Identifying the Phytotoxicity of Biosynthesized Metal Oxide Nanoparticles and Their Impact on Antioxidative Enzymatic Activity in Maize Under Drought Stress
Abstract
1. Introduction
2. Results
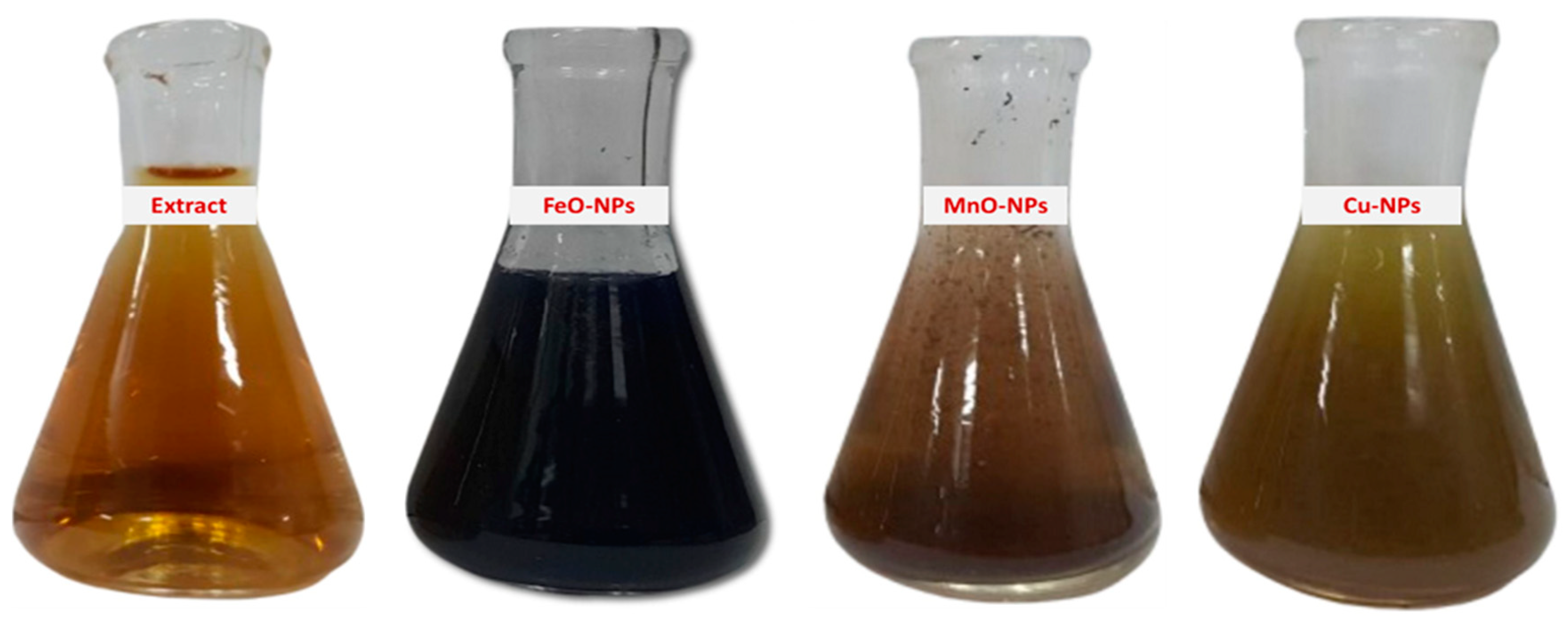
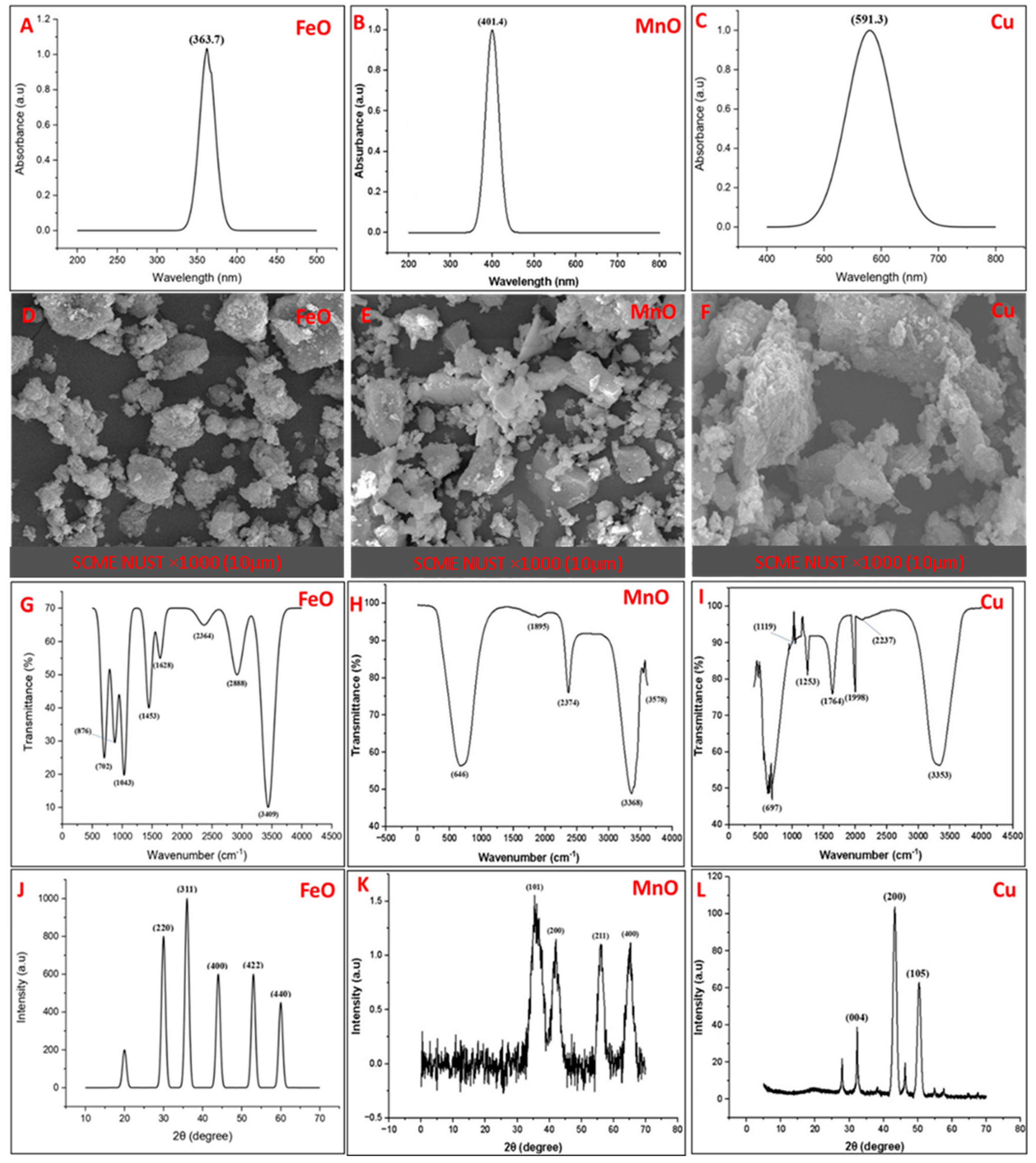
2.1. Biosynthesis and Characterization of Nanoparticles
2.2. Optimization of Nanoparticles
2.2.1. Morphological Growth
2.2.2. Chlorophyll Contents
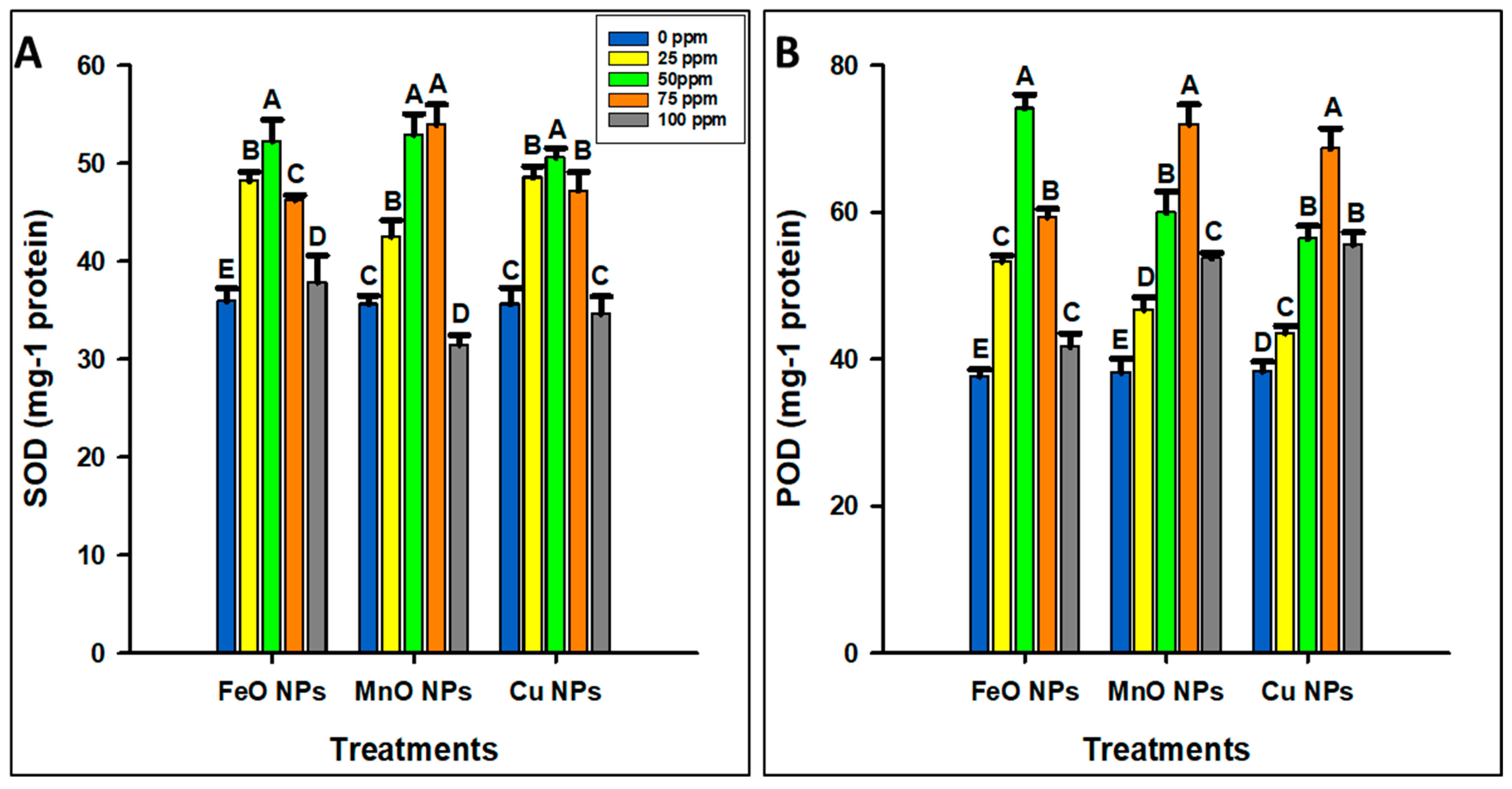
2.2.3. Antioxidant
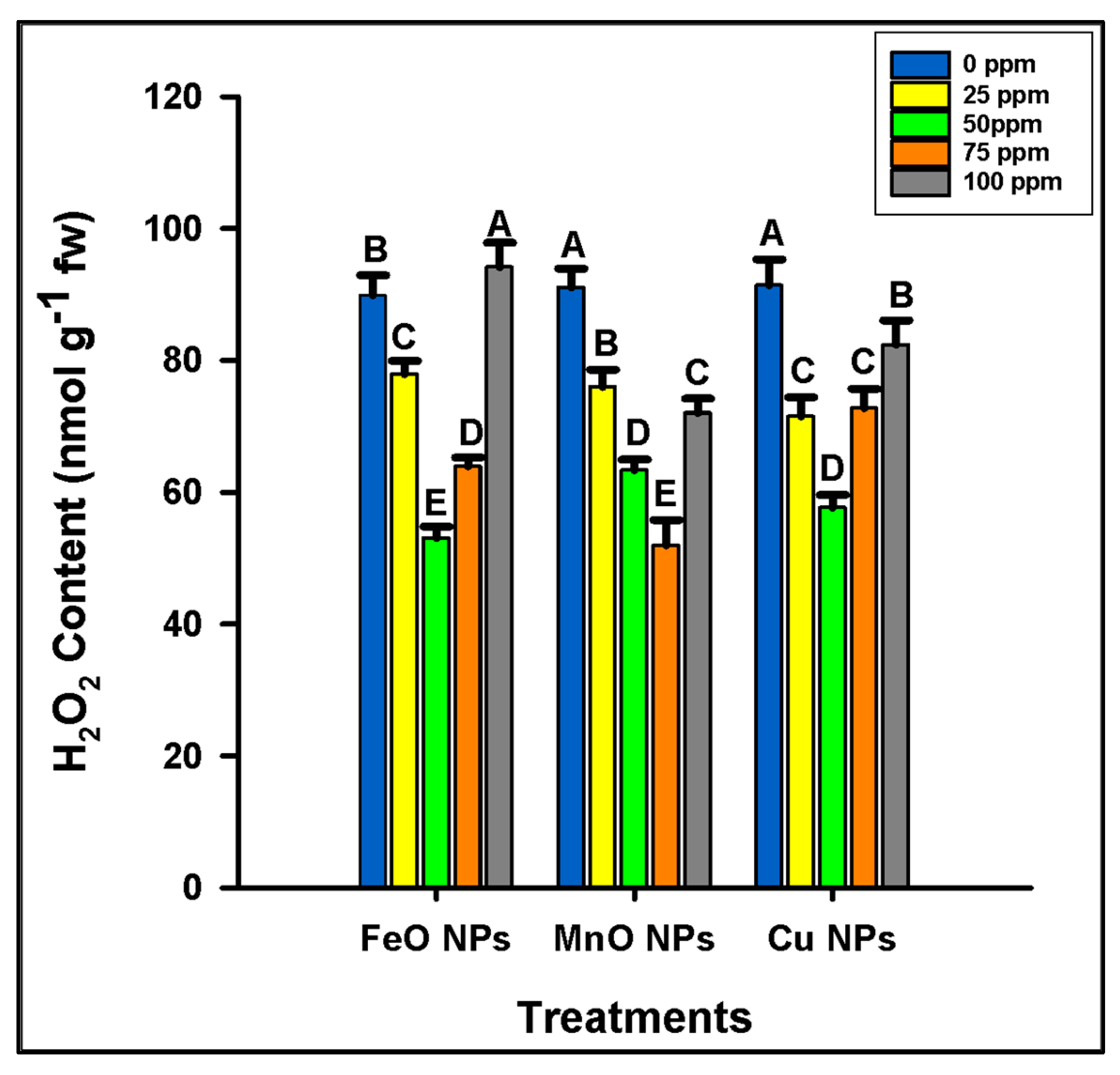
2.2.4. Oxidative Stress
2.3. Experiment Results
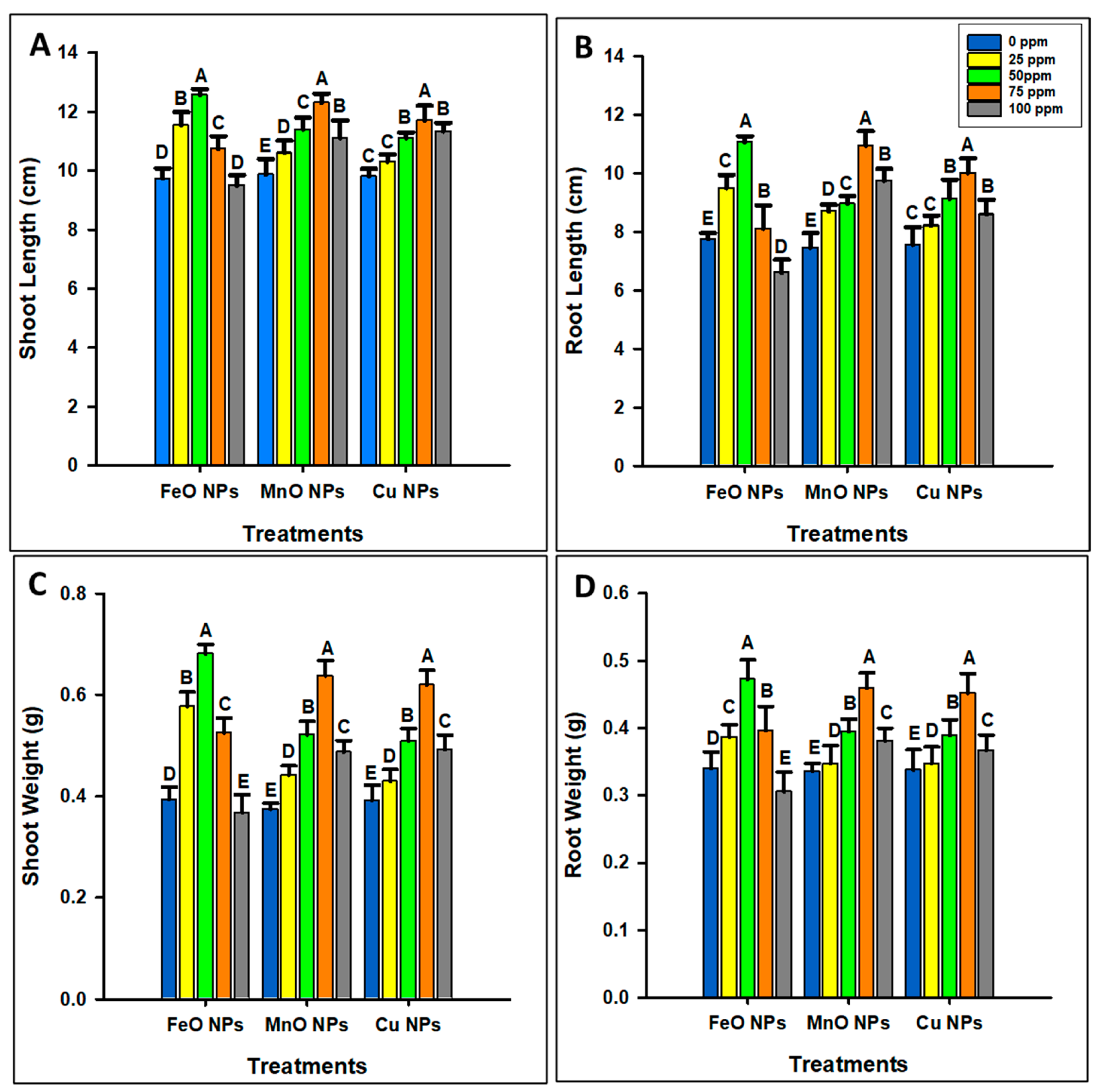
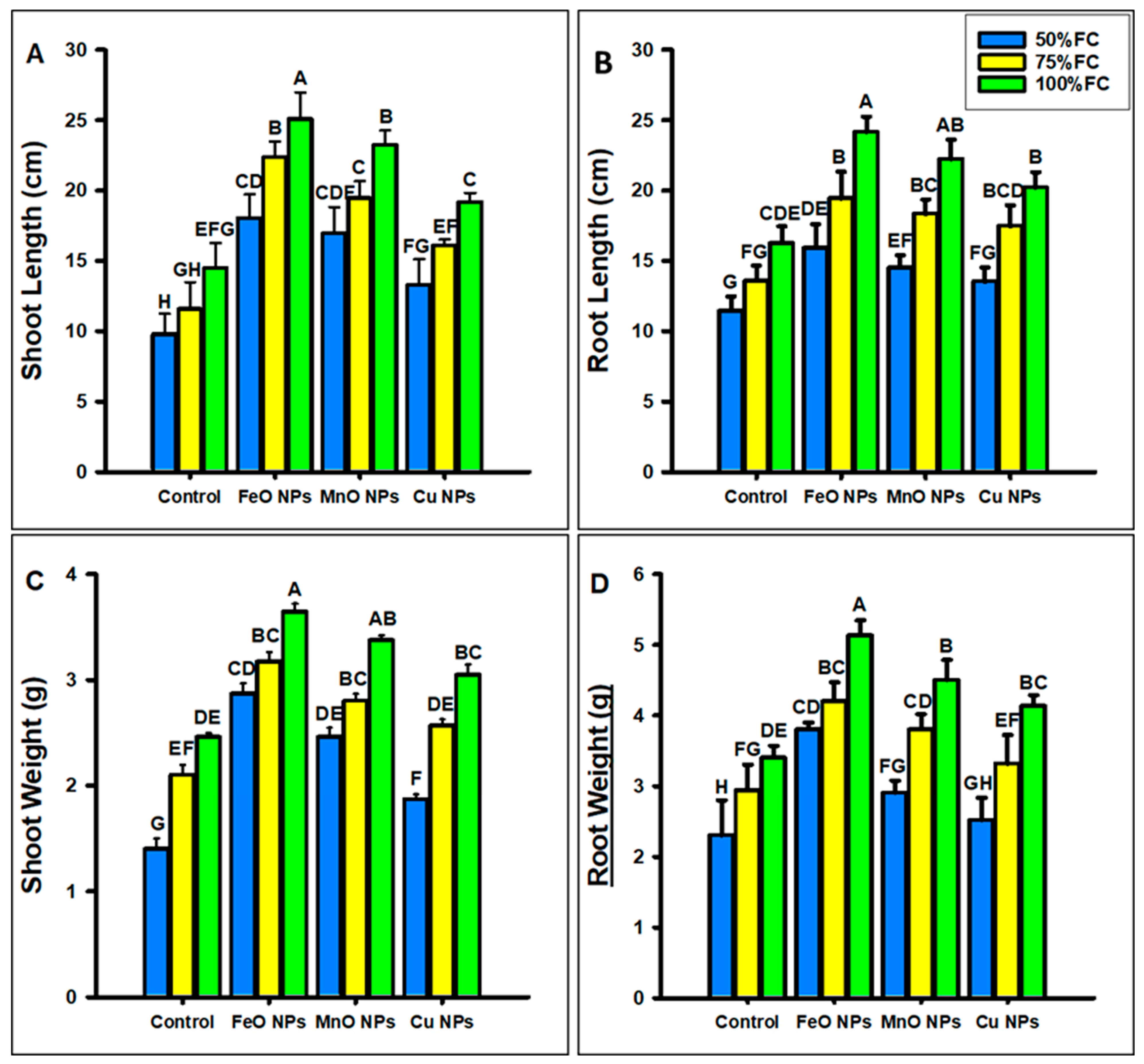
2.3.1. Morphological Parameter
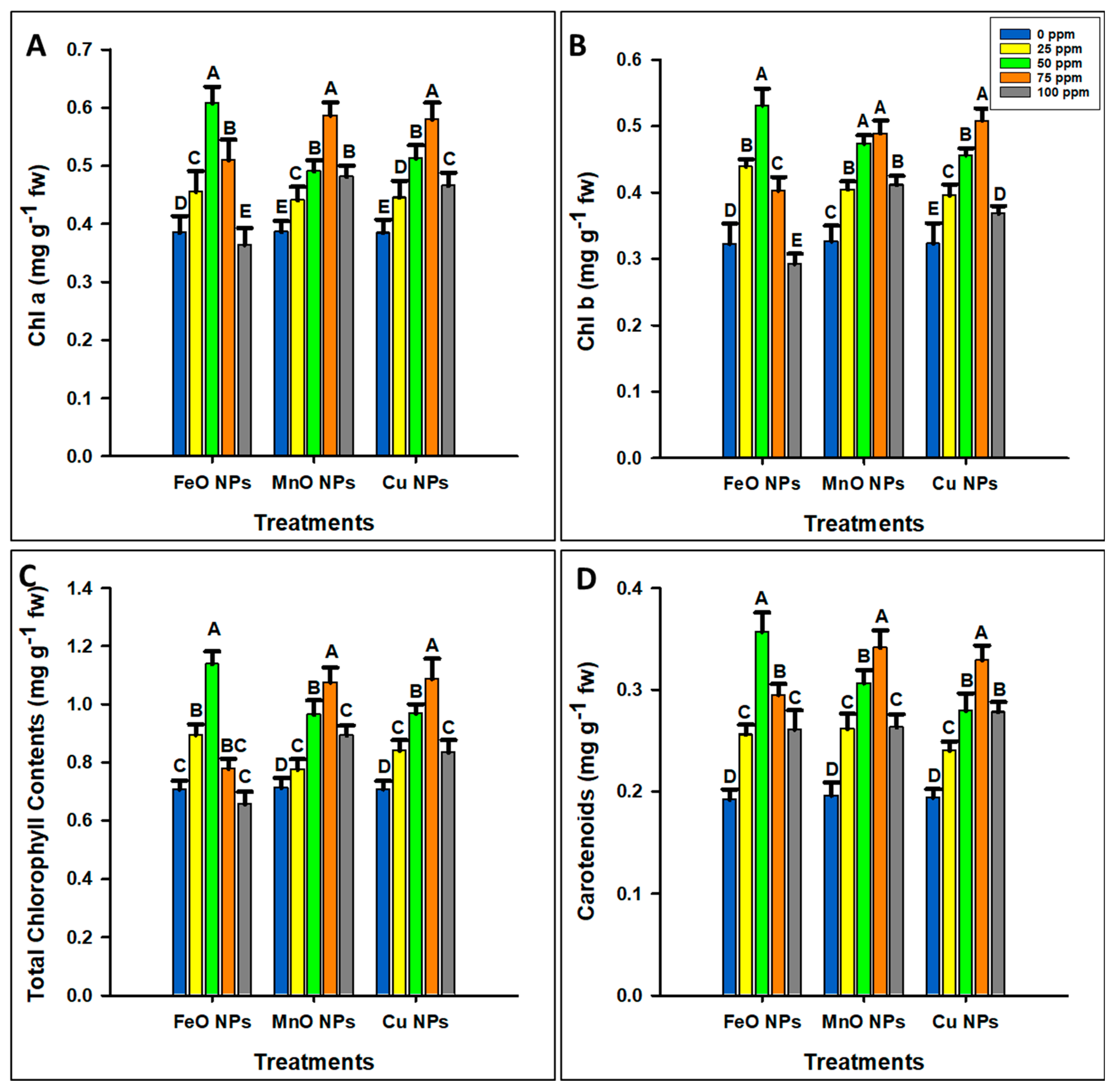
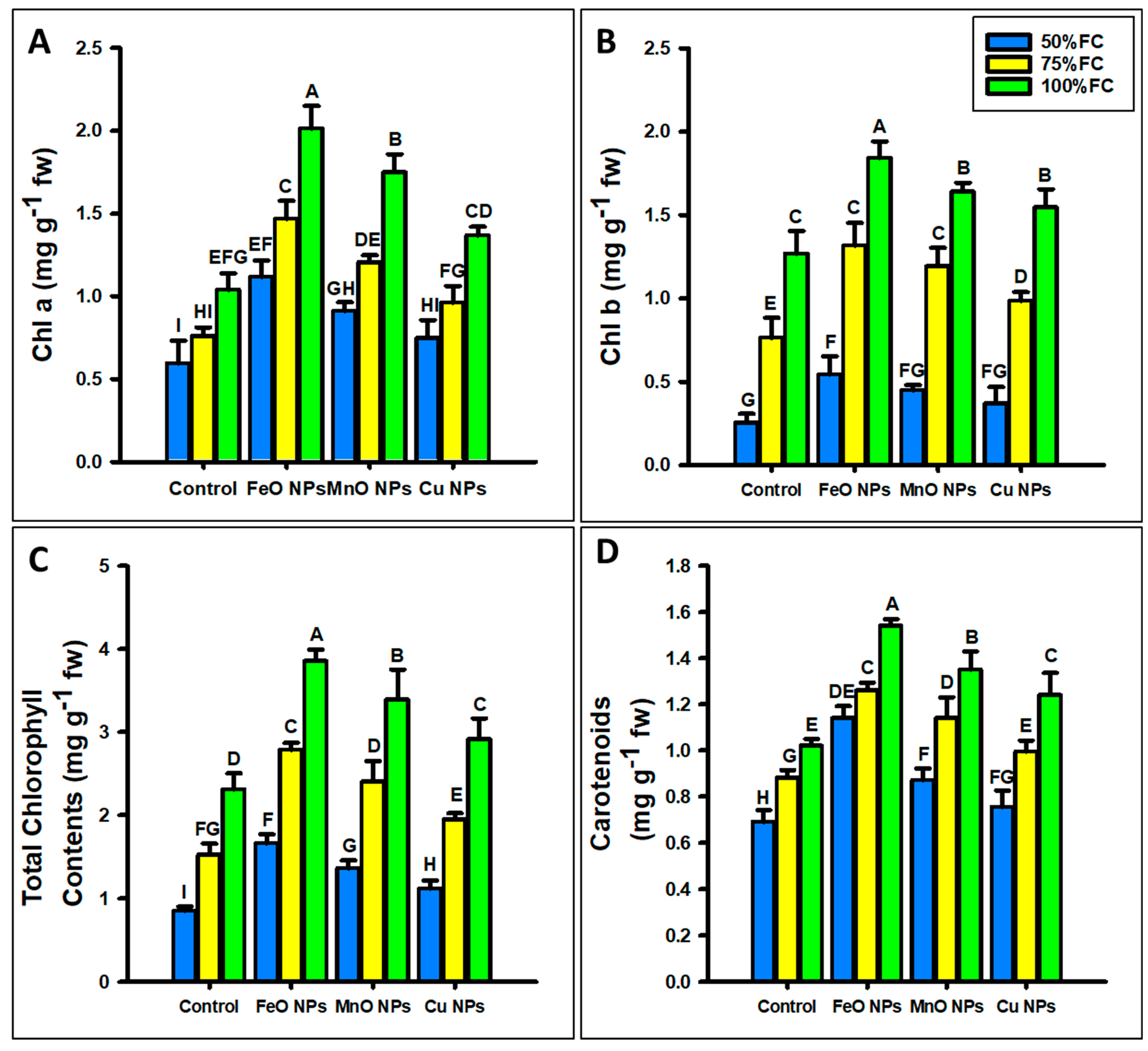
2.3.2. Photosynthetic Pigments and SPAD Values
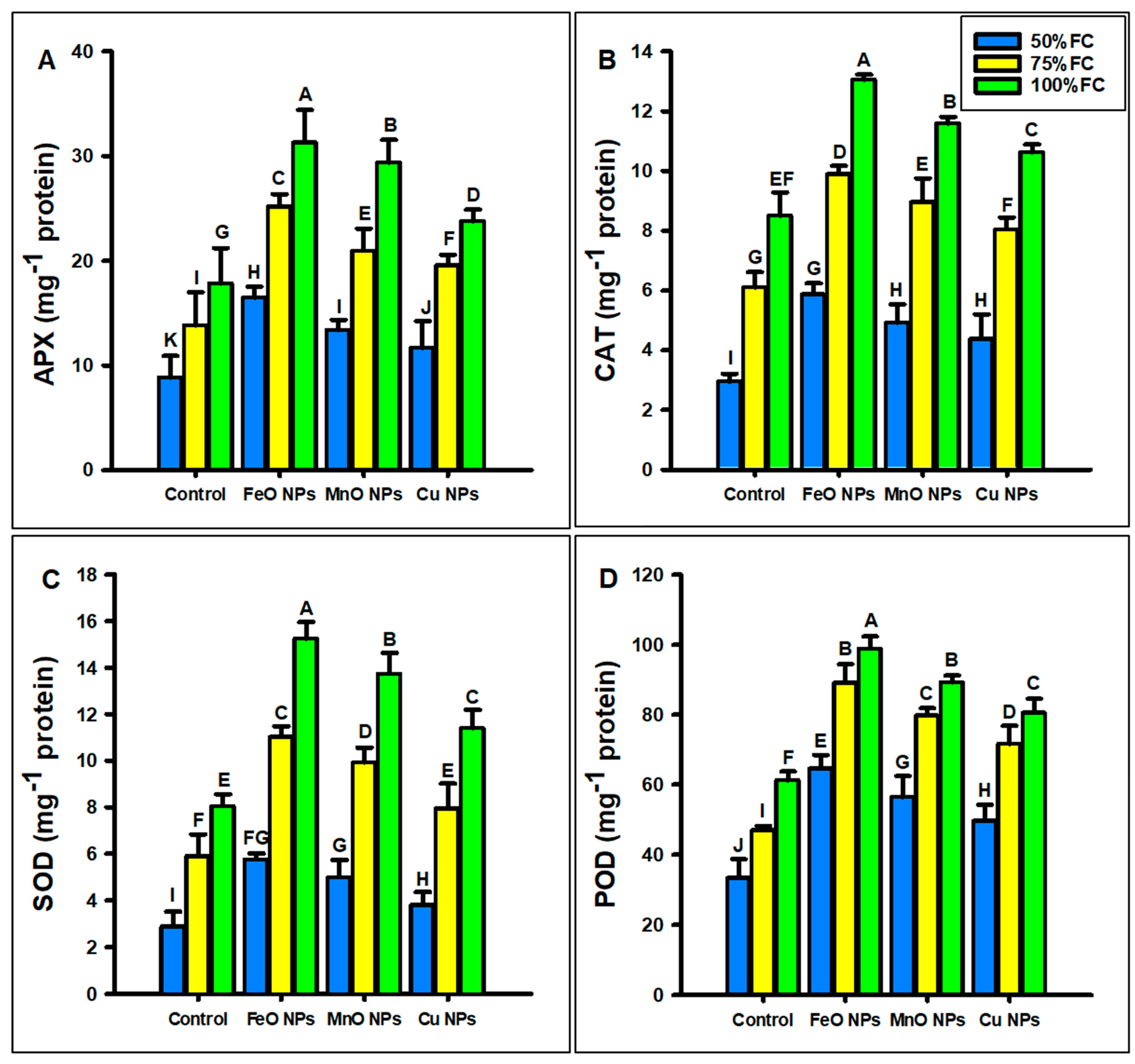
2.3.3. Antioxidant Parameters
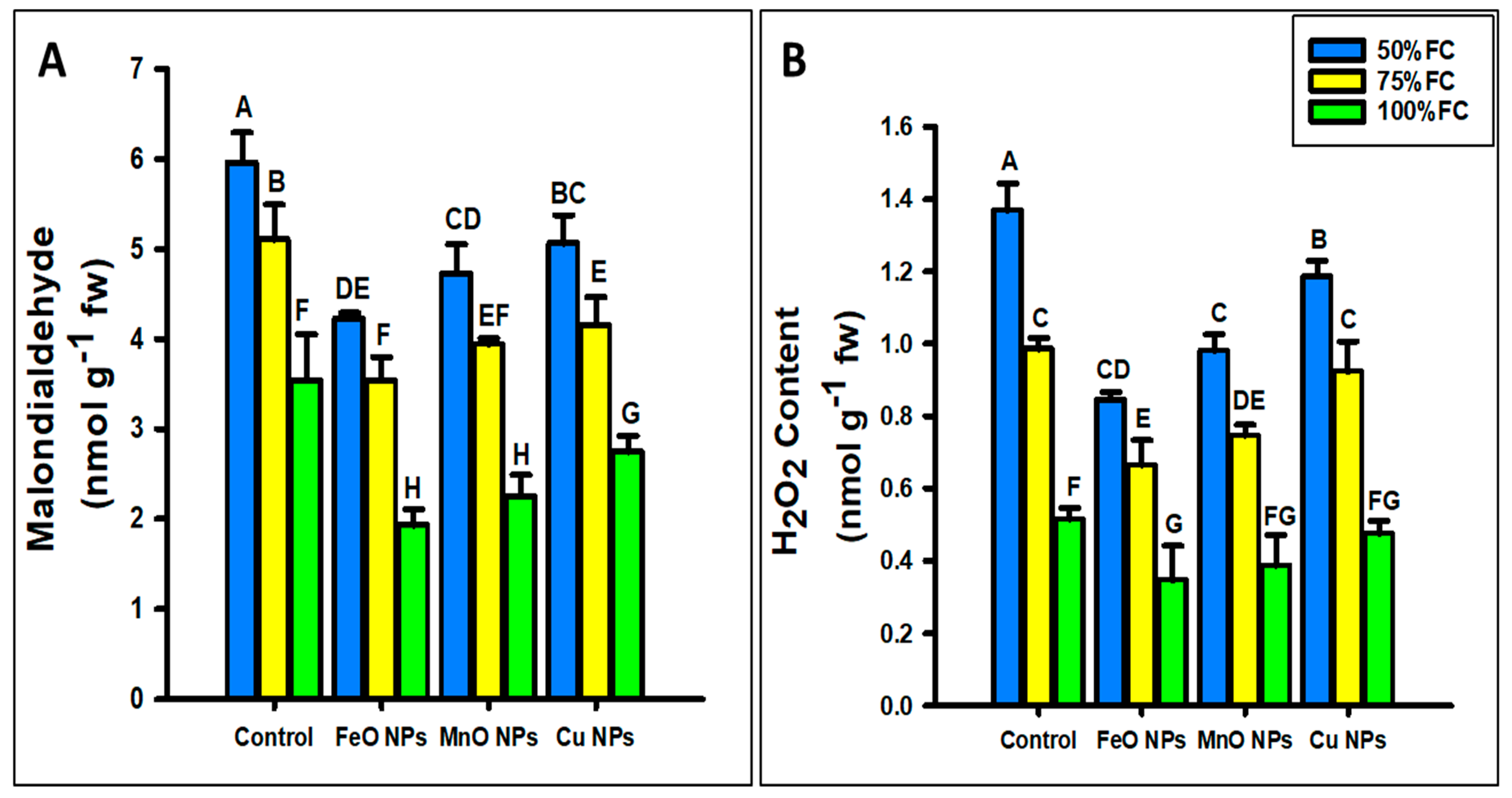
2.3.4. Oxidative Stress Parameters
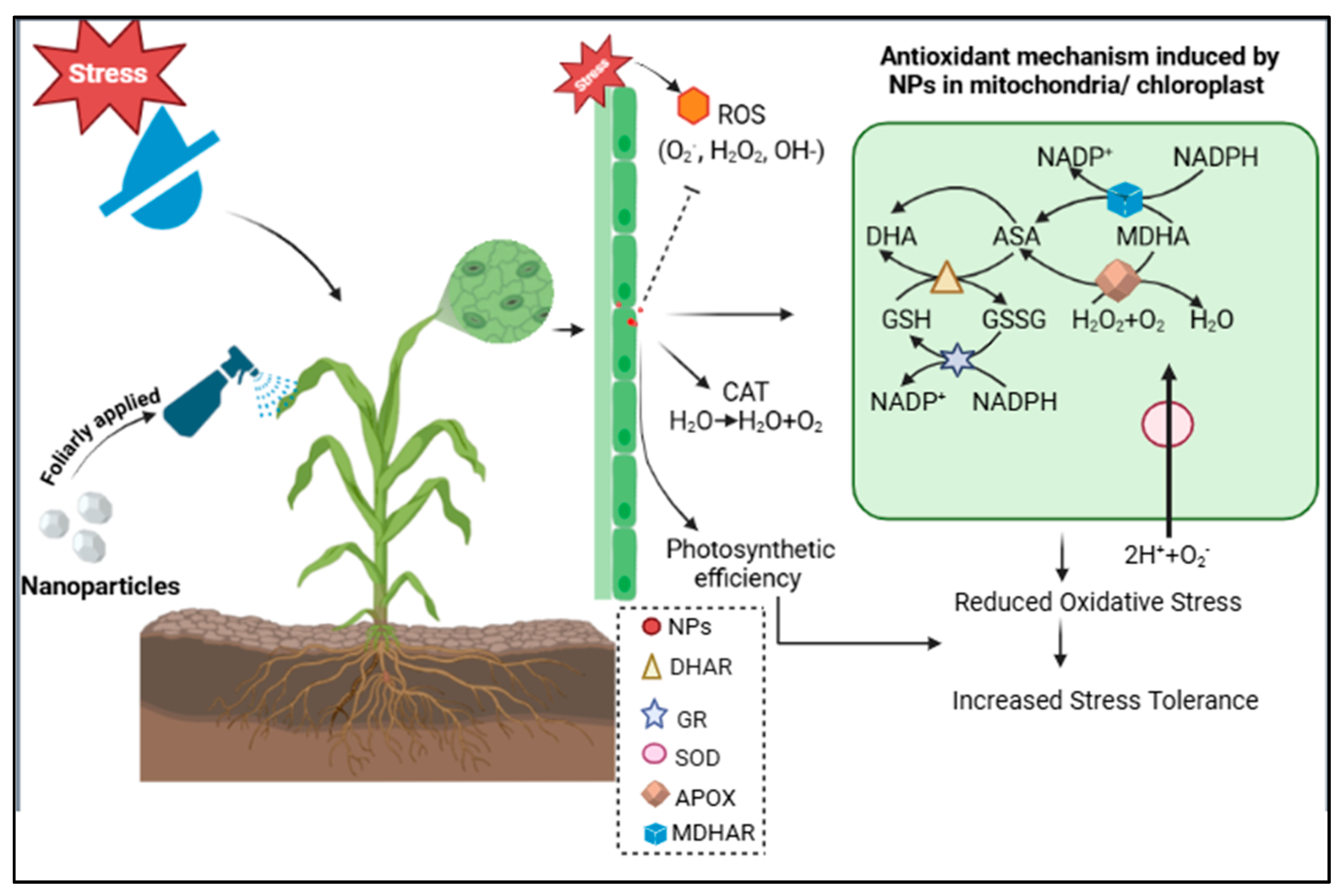
3. Discussion
4. Materials and Methods
4.1. Biosynthesis of Nanoparticles
4.2. Characterization of Nanoparticles
4.3. Optimization of Nanomaterials
4.4. Experimental Conditions
4.5. Morphological Parameters
4.6. Photosynthetic Parameters
4.7. Antioxidant Estimation
4.8. Oxidative Stress Estimation
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahid, I. Analysis of the Decreasing Food Security Situation and Impact on Household in Pakistan; Universidade de São Paulo: São Paulo, Brazil, 2023. [Google Scholar]
- Mahendri, I.G.A.P.; Saptati, R.A.; Adnyana, I.P.C.P. The Demand of Maize by Feed Mill in Indonesia During the COVID-19 Pandemic. In Proceedings of the 3rd International Conference on Environmentally Sustainable Animal Industry 2022 (ICESAI 2022), Malang, Indonesia, 13 October 2022; Atlantis Press: Amsterdam, The Netherlands, 2023; pp. 118–131. [Google Scholar]
- Ahmad, W.; Bibi, N.; Sanwal, M.; Ahmed, R.; Jamil, M.; Kalsoom, R.; Arif, M.; Fahad, S. Cereal Crops in the Era of Climate Change: An Overview. In Environment, Climate, Plant and Vegetation Growth; Springer: Cham, Switzerland, 2024; pp. 609–630. [Google Scholar]
- Széles, A.; Horváth, É.; Simon, K.; Zagyi, P.; Huzsvai, L. Maize Production Under Drought Stress: Nutrient Supply, Yield Prediction. Plants 2023, 12, 3301. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, J.; Orellana, A.E.M.; Kilian, W.; Moryl, A.; Cielecka, N.; Michałowska, K.; Policht-Latawiec, A.; Michalski, A.; Bednarek, A.; Włóka, A. Between Flood and Drought: How Cities Are Facing Water Surplus and Scarcity. J. Environ. Manag. 2023, 345, 118557. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, A.; Haidri, I.; Shafqat, U.; Khan, I.; Iqbal, M.; Mahmood, F.; Hassan, M.U. Impact of Biogenic Zinc Oxide Nanoparticles on Physiological and Biochemical Attributes of Pea (Pisum sativum L.) Under Drought Stress. Physiol. Mol. Biol. Plants 2025, 31, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Ghadirnezhad Shiade, S.R.; Fathi, A.; Taghavi Ghasemkheili, F.; Amiri, E.; Pessarakli, M. Plants’ Responses Under Drought Stress Conditions: Effects of Strategic Management Approaches—A Review. J. Plant Nutr. 2023, 46, 2198–2230. [Google Scholar] [CrossRef]
- Moore, T.D.; Martin-Creuzburg, D.; Yampolsky, L.Y. Diet Effects on Longevity, Heat Tolerance, Lipid Peroxidation and Mitochondrial Membrane Potential in Daphnia. Oecologia 2023, 202, 151–163. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, Y.; Wakisaka, M.; Yang, Z.; Yin, Y.; Fang, W.; Xu, Y.; Omura, T.; Yu, R.; Zheng, A.L.T. Mitigation of Oxidative Stress Damage Caused by Abiotic Stress to Improve Biomass Yield of Microalgae: A Review. Sci. Total Environ. 2023, 896, 165200. [Google Scholar]
- Jarin, A.S.; Islam, M.M.; Rahat, A.; Ahmed, S.; Ghosh, P.; Murata, Y. Drought Stress Tolerance in Rice: Physiological and Biochemical Insights. Int. J. Plant Biol. 2024, 15, 692–718. [Google Scholar] [CrossRef]
- Garg, D.; Sridhar, K.; Stephen Inbaraj, B.; Chawla, P.; Tripathi, M.; Sharma, M. Nano-Biofertilizer Formulations for Agriculture: A Systematic Review on Recent Advances and Prospective Applications. Bioengineering 2023, 10, 1010. [Google Scholar] [CrossRef]
- Nguyen, N.P.U.; Dang, N.T.; Doan, L.; Nguyen, T.T.H. Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’Methods—A Review. Processes 2023, 11, 2617. [Google Scholar] [CrossRef]
- Haidri, I.; Ishfaq, A.; Shahid, M.; Shahzad, T.; Hussain, S.; Mahmood, F. Green Nanoparticles for Textile Wastewater Treatment: The Current Insights. In Microbes Based Approaches for the Management of Hazardous Contaminants; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 277–292. [Google Scholar]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; Alderhami, S.A. Revisiting the Green Synthesis of Nanoparticles: Uncovering Influences of Plant Extracts as Reducing Agents for Enhanced Synthesis Efficiency and Its Biomedical Applications. Int. J. Nanomed. 2023, 18, 4727–4750. [Google Scholar] [CrossRef]
- Yadav, M.; George, N.; Dwibedi, V. Emergence of Toxic Trace Elements in Plant Environments: Insights into Potential of Silica Nanoparticles for Mitigation of Metal Toxicity in Plants. Environ. Pollut. 2023, 333, 122112. [Google Scholar] [PubMed]
- Voloshyna, I.M.; Netiaha, Y.M.; Nechaiuk, Y.V.; Khomenko, V.G.; Shkotova, L.V. The Influence of Metal Nanoparticles on Plants. Biopolym. Cell 2024, 40, 83–95. [Google Scholar]
- Semenova, N.A.; Burmistrov, D.E.; Shumeyko, S.A.; Gudkov, S.V. Fertilizers Based on Nanoparticles as Sources of Macro-and Microelements for Plant Crop Growth: A Review. Agronomy 2024, 14, 1646. [Google Scholar] [CrossRef]
- Chauhan, A.; Anand, J.; Parkash, V.; Rai, N. Biogenic Synthesis: A Sustainable Approach for Nanoparticles Synthesis Mediated by Fungi. Inorg. Nano-Metal Chem. 2023, 53, 460–473. [Google Scholar]
- Tombuloglu, G.; Tombuloglu, H.; Slimani, Y.; Almessiere, M.A.; Baykal, A.; Bostancioglu, S.M.; Kirat, G.; Ercan, I. Effects of Foliar Iron Oxide Nanoparticles (Fe3O4) Application on Photosynthetic Parameters, Distribution of Mineral Elements, Magnetic Behaviour, and Photosynthetic Genes in Tomato (Solanum lycopersicum Var. Cerasiforme) Plants. Plant Physiol. Biochem. 2024, 210, 108616. [Google Scholar]
- Ishfaq, A.; Shahid, M.; Nawaz, M.; Ibrar, D.; Hussain, S.; Shahzad, T.; Mahmood, F.; Rais, A.; Gul, S.; Gaafar, A.-R.Z. Remediation of Wastewater by Biosynthesized Manganese Oxide Nanoparticles and Its Effects on Development of Wheat Seedlings. Front. Plant Sci. 2023, 14, 1263813. [Google Scholar]
- Liu, A.; Xiao, W.; Lai, W.; Wang, J.; Li, X.; Yu, H.; Zha, Y. Potential Application of Selenium and Copper Nanoparticles in Improving Growth, Quality, and Physiological Characteristics of Strawberry Under Drought Stress. Agriculture 2024, 14, 1172. [Google Scholar] [CrossRef]
- Khan, A.R.; Fan, X.; Salam, A.; Azhar, W.; Ulhassan, Z.; Qi, J.; Liaquat, F.; Yang, S.; Gan, Y. Melatonin-Mediated Resistance to Copper Oxide Nanoparticles-Induced Toxicity by Regulating the Photosynthetic Apparatus, Cellular Damages and Antioxidant Defense System in Maize Seedlings. Environ. Pollut. 2023, 316, 120639. [Google Scholar]
- Alhaithloul, H.A.S.; Ali, B.; Alghanem, S.M.S.; Zulfiqar, F.; Al-Robai, S.A.; Ercisli, S.; Yong, J.W.H.; Moosa, A.; Irfan, E.; Ali, Q. Effect of Green-Synthesized Copper Oxide Nanoparticles on Growth, Physiology, Nutrient Uptake, and Cadmium Accumulation in Triticum aestivum (L.). Ecotoxicol. Environ. Saf. 2023, 268, 115701. [Google Scholar]
- Kandhol, N.; Jain, M.; Tripathi, D.K. Nanoparticles as Potential Hallmarks of Drought Stress Tolerance in Plants. Physiol. Plant. 2022, 174, e13665. [Google Scholar]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Desoky, E.-S.M.; Babalghith, A.O.; El-Tahan, A.M.; Ibrahim, O.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A. Role of Nanoparticles in Enhancing Crop Tolerance to Abiotic Stress: A Comprehensive Review. Front. Plant Sci. 2022, 13, 946717. [Google Scholar]
- Shafqat, U.; Hussain, S.; Shahzad, T.; Shahid, M.; Mahmood, F. Elucidating the Phytotoxicity Thresholds of Various Biosynthesized Nanoparticles on Physical and Biochemical Attributes of Cotton. Chem. Biol. Technol. Agric. 2023, 10, 30. [Google Scholar]
- Ghorbani, A.; Emamverdian, A.; Pehlivan, N.; Zargar, M.; Razavi, S.M.; Chen, M. Nano-Enabled Agrochemicals: Mitigating Heavy Metal Toxicity and Enhancing Crop Adaptability for Sustainable Crop Production. J. Nanobiotechnol. 2024, 22, 91. [Google Scholar]
- Shafqat, U.; Yasin, M.U.; Shahid, M.; Hussain, S.; Shahzad, T.; Mahmood, F.; Ishfaq, A.; Nawaz, M.; Shah, A.N.; Ali, H.M. Evaluating the Impact of Biogenic Nanoparticles and Pesticide Application in Controlling Cotton Leaf Curl Virus Disease (CLCuD) in Cotton (Gossypium hirsutum L.). Chem. Biol. Technol. Agric. 2024, 11, 149. [Google Scholar]
- Murgueitio-Herrera, E.; Falconí, C.E.; Cumbal, L.; Gómez, J.; Yanchatipán, K.; Tapia, A.; Martínez, K.; Sinde-Gonzalez, I.; Toulkeridis, T. Synthesis of Iron, Zinc, and Manganese Nanofertilizers, Using Andean Blueberry Extract, and Their Effect in the Growth of Cabbage and Lupin Plants. Nanomaterials 2022, 12, 1921. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Shahid, M.; Mahmood, F.; Shahzad, T.; Azeem, F.; Hussain, S.; Algarni, T.S.; Elshikh, M.S.; Onazi, W.A.; Mustafa, S. Biosynthesis of Copper Nanoparticles Using Bacillus Flexus and Estimation of Their Potential for Decolorization of Azo Dyes and Textile Wastewater Treatment. J. King Saud Univ. 2024, 36, 103309. [Google Scholar]
- Yin, Z.; Lee, C.; Cho, S.; Yoo, J.; Piao, Y.; Kim, Y.S. Facile Synthesis of Oxidation-Resistant Copper Nanowires toward Solution-Processable, Flexible, Foldable, and Free-Standing Electrodes. Small 2014, 10, 5047–5052. [Google Scholar] [PubMed]
- Elumalai, P.; Rajamohan, R.; Purayil, A.T.V.; Menon, V.; Srivatsan, R.P.; Kumar, A.S.; Lakshminarayanan, S.; Mainupriya, S.; Nandi, S.; Gao, X. Biosurfactant and Iron Oxide Nanoparticle-Assisted Bioremediation of Soil Co-Contaminated with Hydrocarbons and Hazardous Heavy Metals. Chem. Eng. J. 2024, 497, 154677. [Google Scholar]
- Osadebe, A.U.; Akinrodoye, T.I.; Ogugbue, C.J.; Okpokwasili, G.C. Green Synthesised Iron Oxide Nanoparticles Decorated on Biochar for Enhanced Natural Attenuation in Simulated Petroleum Compromised Soil. Nanotechnol. Environ. Eng. 2022, 7, 517–528. [Google Scholar]
- Saod, W.M.; Hamid, L.L.; Alaallah, N.J.; Ramizy, A. Biosynthesis and Antibacterial Activity of Manganese Oxide Nanoparticles Prepared by Green Tea Extract. Biotechnol. Rep. 2022, 34, e00729. [Google Scholar]
- Nieto-Maldonado, A.; Bustos-Guadarrama, S.; Espinoza-Gomez, H.; Flores-López, L.Z.; Ramirez-Acosta, K.; Alonso-Nuñez, G.; Cadena-Nava, R.D. Green Synthesis of Copper Nanoparticles Using Different Plant Extracts and Their Antibacterial Activity. J. Environ. Chem. Eng. 2022, 10, 107130. [Google Scholar]
- Rama, P.; Thangapushbam, V.; Sivakami, S.; Jothika, M.; Mariselvi, P.; Sundaram, R.; Muthu, K. Preparation, Characterization of Green Synthesis FeO Nanoparticles and Their Photocatalytic Activity Towards Basic Fuschin Dye. J. Indian Chem. Soc. 2024, 101, 101142. [Google Scholar]
- El-Sawah, A.A.; El-Naggar, N.E.-A.; Eldegla, H.E.; Soliman, H.M. Green Synthesis of Collagen Nanoparticles by Streptomyces Xinghaiensis NEAA-1, Statistical Optimization, Characterization, and Evaluation of Their Anticancer Potential. Sci. Rep. 2024, 14, 3283. [Google Scholar]
- Francis, D.V.; Abdalla, A.K.; Mahakham, W.; Sarmah, A.K.; Ahmed, Z.F.R. Interaction of Plants and Metal Nanoparticles: Exploring Its Molecular Mechanisms for Sustainable Agriculture and Crop Improvement. Environ. Int. 2024, 190, 108859. [Google Scholar]
- Banerjee, A.; Roychoudhury, A. Explicating the Cross-Talks Between Nanoparticles, Signaling Pathways and Nutrient Homeostasis During Environmental Stresses and Xenobiotic Toxicity for Sustainable Cultivation of Cereals. Chemosphere 2022, 286, 131827. [Google Scholar] [PubMed]
- Ibrahim, S.O.; Lukman, H.Y.; Abdulkadir, F.R.; Bello, M.Y.; Ayipo, Y.O.; Babamale, H.F.; Zubair, M.F.; Atolani, O. Nanoparticles as Antioxidant Agents: A Comprehensive Review. Al-Bahir J. Eng. Pure Sci. 2024, 5, 3. [Google Scholar]
- Sarkar, A.; Ghosh, M.; Sil, P.C. Nanotoxicity: Oxidative Stress Mediated Toxicity of Metal and Metal Oxide Nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 730–743. [Google Scholar]
- Wahab, A.; Batool, F.; Muhammad, M.; Zaman, W.; Mikhlef, R.M.; Naeem, M. Current Knowledge, Research Progress, and Future Prospects of Phyto-Synthesized Nanoparticles Interactions with Food Crops Under Induced Drought Stress. Sustainability 2023, 15, 14792. [Google Scholar] [CrossRef]
- Haidri, I.; Ishfaq, A.; Shahid, M.; Hussain, S.; Shahzad, T.; Shafqat, U.; Mustafa, S.; Mahmood, F. Enhancement of Antioxidants’ Enzymatic Activity in the Wheat Crop by Shewanela Sp. Mediated Zinc Oxide Nanoparticles Against Heavy Metals Contaminated Wastewater. J. Soil Sci. Plant Nutr. 2024, 24, 7068–7089. [Google Scholar]
- Pooja; Munjal, R. Oxidative Stress and Antioxidant Defense in Plants Under High Temperature. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 337–352. [Google Scholar]
- Ahmad, I.; Ahmad, W.; Nepal, J.; Junaid, M.B.; Bukhari, N.A.; Usman, M.; Ahmad, N.; Khan, R.N. Synergistic Enhancement of Maize Crop Yield and Nutrient Assimilation via Zinc Oxide Nanoparticles and Phosphorus Fertilization. J. Sci. Food Agric. 2024, 104, 6733–6745. [Google Scholar]
- Zeeshan, M.; Wang, X.; Salam, A.; Wu, H.; Li, S.; Zhu, S.; Chang, J.; Chen, X.; Zhang, Z.; Zhang, P. Selenium Nanoparticles Boost the Drought Stress Response of Soybean by Enhancing Pigment Accumulation, Oxidative Stress Management and Ultrastructural Integrity. Agronomy 2024, 14, 1372. [Google Scholar] [CrossRef]
- Nair, N.M.; Jahanara, M.M.; Ray, D.; Swaminathan, P. Photoresponse of a Printed Transparent Silver Nanowire-Zinc Oxide Nanocomposite. Flex. Print. Electron. 2021, 6, 45004. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sarkhosh, A.; Balal, R.M.; Rauf, S.; Khan, N.; Altaf, M.A.; Camara-Zapata, J.M.; Garcia-Sanchez, F.; Shahid, M.A. Silicon Nanoparticles Mitigate Hypoxia-Induced Oxidative Damage by Improving Antioxidants Activities and Concentration of Osmolytes in Southern Highbush Blueberry Plants. Agronomy 2021, 11, 2143. [Google Scholar] [CrossRef]
- Rath, A.; Das, A.B. Chromium Stress Induced Oxidative Burst in Vigna mungo (L.) Hepper: Physio-Molecular and Antioxidative Enzymes Regulation in Cellular Homeostasis. Physiol. Mol. Biol. Plants 2021, 27, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Sun, R.; Lv, R.; Du, T.; Li, Y.; Zhang, X.; Sheng, R.; Qi, Y. Multienzymatic Antioxidant Activity of Manganese-Based Nanoparticles for Protection Against Oxidative Cell Damage. ACS Biomater. Sci. Eng. 2022, 8, 638–648. [Google Scholar] [PubMed]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the Enzymatic and Non-Enzymatic Antioxidant Defense by Applying Iron Oxide Nanoparticles in Dracocephalum moldavica L. Plant Under Salinity Stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Raza, M.A.; Rizwan, M.; Tariq, R.; Ali, S.; Huang, L. Silver Nanoparticles Improved the Plant Growth and Reduced the Sodium and Chlorine Accumulation in Pearl Millet: A Life Cycle Study. Environ. Sci. Pollut. Res. 2021, 28, 13712–13724. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alkhatib, F.M.; Alzahrani, S.O.; Shafi, M.E.; Abdel-Hamid, S.E.; Taha, T.F.; Aboelenin, S.M.; Soliman, M.M.; Ahmed, N.H. Impact of Mycogenic Zinc Nanoparticles on Performance, Behavior, Immune Response, and Microbial Load in Oreochromis Niloticus. Saudi J. Biol. Sci. 2021, 28, 4592–4604. [Google Scholar] [CrossRef]
- Irshad, M.A.; Rehman, M.Z.U.; Anwar-Ul-Haq, M.; Rizwan, M.; Nawaz, R.; Shakoor, M.B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P.; Ali, S. Effect of Green and Chemically Synthesized Titanium Dioxide Nanoparticles on Cadmium Accumulation in Wheat Grains and Potential Dietary Health Risk: A Field Investigation. J. Hazard. Mater. 2021, 415, 125585. [Google Scholar] [CrossRef] [PubMed]
- Atanda, S.A.; Shaibu, R.O.; Agunbiade, F.O. Nanoparticles in Agriculture: Balancing Food Security and Environmental Sustainability. Discov. Agric. 2025, 3, 26. [Google Scholar] [CrossRef]
- Ahmed, I.; Zia, M.A.; Afzal, H.; Ahmed, S.; Ahmad, M.; Akram, Z.; Sher, F.; Iqbal, H.M.N. Socio-Economic and Environmental Impacts of Biomass Valorisation: A Strategic Drive for Sustainable Bioeconomy. Sustainability 2021, 13, 4200. [Google Scholar] [CrossRef]
- Firisa, S.G.; Muleta, G.G.; Yimer, A.A. Synthesis of nickel oxide nanoparticles and copper-doped nickel oxide nanocomposites using phytolacca dodecandra l’herit leaf extract and evaluation of its antioxidant and photocatalytic activities. ACS Omega 2022, 7, 44720–44732. [Google Scholar] [PubMed]
- Jassim, S.M.; Mohammed, A.A.; Kareem, M.M.; Khodair, Z.T. Synthesis and characterization of Cr-doped cadmium oxide thin films for NH3 gas-sensing applications. Bull. Mater. Sci. 2024, 47, 104. [Google Scholar]
- Palta, J.P. Leaf Chlorophyll Content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar]
- Zhang, X.Z. The Measurement and Mechanism of Lipid Peroxidation and SOD, POD and CAT Activities in Biological System. In Research Methodology of Crop Physiology; China Agriculture Press: Beijing, China, 1992; pp. 208–211. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar]
- Zhang, J.; Kirkham, M.B. Drought-Stress-Induced Changes in Activities of Superoxide Dismutase, Catalase, and Peroxidase in Wheat Species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizwan, H.M.; Shafqat, U.; Ishfaq, A.; Batool, F.; Mahmood, F.; Su, Q.; Yaseen, N.; Raza, T.; Altihani, F.A. Identifying the Phytotoxicity of Biosynthesized Metal Oxide Nanoparticles and Their Impact on Antioxidative Enzymatic Activity in Maize Under Drought Stress. Plants 2025, 14, 1075. https://doi.org/10.3390/plants14071075
Rizwan HM, Shafqat U, Ishfaq A, Batool F, Mahmood F, Su Q, Yaseen N, Raza T, Altihani FA. Identifying the Phytotoxicity of Biosynthesized Metal Oxide Nanoparticles and Their Impact on Antioxidative Enzymatic Activity in Maize Under Drought Stress. Plants. 2025; 14(7):1075. https://doi.org/10.3390/plants14071075
Chicago/Turabian StyleRizwan, Hafiz Muhammad, Usman Shafqat, Aneeza Ishfaq, Fatima Batool, Faisal Mahmood, Qitao Su, Nimra Yaseen, Tehziba Raza, and Faizah Amer Altihani. 2025. "Identifying the Phytotoxicity of Biosynthesized Metal Oxide Nanoparticles and Their Impact on Antioxidative Enzymatic Activity in Maize Under Drought Stress" Plants 14, no. 7: 1075. https://doi.org/10.3390/plants14071075
APA StyleRizwan, H. M., Shafqat, U., Ishfaq, A., Batool, F., Mahmood, F., Su, Q., Yaseen, N., Raza, T., & Altihani, F. A. (2025). Identifying the Phytotoxicity of Biosynthesized Metal Oxide Nanoparticles and Their Impact on Antioxidative Enzymatic Activity in Maize Under Drought Stress. Plants, 14(7), 1075. https://doi.org/10.3390/plants14071075





