F.J.; Moreno, G.; Solla, A. Changes in carbohydrates induced by drought and waterlogging in Castanea sativa. Trees 2019, 34, 579–591. [Google Scholar] [CrossRef]
- Lourenço, L.F.G.; Guerreiro, R.G.O.; Romagnolo, M.B.; Pastorini, L.; Souza, L.A. Efeito do alagamento e da seca sobre o crescimento e fisiologia de Peltophorum dubium (Fabaceae). Rev. Agronegocio Ambiente 2024, 17, e12664. [Google Scholar] [CrossRef]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef] [PubMed]
- Rehem, B.C.; De Almeida, A.A.F.; Mielke, M.S.; Gomes, F.P.; Valle, R.R. Photosynthetic and growth responses of Theobroma cacao L. clones to waterlogging. J. Trop. Agric. 2010, 48, 17–22. [Google Scholar]
- Almeida, J.; Tezara, W.; Herrera, A. Physiological responses to drought and experimental water deficit and waterlogging of four clones of cacao (Theobroma cacao L.) selected for cultivation in Venezuela. Agric. Water Manag. 2016, 171, 80–88. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhao, C.Y.; Li, J.; Li, J.Y.; Peng, G. Morphological, physiological, and biochemical responses of Populus euphratica to soil flooding. Photosynthetica 2015, 53, 110–117. [Google Scholar] [CrossRef]
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Kolb, R.M.; Medri, M.E.; Bianchini, E.; Pimenta, J.A.; Giloni, P.C.; Correa, G.T. Anatomia ecológica de Sebastiania commersoniana (Baillon) Smith & Downs (Euphorbiaceae) submetida ao alagamento. Braz. J. Bot. 1998, 21, 3. [Google Scholar] [CrossRef]
- Melo, L.A.; Magalhães, P.C. Influência da aplicação de cálcio e alagamento do solo sobre características anatômicas das folhas de milho (Zea mays L.) “Saracura” BRS-4154. Ver. Bras. Milho Sorgo. 2010, 3, 333–342. [Google Scholar] [CrossRef]
- Melo, L.A.D.; Melo, H.C.D.; Davide, A.C.; Castro, E.M.D.; Santos, J.D.P.D. Estaquia e efeito da deficiência hídrica ou inundação sobre características morfoanatômicas de Cestrum axillare Vell. Cienc. Florest. 2017, 27, 325–337. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Nogueira, N.O.; Martins, L.D.; Tomaz, M.A.; Andrade, F.V.; Passos, R.R. Teor de nitrogênio, clorofila e relação clorofila-carotenoide em café arábica em solo submetido a diferentes corretivos de acidez. Rev. Bras. Ciênc. Agrárias 2013, 8, 390–395. [Google Scholar] [CrossRef]
- Nascimento, R.; Deuner, S.; Ferreira, L.S.; Badinelli, P.G.; Kerber, R.S. Crescimento e teores de clorofila e carotenóides em três cultivares de soja em função da adubação com magnésio. Rev. Ceres 2009, 56, 3. [Google Scholar]
- Zaidi, P.H.; Maniselvan, P.; Yadav, P.; Singh, A.K.; Sultana, R.; Dureja, P.; Singh, R.P.; Srinivasan, G. Stress-adaptive changes in tropical maize (Zea mays L.) under excessive soil moisture estress. Maydica 2007, 52, 159–171. [Google Scholar]
- de Oliveira Freire, J.L.; Cavalcante, L.F.; do Nascimento, R.; Rebequi, A.M. Teores de clorofila e composição mineral foliar do maracujazeiro irrigado com águas salinas e biofertilizante. Rev. Ciênc. Agrárias 2013, 36, 57–70. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Casali, V.W.D.; Souza, M.A.; Cecon, P.R. Disponibilidade de água no solo e crescimento de Artemísia. Hortic. Bras. 2003, 21, 726–730. [Google Scholar] [CrossRef]
- Streit, N.M.; Canterle, L.P.; Canto, M.W.; Hecktheuer, L.H.H. As clorofilas. Ciênc. Rural 2005, 35, 748–755. [Google Scholar] [CrossRef]
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312. [Google Scholar] [CrossRef]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suites of plants traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef]
- Tamang, G.; Fukao, T. Plant adaptation to multiple stresses during submergence and following desubmergence. Int. J. Mol. Sci. 2015, 16, 30164–30180. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M. Effects of drought stress on phenolic accumulation in greenhouse-grown olive trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Ferner, E.; Rennenberg, H.; Kreuzwieser, J. Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol. 2012, 32, 135–145. [Google Scholar] [CrossRef]
- Qin, X.; Li, F.; Chen, X.; Xie, Y. Growth responses and non-structural carbohydrates in three wetland macrophyte species following submergence and de-submergence. Acta Physiol. Plant. 2013, 35, 2069–2074. [Google Scholar] [CrossRef]
- Gangola, M.P.; Ramadoss, B.R. Sugars play a critical role in abiotic stress tolerance in plants. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Wani, S.H., Ed.; Academic Press: London, UK, 2018; pp. 17–38. [Google Scholar] [CrossRef]
- Sasidharan, R.; Hartman, S.; Liu, Z.; Martopawiro, S.; Sajeev, N.; Van Veen, H.; Voesenek, L.A. Signal dynamics and interactions during flooding stress. Plant Physiol. 2018, 176, 2069–2074. [Google Scholar] [CrossRef]
- Ye, X.Q.; Meng, J.L.; Zeng, B.; Wu, M. Improved flooding tolerance and carbohydrate status of flood-tolerant plant Arundinella anomala at lower water temperature. PLoS ONE 2018, 13, e0192608. [Google Scholar] [CrossRef] [PubMed]
- Winkel, A.; Visser, E.J.; Colmer, T.D.; Brodersen, K.P.; Voesenek, L.A.; Sand-Jensen, K.; Pedersen, O. Leaf gas films, underwater photosynthesis and plant species distributions in a flood gradient. Plant Cell Environ. 2016, 39, 2069–2074. [Google Scholar] [CrossRef] [PubMed]









| Genotype | Origin | Parent | Ancestry | Pollination | Fruit Color and Formation |
|---|---|---|---|---|---|
| Pará | Bahia | Undefined | Forastero | SC | Y/AM |
| TSH-1188 | Trinidad and Tobago | IMC67, ICS1, SCA6, and P18 | Amazônico/Trinitário | SI | R/EL |
| Esfip-02 | Espírito Santo Region | TSH-565 and IMC-67 | Trinitário/Forastero | SI | R/AL |
| Cepec-2002 | Brasileira Farm, Uruçuca-BA | Sca-6 and Comum ** | Amazônico/Amazônico | SC | Y/AM |
| SJ-02 | São José Farm, Itajuipe-BA | IMC-67 and ICS-01 | Amazônico/Trinitário | SC | Y/AL |
| PS-1319 | Porto Seguro Farm, Ilhéus-BA | ICS-01 and PA-150 | Amazônico/Trinitário | SC | Y-V/AM |
| Genotypes | ||||||
|---|---|---|---|---|---|---|
| Treatment | Cepec-2002 | SJ-02 | Esfip-02 | Pará | TSH-1188 | Means |
| Stomatal density | ||||||
| Non-flooded | 35.96 bA | 33.70 bC | 35.16 bB | 34.83 bA | 40.48 aA | |
| Flooded | 25.16 cB | 42.74 aA | 43.38 aA | 32.25 bA | 29.35 bC | |
| Recovered | 33.54 bA | 37.58 aB | 32.74 bB | 31.61 bA | 35.64 aB | |
| DP/DE ratio | ||||||
| Non-flooded | 0.92 aA | 1.0 aA | 1.0 aA | 1.05 aA | 0.97 aA | |
| Flooded | 1.07 aA | 0.75 bB | 0.97 aA | 0.95 aA | 0.85 bA | |
| Recovered | 0.97 aA | 0.95 aA | 1.0 aA | 1.0 aA | 0.97 aA | |
| Lenticel length | ||||||
| Non-flooded | 0 aB | 0 aC | 0 aB | 0 aC | 0 aB | |
| Flooded | 0.55 aA | 0.65 aA | 0.63 aA | 0.61 aA | 0.58 aA | |
| Recovered | 0.54 bA | 0.50 bB | 0.67 aA | 0.50 bB | 0.58 bA | |
| Lenticel height | ||||||
| Non-flooded | 0 | 0 | 0 | 0 | 0 | 0 C |
| Flooded | 0.55 | 0.65 | 0.63 | 0.61 | 0.58 | 0.22 A |
| Recovered | 0.54 | 0.5 | 0.67 | 0.5 | 0.58 | 0.15 B |
| Genotypes | ||||||
|---|---|---|---|---|---|---|
| Environment | Cepec-2002 | SJ-02 | Esfip-02 | Pará | TSH-1188 | Means |
| Chl a [μg mL−1] | ||||||
| Non-flooded | 9.12 | 10.24 | 11.15 | 9.27 | 6.9 | 9.33 A |
| Flooded | 7.46 | 5.7 | 5.85 | 6.58 | 6.57 | 6.43 B |
| Chl b [μg mL−1] | ||||||
| Non-flooded | 10.0925 | 11.57 | 13.14 | 9.8725 | 7.415 | 10.418 A |
| Flooded | 9.5125 | 6.0025 | 6.8125 | 7.6975 | 8.425 | 7.69 B |
| Chl total [μg mL−1] | ||||||
| Non-flooded | 19.2 | 21.8 | 24.28 | 19.14 | 14.31 | 19.75 A |
| Flooded | 16.96 | 11.7 | 12.66 | 14.28 | 14.99 | 14.12 B |
| Total carotenoids [μg mL−1] | ||||||
| Non-flooded | 619.68 | 555.83 | 684.9 | 513.76 | 579.59 | 590.75 A |
| Flooded | 551.88 | 392.23 | 616.04 | 469.39 | 472.13 | 500.33 B |
| Means | 585.78 a | 474.03 b | 650.47 a | 491.58 b | 525.86 b | |
| Genotype | ||||||
|---|---|---|---|---|---|---|
| Environment | Esfip-02 | Cepec-2002 | SJ-02 | Pará | TSH-1188 | Means |
| RS Leaf [mg g−1] | ||||||
| Non-flooded | 43.40 aC | 42.51 aC | 38.11 aB | 37.91 aC | 45.65 aC | |
| Flooded | 77.42 bA | 77.75 bA | 58.42 cA | 78.49 bA | 101.12 aA | |
| Recovered | 60.36 aB | 63.05 Ab | 57.23 aA | 53.49 aB | 65.62 aB | |
| TSS Leaf [mg g−1] | ||||||
| Non-flooded | 60.83 aB | 54.33 aB | 52.33 aB | 50.73 aC | 70.42 aB | |
| Flooded | 134.47 aA | 137.43 aA | 115.26 bA | 108.14 bA | 98.43 bA | |
| Recovered | 61.61 aB | 73.63 aB | 65.24 aB | 79.98 aB | 69.80 aB | |
| Starch Leaf | ||||||
| Non-flooded | 120.99 | 127.13 | 127.98 | 120.63 | 119.97 | 123.34 C |
| Flooded | 150.54 | 155.7 | 157.58 | 144.74 | 147.82 | 151.28 A |
| Recovered | 138.13 | 139.79 | 137.18 | 144.42 | 147.76 | 141.46 B |
| Starch Root | ||||||
| Non-flooded | 143.98 | 130.15 | 152.65 | 132.14 | 150.3 | 141.84 C |
| Flooded | 182.01 | 124.48 | 188.29 | 156.26 | 190.8 | 168.36 B |
| Recovered | 211.94 | 167.66 | 187.02 | 178.63 | 197.31 | 188.51 A |
| Means | 179.31 a | 140.76 b | 175.99 a | 155.67 b | 179.47 a | |
| TSS Root | ||||||
| Non-flooded | 52.26 | 27.32 | 37.5 | 31.27 | 32.55 | 36.18 C |
| Flooded | 104.09 | 95.96 | 70.84 | 89.87 | 72.87 | 86.72 A |
| Recovered | 83.31 | 42.02 | 81.08 | 56.23 | 71.66 | 67.46 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Pinto, M.L.P.B.; Oliveira, V.d.S.; Crasque, J.; Cerri Neto, B.; Ferreira, T.R.; Souza, C.A.S.; Falqueto, A.R.; Souza, T.C.d.; Machado Filho, J.A.; Arantes, L.d.O.; et al. Physiological and Morphological Response Mechanisms of Theobroma cacao L. Rootstocks Under Flooding and Evaluation of Their Adaptability. Plants 2026, 15, 122. https://doi.org/10.3390/plants15010122
Pinto MLPB, Oliveira VdS, Crasque J, Cerri Neto B, Ferreira TR, Souza CAS, Falqueto AR, Souza TCd, Machado Filho JA, Arantes LdO, et al. Physiological and Morphological Response Mechanisms of Theobroma cacao L. Rootstocks Under Flooding and Evaluation of Their Adaptability. Plants. 2026; 15(1):122. https://doi.org/10.3390/plants15010122
Chicago/Turabian StylePinto, Maria Luiza Pereira Barbosa, Vinicius de Souza Oliveira, Jeane Crasque, Basílio Cerri Neto, Thayanne Rangel Ferreira, Carlos Alberto Spaggiari Souza, Antelmo Ralph Falqueto, Thiago Corrêa de Souza, José Altino Machado Filho, Lúcio de Oliveira Arantes, and et al. 2026. "Physiological and Morphological Response Mechanisms of Theobroma cacao L. Rootstocks Under Flooding and Evaluation of Their Adaptability" Plants 15, no. 1: 122. https://doi.org/10.3390/plants15010122
APA StylePinto, M. L. P. B., Oliveira, V. d. S., Crasque, J., Cerri Neto, B., Ferreira, T. R., Souza, C. A. S., Falqueto, A. R., Souza, T. C. d., Machado Filho, J. A., Arantes, L. d. O., Dias, C. d. S., de Santana, E. N., Tesch Kuhlcamp, K., & Dousseau-Arantes, S. (2026). Physiological and Morphological Response Mechanisms of Theobroma cacao L. Rootstocks Under Flooding and Evaluation of Their Adaptability. Plants, 15(1), 122. https://doi.org/10.3390/plants15010122









