Jasmonic Acid-Mediated Antioxidant Defense Confers Chilling Tolerance in Okra (Abelmoschus esculentus L.)
Abstract
1. Introduction
2. Results
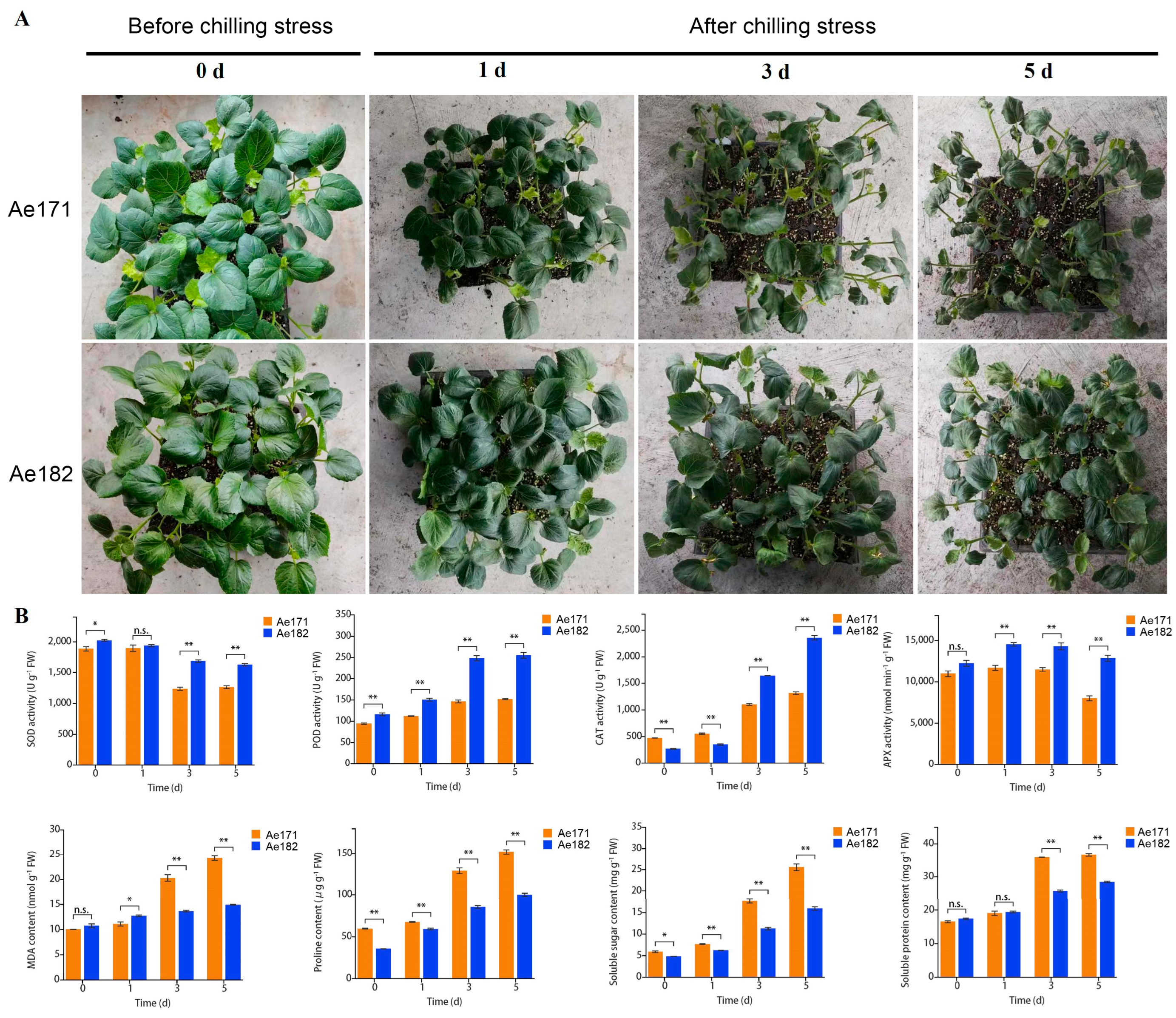
2.1. Phenotypic and Physiological Analyses of Chilling-Sensitive and Chilling-Tolerant Okra Varieties Under Chilling Stress
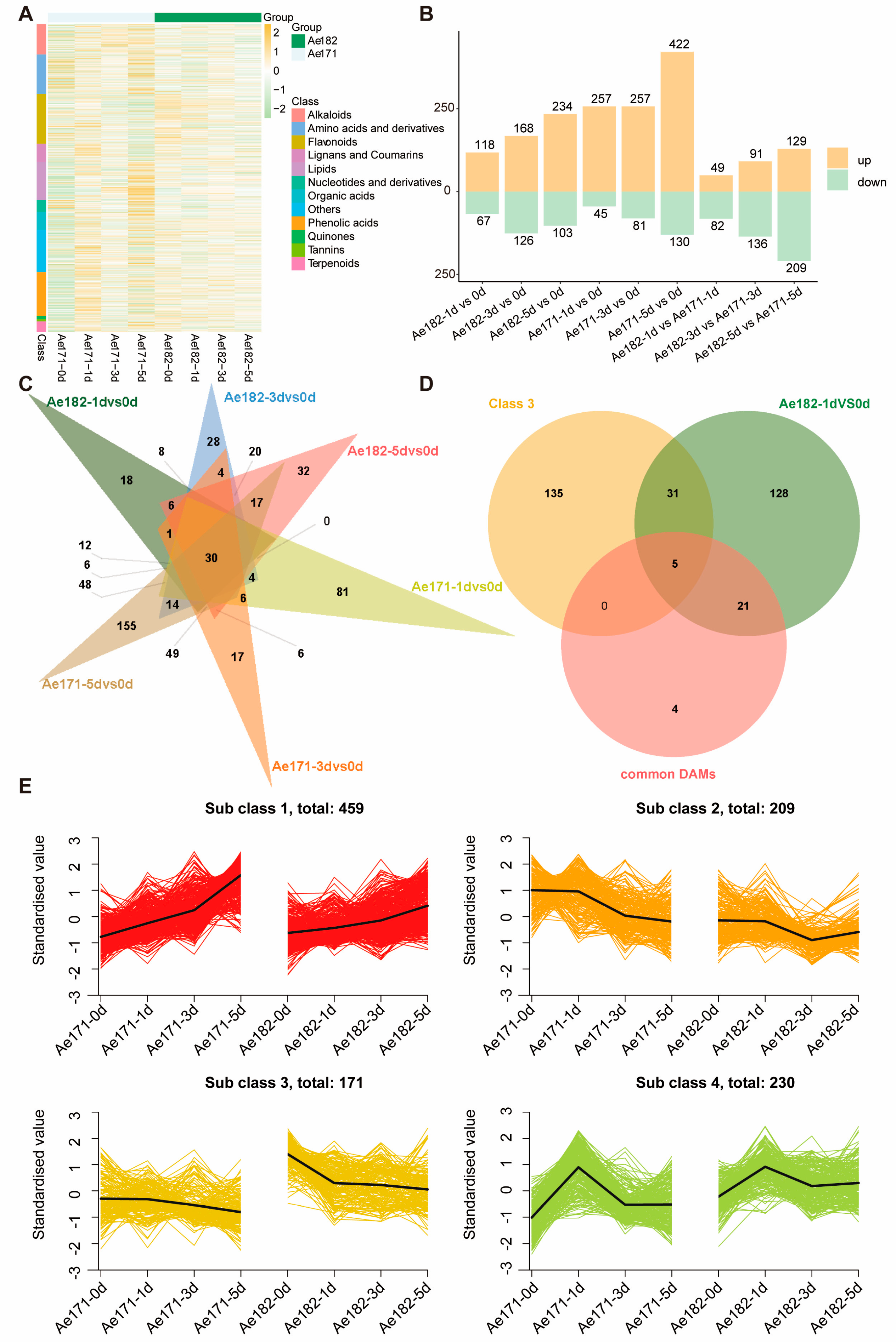
2.2. Metabolomic Profiles of Ae171 and Ae182 in Response to Chilling Stress
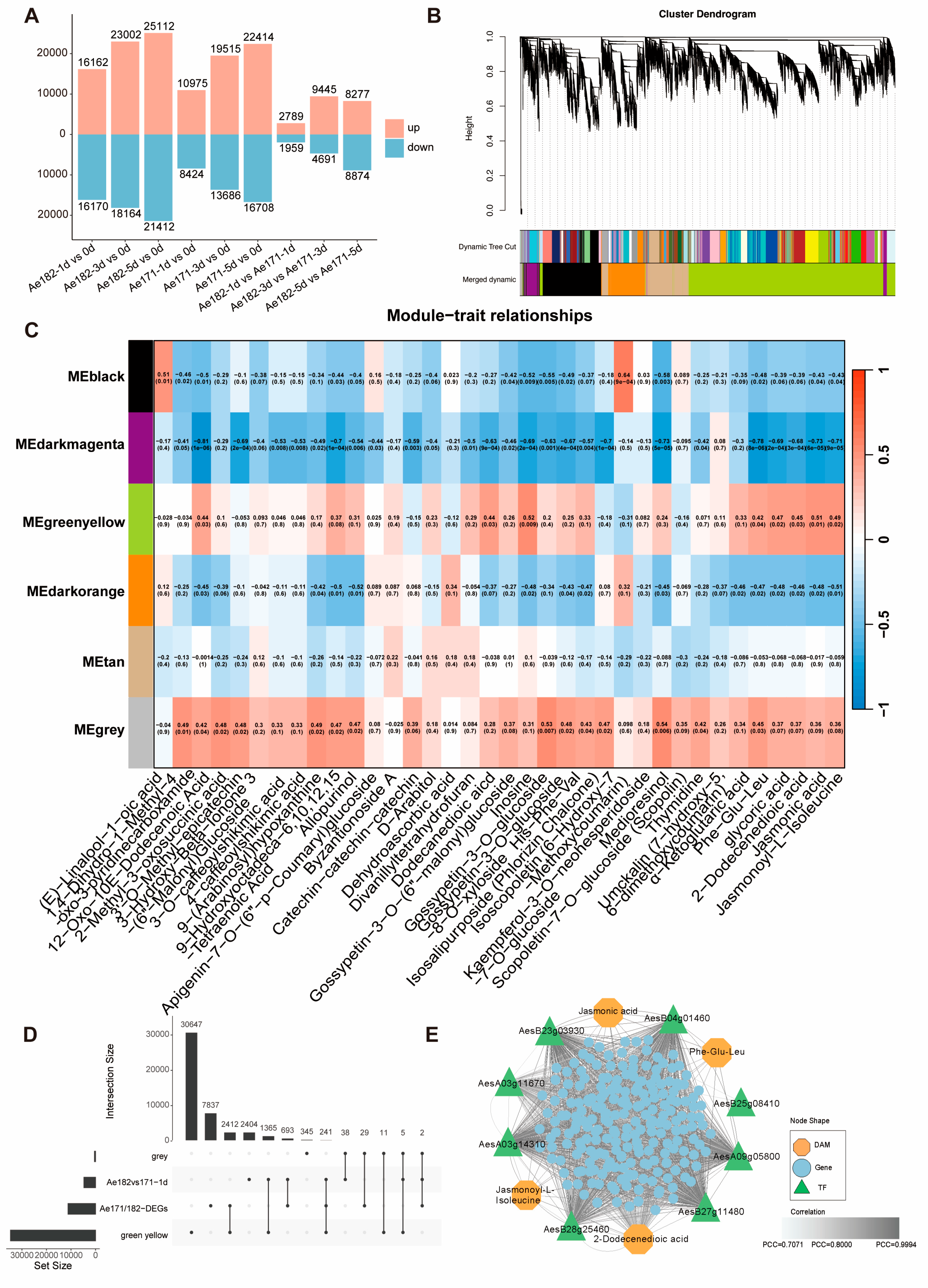
2.3. Ae171 and Ae182 Show Distinct Transcriptomic Alterations in Response to Chilling Stress
2.4. Key Gene Response to Chilling Stress Was Identified Using WGCNA in Orka
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Chilling Stress Treatment
4.2. Physiological Measurements
4.3. Transcriptome Analysis
4.4. Metabolome Analysis
4.5. Combined Transcriptome and Metabolome Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Jiang, Y.; Zhou, W.; Liu, Y.; Li, J.; Lin, M. Research Progress and Utilization of Okra. Sci. Technol. Food Ind. 2018, 39, 329–333. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Deng, J.; Wu, Y.; Liu, L.; Tu, Y.; Zhou, Y. Extraction and Antioxidant Activity in vitro of Okra Flavonoids. Food Sci. 2014, 35, 121–125. [Google Scholar]
- Wang, J.; Liu, Y.; Xie, W.; Shi, D.; Zhao, X. Research progress on biological characteristics, cultivation and breeding in Abelmoschus esculentus. North Hortic. 2016, 9, 194–197. [Google Scholar]
- Miao, J.; Ren, T. The cultivation and management techniques of okra. Agric. Henan 2024, 20. [Google Scholar]
- Liu, D.; Ye, H.; Liu, G. Research progress on the application value and cultivation techniques of Okra (Abelmoschus esculentus L.). J. Anhui Agri 2006, 34, 4. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuscan, G.A.; Muchero, W.; Chen, J.-G. Role of Reactive Oxygen Species and Hormones in Plant Responses to Temperature Changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. Nmcd 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, L.; Liu, Z.; Zhang, S.; Zheng, Z.; Huang, F.; Liu, X. Effects of low temperature stress on physiological characteristics of Abelmoschus esculentus seedlings. Acta Agric. Jiangxi 2020, 32, 41–44. [Google Scholar] [CrossRef]
- George, I.S.; Pascovici, D.; Mirzaei, M.; Haynes, P.A. Quantitative proteomic analysis of cabernet sauvignon grape cells exposed to thermal stresses reveals alterations in sugar and phenylpropanoid metabolism. Proteomics 2015, 15, 3048–3060. [Google Scholar] [CrossRef]
- Zhu, A.; Li, W.; Ye, J.; Sun, X.; Ding, Y.; Cheng, Y. Microarray Expression Profiling of Postharvest Ponkan Mandarin (Citrus reticulata) Fruit under Cold Storage Reveals Regulatory Gene Candidates and Implications on Soluble Sugars Metabolism. J. Integr. Plant Biol. 2011, 53, 358–374. [Google Scholar] [PubMed]
- Chen, W.P.; Li, P.H. Membrane stabilization by abscisic acid under cold aids proline in alleviating chilling injury in maize (Zea mays L.) cultured cells. Plant Cell Environ. 2002, 25, 955–962. [Google Scholar]
- Gothandam, K.M.; Nalini, E.; Karthikeyan, S.; Shin, J.S. OsPRP3, a flower specific proline-rich protein of rice, determines extracellular matrix structure of floral organs and its overexpression confers cold-tolerance. Plant Mol. Biol. 2010, 72, 125–135. [Google Scholar] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegen, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar]
- Chen, Y.; Yan, L.; Liu, Z.; Yu, J. Effect of exogenous NO on growth and physiological characteristics of Okra (Abelmoschus esculentus L.) seedlings under low temperature stress. Acta Agric. Boreali-Occident. Sin. 2022, 31, 1462–1469. [Google Scholar]
- Agarwal, P.K.; Jha, B. Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol. Plant. 2010, 54, 201–212. [Google Scholar]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate regulates the inducer of cbf expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar]
- Yu, M.M.; Wang, R.; Xia, J.Q.; Li, C.; Xu, Q.H.; Cang, J.; Wang, Y.Y.; Zhang, D. JA-induced TaMPK6 enhanced the freeze tolerance of Arabidopsis thaliana through regulation of ICE-CBF-COR module and antioxidant enzyme system. Plant Sci. Int. J. Exp. Plant Biol. 2023, 329, 111621. [Google Scholar]
- Zheng, L.; Li, B.; Zhang, G.; Zhou, Y.; Gao, F. Jasmonate enhances cold acclimation in jojoba by promoting flavonol synthesis. Hortic. Res. 2024, 11, uhae125. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Guo, X.; Dai, D.; Hu, Y.; Zhang, Y.; Guo, W. Effect of salicylic acid on germination and growth of Okra under chilling stress. J. Henan Agric. Sci. 2016, 45, 126–129. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [PubMed]
- Gusain, S.; Joshi, S.; Joshi, R. Sensing, signalling, and regulatory mechanism of cold-stress tolerance in plants. Plant Physiol. Biochem. 2023, 197, 107646. [Google Scholar]
- Xie, Z.; Nolan, T.M.; Jiang, H.; Yin, Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar]
- Roychowdhury, R.; Prakash Das, S.; Gupta, A.; Parihar, P.; Chandrasekhar, K.; Sarker, U.; Kumar, A.; Pandurang Ramrao, D.; Sudhakar, C. Multi-Omics Pipeline and Omics-Integration Approach to Decipher Plant’s Abiotic Stress Tolerance Responses. Genes 2023, 14, 1281. [Google Scholar] [CrossRef]
- Cheng, Y.; Ban, Q.; Mao, J.; Lin, M.; Zhu, X.; Xia, Y.; Cao, X.; Zhang, X.; Li, Y. Integrated Metabolomic and Transcriptomic Analysis Reveals That Amino Acid Biosynthesis May Determine Differences in Cold-Tolerant and Cold-Sensitive Tea Cultivars. Int. J. Mol. Sci. 2023, 24, 1907. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, M.; Xu, K.; Li, J.; Li, S.; Zhang, S.; Yang, X. Integrated transcriptomics and metabolomics analyses provide insights into cold stress response in wheat. Crop J. 2019, 7, 857–866. [Google Scholar]
- Bian, H.; Zhou, Q.; Du, Z.; Zhang, G.; Han, R.; Chen, L.; Tian, J.; Li, Y. Integrated Transcriptomics and Metabolomics Analysis of the Fructan Metabolism Response to Low-Temperature Stress in Garlic. Genes 2023, 14, 1290. [Google Scholar] [CrossRef]
- Li, Z.; Huang, C.; Zhao, J.; Yu, G.; Huang, T.; Peng, Y.; Hassan, M.J. A bermudagrass variant exhibits strong tolerance to low temperature associated with enhanced sugar metabolism and cold-responsive pathways. Crop Sci. 2023, 63, 2553–2568. [Google Scholar]
- Ali, R.; Wei, S.; Azhar, H.M.; Saher, M.S.; Xuekun, Z.; Yong, C.; Xiling, Z.; Yan, L. Integrated Analysis of Metabolome and Transcriptome Reveals Insights for Cold Tolerance in Rapeseed (Brassica napus L.). Front. Plant Sci. 2021, 12, 721681. [Google Scholar]
- Xin, W.; Yue, L.; Zhongkui, H.; Yuning, C.; Dongxin, H.; Yanping, K.; Zhihui, W.; Liying, Y.; Huifang, J.; Yong, L.; et al. Integrated Transcriptomics and Metabolomics Analysis Reveal Key Metabolism Pathways Contributing to Cold Tolerance in Peanut. Front. Plant Sci. 2021, 12, 752474. [Google Scholar]
- Tao, Y.; Meng, S.; Rui, S.; Xiaozhong, W.; Xuedan, L.; Yunhua, X.; Huabing, D.; Xiong, L.; Wenbang, T.; Guilian, Z. Transcriptomic Profiling of Cold Stress-Induced Differentially Expressed Genes in Seedling Stage of Indica Rice. Plants 2023, 12, 2675. [Google Scholar] [CrossRef]
- Hualong, L.; Wei, X.; Yinglin, W.; Dezhuang, Z.; Jingguo, W.; Hongliang, Z.; Luomiao, Y.; Shoujun, N.; Detang, Z. An integrated analysis of the rice transcriptome and lipidome reveals lipid metabolism plays a central role in rice cold tolerance. BMC Plant Biol. 2022, 22, 91. [Google Scholar]
- Congping, X.; Zihao, G.; Yuxiao, H.; Haizhen, Y.; Jie, L.; Xingquan, Z. Integrated Transcriptomics and Metabolomics Analyses Provide Insights into Qingke in Response to Cold Stress. J. Agric. Food Chem. 2023, 71, 18345–18358. [Google Scholar]
- Yonghong, L.; Qihang, T.; Zhaoyuan, W.; Jie, L.; Shiyuan, L.; Ruifeng, C.; Hu, C.; Guojian, L. Integrated analysis of transcriptomics and metabolomics of peach under cold stress. Front. Plant Sci. 2023, 14, 1153902. [Google Scholar]
- Qi, L.; Yifang, Z.; Xue, D.; Lamei, Z.; Yijun, Z.; Fei, G. Integrated metabolomics and transcriptomics analysis reveals that the change of apoplast metabolites contributes to adaptation to winter freezing stress in Euonymus japonicus. Plant Physiol. Biochem. 2023, 202, 107924. [Google Scholar]
- Lu, L.; Yang, W.; Dong, Z.; Tang, L.; Liu, Y.; Xie, S.; Yang, Y. Integrated Transcriptomic and Metabolomics Analyses Reveal Molecular Responses to Cold Stress in Coconut (Cocos nucifera L.) Seedlings. Int. J. Mol. Sci. 2023, 24, 14563. [Google Scholar] [CrossRef]
- Jun, W.Y.; Li, W.L.; Hong, S.M.; Ze, L.; Feng, T.X.; An, L.J. Transcriptomic and metabolomic insights on the molecular mechanisms of flower buds in responses to cold stress in two Camellia oleifera cultivars. Front. Plant Sci. 2023, 14, 1126660. [Google Scholar]
- Xiaoxu, Y.; Chang, L.; Mengdi, L.; Yanmei, L.; Zhishan, Y.; Guojun, F.; Dajun, L. Integrated transcriptomics and metabolomics analysis reveals key regulatory network that response to cold stress in common Bean (Phaseolus vulgaris L.). BMC Plant Biol. 2023, 23, 85. [Google Scholar]
- Mingyue, Z.; Jieyang, J.; Ting, G.; Na, Z.; Tingting, J.; Jingming, W.; Qiuyan, B.; Wilfried, S.; Chuankui, S. Glucosyltransferase CsUGT78A14 Regulates Flavonols Accumulation and Reactive Oxygen Species Scavenging in Response to Cold Stress in Camellia sinensis. Front. Plant Sci. 2019, 10, 1675. [Google Scholar]
- Zhu, Z.; Wei, L.; Guo, L.; Bao, H.; Wang, X.; Kear, P.; Wang, Z.; Zhu, G. Integrated Full-Length Transcriptome and Metabolome Profiling Reveals Flavonoid Regulation in Response to Freezing Stress in Potato. Plants 2023, 12, 2054. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261 e212. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.; Chini, A.; Hamberg, M.; Adie, B.; Porzel, A.; Kramell, R.; Miersch, O.; Wasternack, C.; Solano, R. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 2009, 5, 344–350. [Google Scholar] [CrossRef]
- Wang, R.; Li, W.; He, Q.; Zhang, H.; Wang, M.; Zheng, X.; Liu, Z.; Wang, Y.; Du, C.; Du, H. The genome of okra (Abelmoschus esculentus) provides insights into its genome evolution and high nutrient content. Hortic. Res. 2023, 10, uhad120. [Google Scholar]
- Guo, X.; Liu, D.; Chong, K. Cold signaling in plants:Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar]
- Chao, X.; Yuting, W.; Huidong, Y.; Yuqing, T.; Buchun, L.; Xinlong, H.; Zhongdong, H. Cold acclimation alleviates photosynthetic inhibition and oxidative damage induced by cold stress in citrus seedlings. Plant Signal. Behav. 2023, 18, 2285169. [Google Scholar]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar]
- Ruelland, E.; Vaultier, M.-N.; Zachowski, A.; Hurry, V. Chapter 2 Cold Signalling and Cold Acclimation in Plants. Adv. Bot. Res. 2009, 49, 35–150. [Google Scholar]
- Xiaomin, D.; Jianxiao, W.; Yan, L.; Shaohua, W.; Shuguang, Y.; Jinquan, C.; Yueyi, C.; Shixin, Z.; Minjing, S.; Weimin, T. Comparative transcriptome analysis reveals phytohormone signalings, heat shock module and ROS scavenger mediate the cold-tolerance of rubber tree. Sci. Rep. 2018, 8, 4931. [Google Scholar]
- Pottosin, I.; Velarde-Buendía, A.M.; Bose, J.; Zepeda-Jazo, I.; Shabala, S.; Dobrovinskaya, O. Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: Implications for plant adaptive responses. J. Exp. Bot. 2014, 65, 1271–1283. [Google Scholar]
- Ding, F.; Wang, C.; Xu, N.; Wang, M.; Zhang, S. Jasmonic acid-regulated putrescine biosynthesis attenuates cold-induced oxidative stress in tomato plants. Sci. Hortic. 2021, 288, 110373. [Google Scholar]
- Ding, F.; Wang, C.; Zhang, S.; Wang, M. A jasmonate-responsive glutathione S-transferase gene SlGSTU24 mitigates cold-induced oxidative stress in tomato plants. Sci. Hortic. 2022, 303, 111231. [Google Scholar]
- Abdullah, S.N.A.; Azzeme, A.M.; Yousefi, K. Fine-Tuning Cold Stress Response Through Regulated Cellular Abundance and Mechanistic Actions of Transcription Factors. Front. Plant Sci. 2022, 13, 850216. [Google Scholar] [CrossRef]
- Dong, Z.; Hao, Y.; Zhao, Y.; Tang, W.; Wang, X.; Li, J.; Wang, L.; Hu, Y.; Guan, X.; Gu, F.; et al. Genome-Wide Analysis of the TCP Transcription Factor Gene Family in Pepper (Capsicum annuum L.). Plants 2024, 13, 641. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernandez-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. Eggnog-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Steinegger, M.; Soding, J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 2017, 35, 1026–1028. [Google Scholar] [CrossRef]
- Buchfink, B.; Reuter, K.; Drost, H.G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernandez-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. Eggnog 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, J.; Zhu, D.; Yin, X.; Du, G.; Qin, Y.; Zhang, Z.; Liu, Z. Jasmonic Acid-Mediated Antioxidant Defense Confers Chilling Tolerance in Okra (Abelmoschus esculentus L.). Plants 2025, 14, 1100. https://doi.org/10.3390/plants14071100
Liu W, Wang J, Zhu D, Yin X, Du G, Qin Y, Zhang Z, Liu Z. Jasmonic Acid-Mediated Antioxidant Defense Confers Chilling Tolerance in Okra (Abelmoschus esculentus L.). Plants. 2025; 14(7):1100. https://doi.org/10.3390/plants14071100
Chicago/Turabian StyleLiu, Weixia, Jielin Wang, Dan Zhu, Xiaomin Yin, Gongfu Du, Yuling Qin, Zhiyuan Zhang, and Ziji Liu. 2025. "Jasmonic Acid-Mediated Antioxidant Defense Confers Chilling Tolerance in Okra (Abelmoschus esculentus L.)" Plants 14, no. 7: 1100. https://doi.org/10.3390/plants14071100
APA StyleLiu, W., Wang, J., Zhu, D., Yin, X., Du, G., Qin, Y., Zhang, Z., & Liu, Z. (2025). Jasmonic Acid-Mediated Antioxidant Defense Confers Chilling Tolerance in Okra (Abelmoschus esculentus L.). Plants, 14(7), 1100. https://doi.org/10.3390/plants14071100




