Heavy Metal Accumulation in Dominant Green Algae Living in a Habitat Under the Influence of Cu Mine Discharge Water
Abstract
1. Introduction
2. Materials and Methods
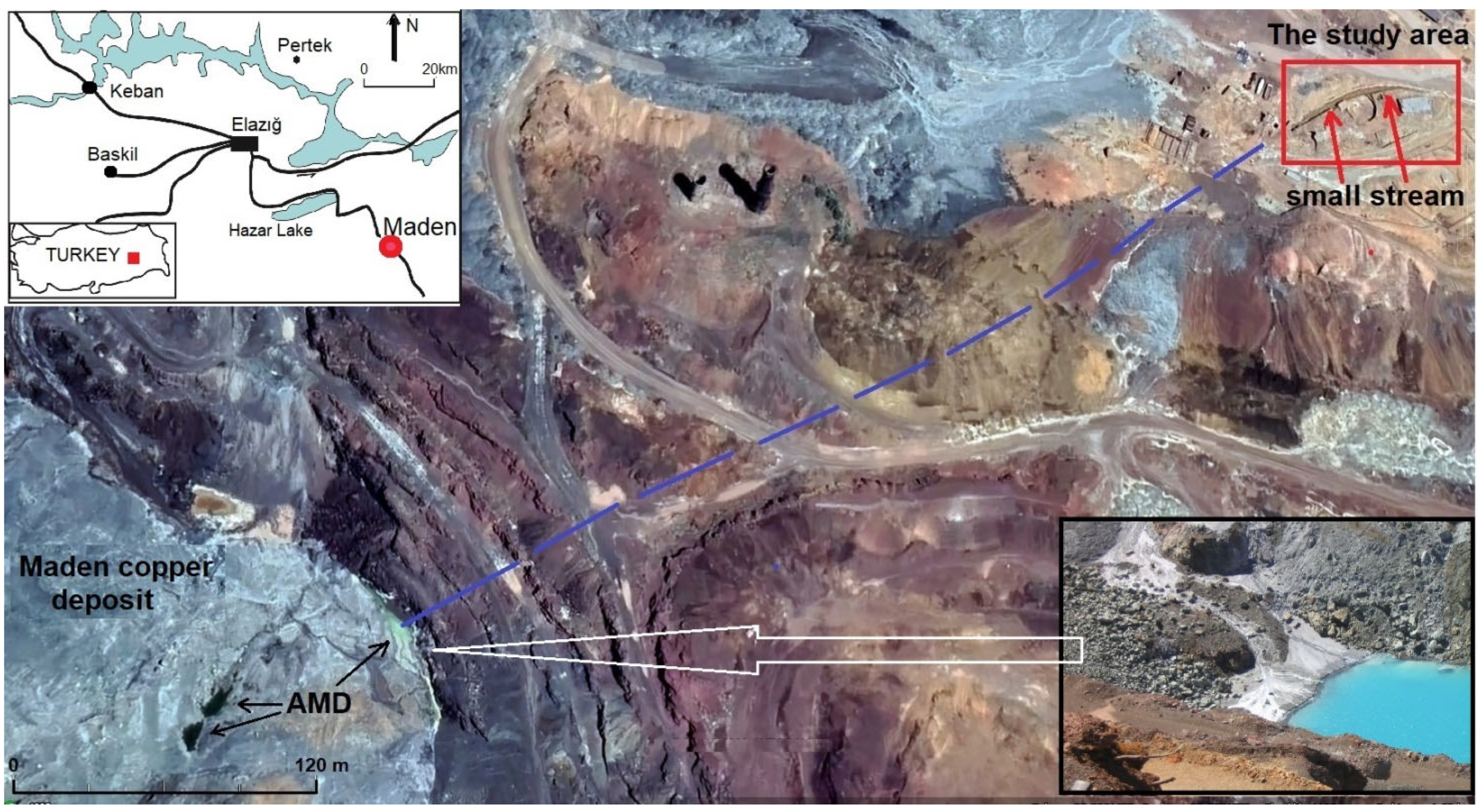
2.1. Sampling Site
2.2. Water and Algae Sample Collection
2.3. Physical and Chemical Analysis of Water Samples
2.4. Algae Sample Preparation and Bioconcentration Factor (BCF) Determination


3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal-organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar]
- Gupta, A.; Joia, J.; Sood, A.; Sood, R.; Sidhu, C. Microbes as potential tool for remediation of heavy metals: A review. J. Microb. Biochem. Technol. 2016, 8, 364–372. [Google Scholar]
- Jin, H.; Yu, Y.; Chen, X. Electrochemical precipitation for water and wastewater treatment. Process Saf. Environ. Prot. 2024, 184, 1011–1016. [Google Scholar]
- Chekroun, K.B.; Baghour, M. The role of algae in phytoremediation of heavy metals: A review. J. Mater. Environ. Sci. 2013, 4, 873–880. [Google Scholar]
- Mehta, P.; Roy, S.; Labelle, S. Phytoremediation of heavy metal and PAH-contaminated brownfield sites. Plant Soil 2005, 272, 277–290. [Google Scholar]
- Kumar, K.S.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311. [Google Scholar]
- Nsimba, R.Y.; West, N.; Boateng, A.A. Structure and Radical Scavenging Activity Relationships of Pyrolytic Lignins. J. Agricultural Food Chem. 2012, 60, 12525–12530. [Google Scholar] [CrossRef]
- Ahalya, N.; Ramachandra, T.V.; Kanamadi, R.D. Biosorption of heavy metals. Res. J. Chem. Environ. 2003, 7, 71–78. [Google Scholar]
- Sekabira, K.; Oryem-Origa, H.; Basamba, T.A.; Mutumba, G.; Kakudidi, E. Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int. J. Environ. Sci. Tech. 2010, 7, 435–446. [Google Scholar]
- Huang, B.; Dai, H.; Zhou, W.; Peng, L.; Li, M.; Wan, R.; He, W. Characteristics of Cd accumulation and distribution in two sweet potato cultivars. Int. J. Phytorem. 2019, 21, 391–398. [Google Scholar]
- Xin, J.L.; Huang, B.F.; Dai, H.W.; Mu, Y.X. Characterization of root morphology and root-derived low molecular weight organic acids in two sweet potato cultivars exposed to cadmium. Arch. Agron. Soil Sci. 2016, 63, 723–734. [Google Scholar]
- Ji, J.; Long, Z.F.; Lin, D.H. Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chem. Eng. J. 2011, 170, 525–530. [Google Scholar]
- Ambasht, R.S.; Ambasht, N.K. Conservation of Soil and Nutrients through Plant Cover on Wetland Margins. In Modern Trends in Applied Aquatic Ecology; Ambasht, R.S., Ambasht, N.K., Eds.; Springer: Boston, MA, USA, 2003; pp. 269–280. [Google Scholar]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Alginate and algal-based beads for the sorption of metal cations: Cu (II) and Pb (II). Int. J. Mol. Sci. 2016, 17, 1453. [Google Scholar] [CrossRef]
- Dwivedi, S. Bioremediation of Heavy Metal by Algae: Current and Future Perspective. J. Adv. Lab. Res. Biol. 2012, 3, 195–199. [Google Scholar]
- Valente, T.; Gomes, C.L. The role of two acidophilic algae as ecological indicators of acid mine drainage sites. J. Iber Geol. 2007, 33, 283–294. [Google Scholar]
- Niyogi, D.K.; Lewis, W.M.; McKnight, D.M. Effects of stress from mine drainage on diversity, biomass, and function of primary producers in mountain streams. Ecosystems 2002, 5, 554–567. [Google Scholar]
- Sasmaz, M.; Uslu Senel, G.; Obek, E. Boron Bioaccumulation by the Dominant Macrophytes Grown in Various Discharge Water Environments. Bull Environ. Contam. Toxicol. 2021, 106, 1050–1058. [Google Scholar]
- Sasmaz, M.; Uslu Senel, G.; Obek, E. Strontium accumulation by the terrestrial and aquatic plants affected by mining and municipal wastewaters (Elazig, Turkey). Environ. Geochem. Health 2021, 43, 2257–2270. [Google Scholar]
- Sasmaz Kislioglu, M. Removal of Ag, Au, and As from Acid Mine Water Using Lemna gibba and Lemna minor—A Performance Analysis. Water 2023, 15, 1293. [Google Scholar] [CrossRef]
- Bicudo, C.E.M.; Menezes, M. Gêneros de Algas de Aguas Continentais do Brasil: Chave para Identificação e Descrições, 2nd ed.; RiMa: São Carlos, Brazil, 2006; 502p. [Google Scholar]
- Zhang, S.; Fu, K.; Gao, S.; Liang, B.; Lu, J.; Fu, G. Bioaccumulation of Heavy Metals in the Water, Sediment, and Organisms from The Sea Ranching Areas of Haizhou Bay in China. Water 2023, 15, 2218. [Google Scholar] [CrossRef]
- Diop, M.; Howsam, M.; Diop, C.; Goossens, J.F.; Diouf, A.; Amara, R. Assessment of trace element contamination and bioaccumulation in algae (Ulva lactuca), mussels (Perna perna), shrimp (Penaeus kerathurus), and fish (Mugil cephalus, Saratherondon melanotheron) along the Senegalese coast. Mar. Pollut. Bull. 2016, 103, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Phetsombat, S.; Kruatrachue, M.; Pokethitiyook, P.; Upatham, S. Toxicity and bioaccumulation of cadmium and lead in Salvinia cucullata. J. Environ. Biol. 2006, 27, 645–652. [Google Scholar] [PubMed]
- Konakci, N. Accumulation Assessment of Mo4+, Pb++, and Cu++ in the Acidic Water of Copper Mines with Lemna minor and Lemna gibba. Water 2024, 16, 975. [Google Scholar] [CrossRef]
- Novis, P.M.; Harding, J.S. Extreme Acidophiles: Freshwater algae associated with acid mine drainage. In Algae and Cyanobacteria In Extreme Environment; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 443–463. [Google Scholar]
- Brake, S.S.; Hasiotis, S.T.; Dannely, H.K. Diatoms in acid mine drainage and their role in the formation of iron-rich stromatolites. Geomicrobiol. J. 2004, 21, 331–340. [Google Scholar] [CrossRef]
- Sutherland, I.W. Microbial exopolysaccharides. In Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; Dumitriu, S., Ed.; Marcel Dekker: New York, NY, USA, 2005; pp. 431–458. [Google Scholar]
- Van Hille, R.; Boshoff, G.A.; Rose, P.D.; Duncan, J.R. A continuous process for the biological treatment of heavy metal contaminated acid mine water. Resources, Conserv. Recycl. 1999, 27, 157–167. [Google Scholar] [CrossRef]
- Freire-Nordi, C.S.; Vieira, A.A.H.; Nascimento, O.R. The metal binding capacity of Anabaena spiroides extracellular polysaccharide: An EPR study. Process Biochem. 2005, 40, 2215–2224. [Google Scholar] [CrossRef]
- Haritonidis, S.; Malea, P. Bioaccumulation of metals by the green alga Ulva rigida from Thermaikos Gulf, Greece. Environ. Pollut. 1999, 104, 365–372. [Google Scholar] [CrossRef]
- Akcali, I.; Kucuksezgin, F. A biomonitoring study: Heavy metals in macroalgae from eastern Aegean coastal areas. Mar. Pollut. Bull. 2011, 62, 637–645. [Google Scholar] [CrossRef]
- Du, T.H.; Bogush, A.; Edwards, P.; Stanley, P.; Lombardi, A.T.; Campos, L.C. Bioaccumulation of metals by algae from acid mine drainage: A case study of Frongoch Mine (UK). Environ. Sci. Pollut. Res. 2022, 29, 32261–32270. [Google Scholar] [CrossRef]
- Massocato, T.F.; Ramos, J.C.; Bascunan, V.L.F.; Simioni, C.; Rorig, L.R.; Barufi, B. Tolerance of Ulothrix sp. LAFIC 010 (Chlorophyta) against high concentration of metals from acid mine drainage. Ecotoxicol. Environ. Saf. 2018, 157, 227–234. [Google Scholar] [PubMed]
- Malakootian, M.; Toolabi, A.; Moussavi, S.G.H.; Ahmadian, M. Equilibrium and Kinetic Modeling of Heavy Metals Biosorption from Three Different Real Industrial Wastewaters Onto Ulothrix zonata Algae. Australian J. Basic App. Sci. 2011, 5, 1030–1037. [Google Scholar]
- Saavedra, R.; Muñoz, R.; Taboada, M.E.; Vega, M.; Bolado, S. Comparative uptake study of arsenic, boron, copper, manganese and zinc from water by diferent green microalgae. Bioresour. Technol. 2018, 263, 49–57. [Google Scholar] [PubMed]
- Pham, T.L.; Dao, T.S.; Bui, H.N.; Pham, T.K.N.; Ngo, T.T.H.; Bui, H.M. Lipid production combined with removal and bioaccumulation of Pb by Scenedesmus sp. Green Alga. Pol. J. Environ. Stud. 2020, 29, 1785–1791. [Google Scholar]
- Li, Y.; Song, S.; Xia, L.; Yin, H.; Meza, J.V.G.; Ju, W. Enhanced Pb (II) removal by algal-based biosorbent cultivated in high-phosphorus cultures. Chem. Eng. J. 2019, 361, 167. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Zhang, C.; Yue, Q.; Li, Y.; Li, C. Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 2010, 252, 149–156. [Google Scholar]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, F.X. Metal uptake by microalgae: Underlying mechanisms and practical applications. Biotechn. Prog. 2012, 28, 299. [Google Scholar] [CrossRef]
- Flouty, R.; Estephane, G. Bioaccumulation and biosorption of copper and lead by a unicellular algae Chlamydomonas reinhardtii in single and binary metal systems: A comparative study. J. Env. Man. 2012, 111, 106. [Google Scholar] [CrossRef]
- Flouty, R.; Khalaf, G. Role of Cu and Pb on Ni bioaccumulation by Chlamydomonas reinhardtii: Validation of the biotic ligand model in binary metal Mixtures. Ecotox. Env. Safety 2015, 113, 79. [Google Scholar] [CrossRef]
| Det. Limit (ppb) | Maden River (ppb) | U. tenuissima (n = 5 Samples) (mg/kg) | BCF | Mining Water (ppb) | U. variabilis (n = 6 Samples) (mg/kg) | BCF | |
|---|---|---|---|---|---|---|---|
| T °C | - | 18.6 ± 1.2 | - | - | 19.3 ± 1.4 | - | - |
| pH | - | 8.42 ± 0.6 | - | - | 5.82 ± 0.4 | - | - |
| EC mS cm−1 | - | 1.55 ± 0.02 | - | - | 2.49 ± 0.03 | - | - |
| HCO3 mg L−1 | - | 345 ± 15 | - | - | 288 ± 18 | - | - |
| NO3− mg L−1 | - | 2.18 ± 0.11 | - | - | 1.88 ± 0.02 | - | - |
| SO4 mg L−1 | - | 61.7 ± 4 | - | - | 122 ± 8 | - | - |
| Cl− mg L−1 | - | 4.88 ± 0.3 | - | - | 6.54 ± 0.5 | - | - |
| F− mg L−1 | - | 0.26 ± 0.02 | - | - | 0.38 ± 0.02 | - | - |
| Total N | - | 0.62 ± 0.05 | - | - | 0.76 ± 0.03 | - | - |
| Kjeldahl N | - | 0.18 ± 0.01 | - | - | 0.23 ± 0.01 | - | - |
| Ag (ppb) | 0.05 | 0.08 ± 0.01 | 130 ± 8 | 1625 | 9.25 ± 0.7 | 1509 ± 78 | 163 |
| Al | 1 | 106 ± 8 | 1.62 ± 0.2 | 0.02 | 24045 ± 36 | 0.47 ± 0.01 | 0.01 |
| As | 0.5 | 0.7 ± 0.01 | 42.5 ± 2 | 61 | 170 ± 10 | 67.3 ± 0.4 | 0.40 |
| Au (ppb) | 0.05 | <0.05 | 55.4 ± 4 | - | 0.7 ± 0.02 | 301 ± 22 | 430 |
| B | 5 | 32 ± 2.1 | 7 ± 0.5 | −0.22 | 840 ± 24 | 21 ± 1.8 | 0.03 |
| Ba | 0.05 | 11.4 ± 0.8 | 60 ± 5 | 5.26 | 450 ± 22 | 53.9 ± 4.2 | 0.12 |
| Cd | 0.05 | 0.17 ± 0.02 | 5.4 ± 0.4 | 32 | 6.2 ± 0.04 | 1.48 ± 0.12 | 0.24 |
| Co | 0.02 | 1.4 ± 0.01 | 64 ± 3 | 64 | 1725 ± 52 | 838 ± 55 | 0.49 |
| Cr | 0.5 | 76.6 ± 4.3 | 107 ± 11 | 1.40 | 200 ± 14 | 164 ± 12 | 0.82 |
| Cu | 0.1 | 0.24 ± 0.02 | 180 ± 15 | 750 | 3562 ± 66 | 6787 ± 128 | 1.91 |
| Fe | 10 | 370 ± 22 | 8.26 ± 0.8 | 0.02 | 115750 ± 87 | 11094 ± 48 | 0.10 |
| Hg | 0.01 | <0.01 | 85 ± 6 | - | 1.8 ± 0.02 | 96 ± 7.2 | 53 |
| La | 0.01 | 0.02 ± 0.01 | 8.09 ± 0.9 | 405 | 9.45 ± 0.6 | 1.01 ± 0.06 | 0.11 |
| Li | 0.05 | <0.05 | 9.27 ± 0.8 | - | 23 ± 1.5 | 4.54 ± 0.3 | 0.20 |
| Mn | 0.05 | 0.14 ± 0.01 | 13 ± 1.4 | 942 | 6460 ± 106 | 525 ± 38 | 0.08 |
| Mo | 0.01 | <0.01 | 6.4 ± 0.5 | - | 30 ± 2.8 | 10.5 ± 0.8 | 0.35 |
| Ni | 0.02 | 0.12 ± 0.01 | 95 ± 7 | 792 | 455 ± 28 | 472 ± 23 | 1.04 |
| Pb | 0.02 | 0.18 ± 0.01 | 68.7 ± 6 | 382 | 285 ± 15 | 84 ± 7 | 0.29 |
| Sb | 0.05 | 0.11 ± 0.01 | 2.09 ± 0.3 | 19 | 18.5 ± 1.2 | 2.87 ± 0.3 | 0.16 |
| Sr | 0.01 | 163 ± 12 | 174 ± 13 | 1.02 | 2585 ± 75 | 26.2 ± 1.8 | 0.01 |
| Th | 0.05 | 0.08±0.01 | 0.6 ± 0.01 | 7.5 | 0.48 ± 0.03 | 0.2 ± 0.01 | 0.42 |
| Ti | 10 | 12 ± 1.3 | 420 ± 33 | 35 | 186 ± 14 | 101 ± 8.4 | 0.54 |
| Tl | 0.01 | 0.06 ± 0.01 | 0.32 ± 0.01 | 5.33 | 6.04 ± 0.5 | 0.37 ± 0.02 | 0.06 |
| U | 0.02 | 0.13 ± 0.01 | 1.4 ± 0.01 | 11 | 4.2 ± 0.2 | 0.11 ± 0.01 | 0.03 |
| V | 0.02 | 1.7 ± 0.02 | 23 ± 2.4 | 14 | 96.4 ± 9 | 91 ± 6.4 | 0.94 |
| W | 0.02 | 0.03 ± 0.01 | 0.2 ± 0.01 | 7 | 0.10 ± 0.01 | 0.2 ± 0.01 | 2.00 |
| Zn | 0.5 | 78.9 ± 12 | 283 ± 14 | 3.6 | 2850 ± 36 | 680 ± 44 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasmaz Kislioglu, M.; Obek, E.; Konakci, N.; Sasmaz, A. Heavy Metal Accumulation in Dominant Green Algae Living in a Habitat Under the Influence of Cu Mine Discharge Water. Plants 2025, 14, 993. https://doi.org/10.3390/plants14070993
Sasmaz Kislioglu M, Obek E, Konakci N, Sasmaz A. Heavy Metal Accumulation in Dominant Green Algae Living in a Habitat Under the Influence of Cu Mine Discharge Water. Plants. 2025; 14(7):993. https://doi.org/10.3390/plants14070993
Chicago/Turabian StyleSasmaz Kislioglu, Merve, Erdal Obek, Nevin Konakci, and Ahmet Sasmaz. 2025. "Heavy Metal Accumulation in Dominant Green Algae Living in a Habitat Under the Influence of Cu Mine Discharge Water" Plants 14, no. 7: 993. https://doi.org/10.3390/plants14070993
APA StyleSasmaz Kislioglu, M., Obek, E., Konakci, N., & Sasmaz, A. (2025). Heavy Metal Accumulation in Dominant Green Algae Living in a Habitat Under the Influence of Cu Mine Discharge Water. Plants, 14(7), 993. https://doi.org/10.3390/plants14070993