Mediated Transformation of Tamarillo (Solanum betaceum) Callus Cell Suspension Cultures: A Novel Platform for Biotechnological Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Induction of Callus
2.2. Vectors, Constructs, and Agrobacterium Strains Used for Transformation
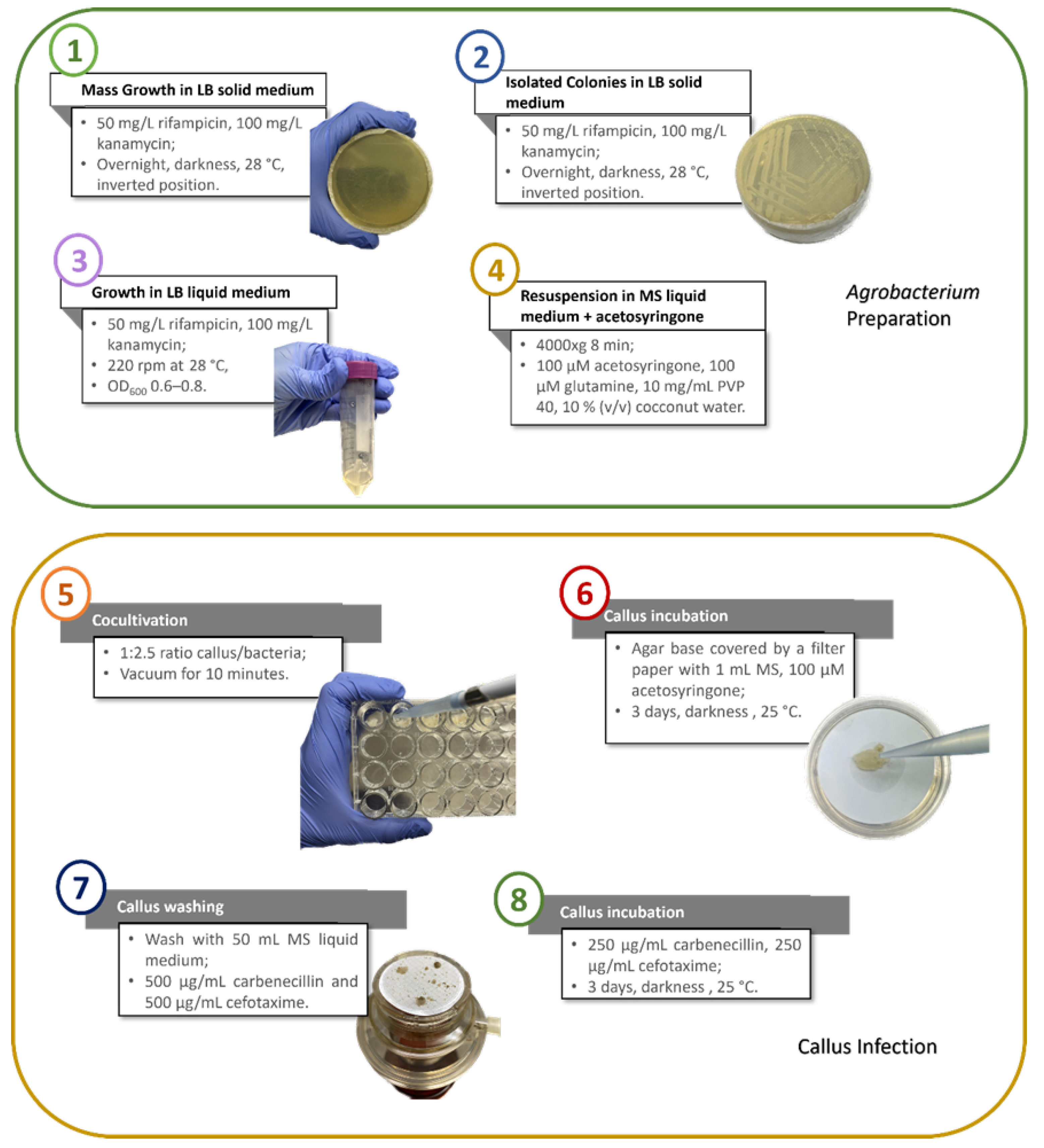
2.3. Cocultivation and Washing of the Callus: Infection in a Multiwell
2.4. Cocultivation and Washing of the Callus: Infection in Liquid Medium
2.5. Selection of Transformed Callus
2.6. Histochemical GUS Assay
2.7. PCR Confirmation of Transgenic Cells
2.8. qPCR Relative Quantification of the gusA Gene Insertion
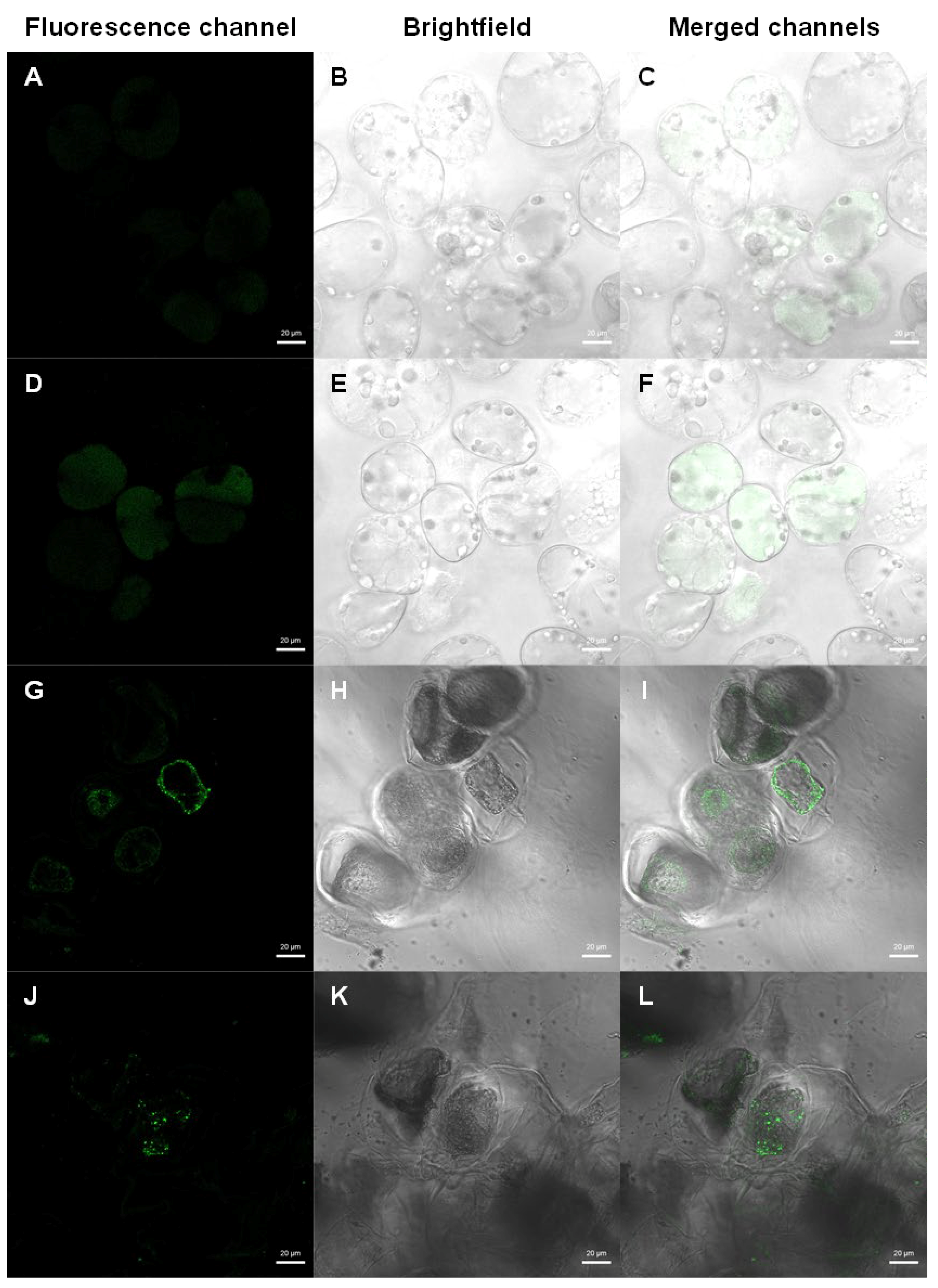
2.9. Detection of Transformed Cells by Fluorescence Microscopy
2.10. Statistical Analysis
3. Results
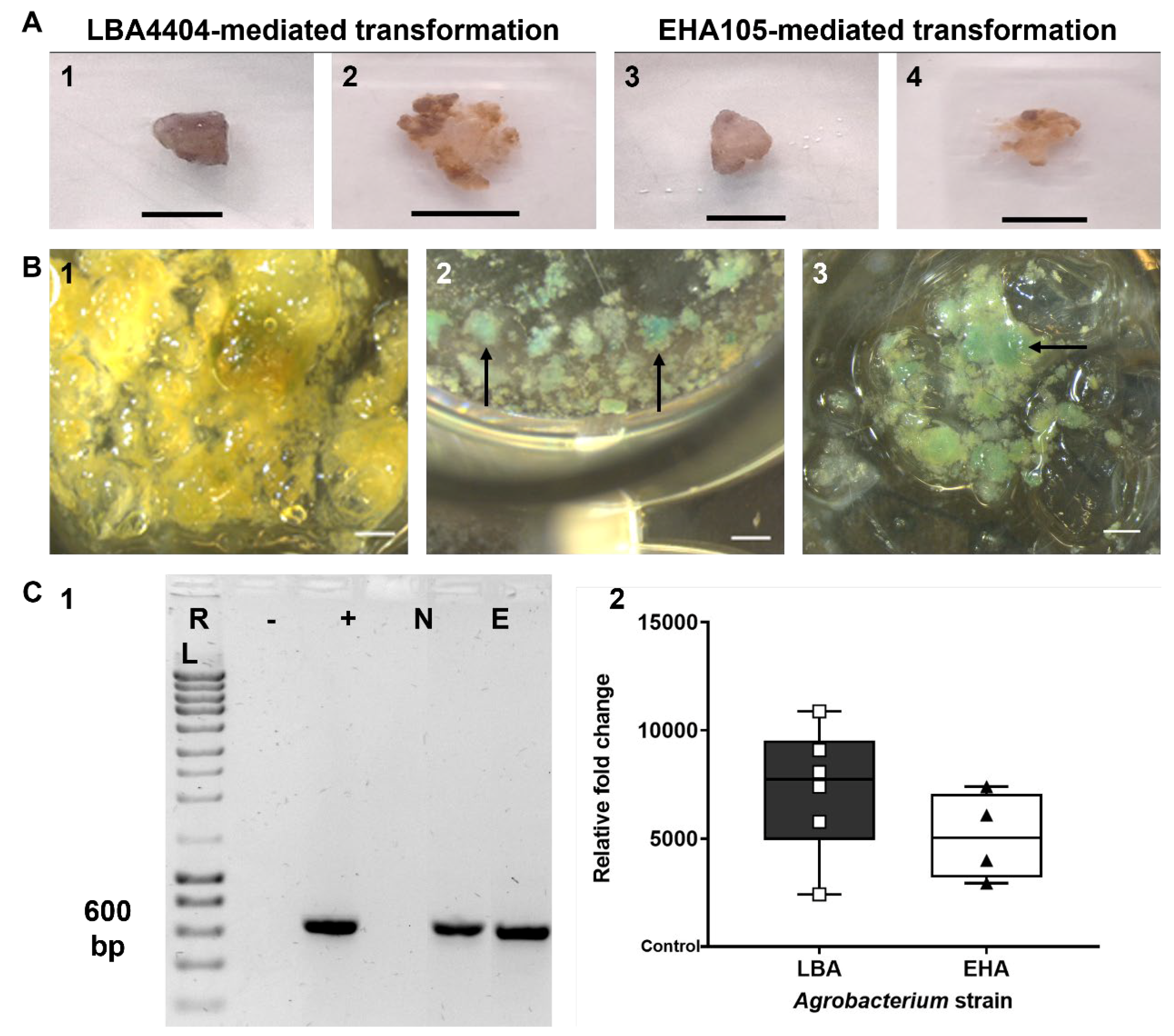
3.1. Transformation of Tamarillo Callus Cells
3.2. Selection of Transformed Callus Cells
3.3. Transformation Confirmation by β-Glucuronidase (GUS) Activity Analysis
3.4. Transformation Confirmation and Efficiency Evaluation by PCR-Based Approaches
3.5. Optimisation of the Protocol Using Liquid Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CaMV35S | Cauliflower mosaic virus 35S promoter |
| CARB | Carbenicillin |
| CEFO | Cefotaxime |
| eyfp | Yellow fluorescent protein gene |
| gusA | β-glucuronidase gene |
| KAN | Kanamycin |
| LB | Luria broth medium |
| MS | Murashige and Skoog basal medium |
| nos | Nopaline synthase promoter |
| nptII | Neomycin phosphotransferase II gene |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| PVP | Polyvinylpyrrolidone |
| qPCR | Quantitative PCR |
| SM | Selective medium |
| TP | Induction medium |
| TS | Suspension liquid medium |
| US FDA | United States Food and Drug Administration |
References
- Motti, R. The Solanaceae family: Botanical features and diversity. In The Wild Solanums Genomes; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–9. [Google Scholar] [CrossRef]
- Bohs, L. Ethnobotany of the genus Cyphomandra (Solanaceae). Econ. Bot. 1989, 43, 143–163. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Tamarillo (Solanum betaceum): Chemical composition, biological properties, and product innovation. Trends Food Sci. Technol. 2020, 95, 45–58. [Google Scholar] [CrossRef]
- Correia, M.; Lopes, T.; Puga, A.P.; Pinto, G.; Canhoto, J.; Correia, S. Morpho-physiological evaluation of Solanum betaceum Cav. in vitro cloned plants: A comparison of different micropropagation methods. Plants 2023, 12, 1884. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, B.; Mota, I.; Veríssimo, P.; Canhoto, J.; Correia, S. Enhancing the production of hydrolytic enzymes in elicited tamarillo (Solanum betaceum Cav.) cell suspension cultures. Plants 2023, 12, 190. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; de Ronne, M.; Yoosefzadeh-Najafabadi, M.; Adamek, K.; Torkamaneh, D.; Jones, A.M.P. Transcriptomic profiling of embryogenic and non-embryogenic callus provides new insight into the nature of recalcitrance in cannabis. Int. J. Mol. Sci. 2023, 24, 14625. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Alves, A.; Caeiro, A.; Correia, S.I.; Veríssimo, P.; Canhoto, J. Establishment and biochemical characterization of tamarillo (Solanum betaceum Cav.) embryogenic cell suspension cultures. Vitr. Cell. Dev. Biol. Plant 2017, 53, 606–618. [Google Scholar] [CrossRef]
- Hellwig, S.; Drossard, J.; Twyman, R.M.; Fischer, R. Plant cell cultures for the production of recombinant proteins. Nat. Biotechnol. 2004, 22, 1415–1422. [Google Scholar] [CrossRef]
- Debnath, N.; Thakur, M.; Negi, N.P.; Gautam, V.; Kumar Yadav, A.; Kumar, D. Insight of oral vaccines as an alternative approach to health and disease management: An innovative intuition and challenges. Biotechnol. Bioeng. 2022, 119, 327–346. [Google Scholar] [CrossRef]
- Komarova, T.V.; Sheshukova, E.V.; Dorokhov, Y.L. Plant-made antibodies: Properties and therapeutic applications. Curr. Med. Chem. 2019, 26, 381–395. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Pal, M.; Kumar, V.; Yadav, R.; Gulati, D.; Yadav, R. Potential and prospects of shikonin production enhancement in medicinal plants. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 775–784. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.-L.; Li, J.; Li, J.-X.; Liu, S.-J.; Huang, L.-Q.; Gao, W.-Y. Production of active compounds in medicinal plants: From plant tissue culture to biosynthesis. Chin. Herb. Med. 2017, 9, 115–125. [Google Scholar] [CrossRef]
- Rebelo, B.A.; Folgado, A.; Ferreira, A.C.; Abranches, R. Production of the SARS-CoV-2 spike protein and its receptor binding domain in plant cell suspension cultures. Front. Plant Sci. 2022, 13, 995429. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Z.; Zhang, Y.; Yi, L.; Duan, F.; Zhao, Q.; Gu, Y.; Wang, S. Proteomics-guided isolation of a novel serine protease with milk-clotting activity from tamarillo (Solanum betaceum Cav.). Food Chem. 2025, 465, 141956. [Google Scholar] [CrossRef]
- Sainsbury, F.; Lomonossoff, G.P. Transient expressions of synthetic biology in plants. Curr. Opin. Plant Biol. 2014, 19, 1–7. [Google Scholar] [CrossRef]
- Wang, P.; Pu, Y.; Abid, M.A.; Kang, L.; Ye, Y.; Zhang, M.; Liang, C.; Wei, Y.; Zhang, R.; Meng, Z. A Rapid and efficient method for isolation and transformation of cotton callus protoplast. Int. J. Mol. Sci. 2022, 23, 8368. [Google Scholar] [CrossRef]
- Burnett, M.J.; Burnett, A.C. Therapeutic recombinant protein production in plants: Challenges and opportunities. Plants People Planet 2020, 2, 121–132. [Google Scholar] [CrossRef]
- Cordeiro, D.; Alves, A.; Ferraz, R.; Casimiro, B.; Canhoto, J.; Correia, S. An efficient Agrobacterium-mediated genetic transformation method for Solanum betaceum Cav. embryogenic callus. Plants 2023, 12, 1202. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Quiñonero, C.; Akdemir, H.; Alburquerque, N.; Pedreño, M.Á.; Burgos, L. Agrobacterium-mediated transformation of Vitis Cv. Monastrell suspension-cultured cells: Determination of critical parameters. Biotechnol. Prog. 2016, 32, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Canhoto, J. Somatic embryogenesis of tamarillo (Solanum betaceum Cav.). In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 171–179. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Vancanneyt, G.; Schmidt, R.; O’Connor-Sanchez, A.; Willmitzer, L.; Rocha-Sosa, M. Construction of an intron-containing marker gene: Splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol. Gen. Genet. MGG 1990, 220, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Hoekema, A.; Hirsch, P.R.; Hooykaas, P.J.; Schilperoort, R.A. A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 1983, 303, 179–180. [Google Scholar] [CrossRef]
- Hood, E.E.; Gelvin, S.B.; Melchers, L.S.; Hoekema, A. New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 1993, 2, 208–218. [Google Scholar] [CrossRef]
- Alves, A. Ensaios de Embriogénese Somática e Transformação Genética em Tamarilho (Cyphomandra betacea [Cav.] Sendt.). Master’s Thesis, Universidade de Coimbra, Coimbra, Portugal, 2012. [Google Scholar]
- Hu, Q.; Kononowicz-Hodges, H.; Nelson-Vasilchik, K.; Viola, D.; Zeng, P.; Liu, H.; Kausch, A.P.; Chandlee, J.M.; Hodges, T.K.; Luo, H. FLP recombinase-mediated site-specific recombination in rice. Plant Biotechnol. J. 2008, 6, 176–188. [Google Scholar] [CrossRef]
- Schauer, S.E.; Schlüter, P.M.; Baskar, R.; Gheyselinck, J.; Bolaños, A.; Curtis, M.D.; Grossniklaus, U. Intronic regulatory elements determine the divergent expression patterns of AGAMOUS-LIKE6 subfamily members in Arabidopsis. Plant J. 2009, 59, 987–1000. [Google Scholar] [CrossRef]
- Cordeiro, D.; Rito, M.; Borges, F.; Canhoto, J.; Correia, S. Selection and validation of reference genes for qPCR analysis of miRNAs and their targets during somatic embryogenesis in tamarillo (Solanum betaceum Cav.). Plant Cell Tissue Organ Cult. (PCTOC) 2020, 143, 109–120. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Atkinson, R.G.; Gardner, R.C. Regeneration of transgenic tamarillo plants. Plant Cell Rep. 1993, 12, 347–351. [Google Scholar] [CrossRef]
- Correia, S.; Alhinho, A.T.; Casimiro, B.; Miguel, C.M.; Oliveira, M.; Veríssimo, P.; Canhoto, J. NEP-TC a rRNA methyltransferase involved on somatic embryogenesis of tamarillo (Solanum betaceum Cav.). Front. Plant Sci. 2019, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.A.; Roberts, S.C. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Mansfield, J.W.; Bennett, M.H. Agrobacterium-mediated transformation of Indica rice genotypes: An assessment of factors affecting the transformation efficiency. Plant Cell Tissue Organ Cult. 2005, 82, 45–55. [Google Scholar] [CrossRef]
- Amoah, B.; Wu, H.; Sparks, C.; Jones, H. Factors influencing Agrobacterium-mediated transient expression of uidA in wheat inflorescence tissue. J. Exp. Bot. 2001, 52, 1135–1142. [Google Scholar] [CrossRef]
- Lopez, E.; Proano, K.; Jadan, M.; Mihai, R. Callus tissue induction and analysis of GUS reporter gene expression in tomato (Solanum lycopersicum L.) transformed with Agrobacterium tumefaciens. Rom. Biotechnol. Lett. 2015, 20, 10205. [Google Scholar]
- Niedbała, G.; Niazian, M.; Sabbatini, P. Modeling Agrobacterium-mediated gene transformation of tobacco (Nicotiana tabacum)—A model plant for gene transformation studies. Front. Plant Sci. 2021, 12, 695110. [Google Scholar] [CrossRef]
- Dhekney, S.; Sessions, S.; Brungart-Rosenberg, M.; Claflin, C.; Li, Z.; Gray, D. Genetic modification of grapevine embryogenic cultures. In Transgenic Plants: Methods and Protocols; Humana Press: New York, NY, USA, 2019; pp. 191–201. [Google Scholar]
- Uranbey, S.; Sevimay, C.; Kaya, M.; Ipek, A.; Sancak, C.; Başalma, D.; Er, C.; Özcan, S. Influence of different co-cultivation temperatures, periods and media on Agrobacterium tumefaciens-mediated gene transfer. Biol. Plant. 2005, 49, 53–57. [Google Scholar] [CrossRef]
- Vasudevan, V.; Siva, R.; Krishnan, V.; Manickavasagam, M. Polyamines, sonication and vacuum infiltration enhances the Agrobacterium-mediated transformation in watermelon (Citrullus lanatus Thunb.). S. Afr. J. Bot. 2020, 128, 333–338. [Google Scholar] [CrossRef]
- Priya, A.M.; Pandian, S.K.; Manikandan, R. The effect of different antibiotics on the elimination of Agrobacterium and high frequency Agrobacterium-mediated transformation of indica rice (Oryza sativa L.). Czech J. Genet. Plant Breed. 2012, 48, 120–130. [Google Scholar] [CrossRef]
- Kumar, N.; Gulati, A.; Bhattacharya, A. L-glutamine and L-glutamic acid facilitate successful Agrobacterium infection of recalcitrant tea cultivars. Appl. Biochem. Biotechnol. 2013, 170, 1649–1664. [Google Scholar] [CrossRef]
- Sheikholeslam, S.N.; Weeks, D.P. Acetosyringone promotes high efficiency transformation of Arabidopsis thaliana explants by Agrobacterium tumefaciens. Plant Mol. Biol. 1987, 8, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J.; Dewey, R.E.; Boss, W.; Phillippy, B.Q.; Qu, R. Enhanced Agrobacterium-mediated transformation efficiencies in monocot cells is associated with attenuated defense responses. Plant Mol. Biol. 2013, 81, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Gerszberg, A.; Grzegorczyk-Karolak, I. Influence of selected antibiotics on the tomato regeneration in in vitro cultures. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 558–564. [Google Scholar] [CrossRef]
- Haddadi, F.; Abd Aziz, M.; Abdullah, S.N.A.; Tan, S.G.; Kamaladini, H. An efficient Agrobacterium-mediated transformation of strawberry cv. Camarosa by a dual plasmid system. Molecules 2015, 20, 3647–3666. [Google Scholar] [CrossRef]
- Khatun, M.; Borphukan, B.; Alam, I.; Keya, C.A.; Khan, H.; Reddy, M.K.; Salimullah, M. An improved Agrobacterium mediated transformation and regeneration protocol for successful genetic engineering and genome editing in eggplant. Sci. Hortic. 2022, 293, 110716. [Google Scholar] [CrossRef]
- Ling, H.-Q.; Kriseleit, D.; Ganal, M. Effect of ticarcillin/potassium clavulanate on callus growth and shoot regeneration in Agrobacterium-mediated transformation of tomato (Lycopersicon esculentum Mill.). Plant Cell Rep. 1998, 17, 843–847. [Google Scholar] [CrossRef]
- Peng, W.; Wang, X.; Wei, H.; Zhang, Z.; Teng, C.; Li, Q.; Lyu, K.; Lyu, S.; Fan, Y. Development of Agrobacterium tumefaciens-mediated transformation of Solanum nigrum and expression of AcMYB110 in S. nigrum. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Rebelo, B.A.; Abranches, R. A simplified protocol for Agrobacterium-mediated transformation of cell suspension cultures of the model species Medicago truncatula A17. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 153, 669–675. [Google Scholar] [CrossRef]
- Martínez Márquez, A.; Morante Carriel, J.; Ramírez-Estrada, K.; Cusidó, R.M.; Sellés-Marchart, S.; Palazon, J.; Pedreño, M.A.; Bru-Martínez, R. A reliable protocol for the stable transformation of non-embryogenic cells cultures of grapevine (Vitis vinifera L.) and Taxus × media. J. Biol. Methods 2015, 2, e21. [Google Scholar] [CrossRef]
| Time | 3 Weeks | 6 Weeks | 9 Weeks | 12 Weeks | 15 Weeks | 18 Weeks | Subsequent Weeks |
|---|---|---|---|---|---|---|---|
| TP solid medium + antibiotics (transformation in the multiwell) | 250 CARB | 250 CARB | 250 CARB | 200 CARB | 100 CARB | 100 CARB | |
| 250 CEFO | 250 CEFO | 250 CEFO | 200 CEFO | -- | -- | ||
| 50 KAN | 100 KAN | 120 KAN | 120 KAN | 120 KAN | 100 KAN | ||
| TP solid medium + antibiotics (liquid medium transformation) | 0 CARB | 0 CARB | 0 CARB | 0 CARB | 0 CARB | 0 CARB | 100 CARB |
| 300 CEFO | 250 CEFO | 250 CEFO | 250 CEFO | 250 CEFO | 250 CEFO | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, R.; Casimiro, B.; Cordeiro, D.; Canhoto, J.; Correia, S. Mediated Transformation of Tamarillo (Solanum betaceum) Callus Cell Suspension Cultures: A Novel Platform for Biotechnological Applications. Plants 2025, 14, 1028. https://doi.org/10.3390/plants14071028
Ferraz R, Casimiro B, Cordeiro D, Canhoto J, Correia S. Mediated Transformation of Tamarillo (Solanum betaceum) Callus Cell Suspension Cultures: A Novel Platform for Biotechnological Applications. Plants. 2025; 14(7):1028. https://doi.org/10.3390/plants14071028
Chicago/Turabian StyleFerraz, Ricardo, Bruno Casimiro, Daniela Cordeiro, Jorge Canhoto, and Sandra Correia. 2025. "Mediated Transformation of Tamarillo (Solanum betaceum) Callus Cell Suspension Cultures: A Novel Platform for Biotechnological Applications" Plants 14, no. 7: 1028. https://doi.org/10.3390/plants14071028
APA StyleFerraz, R., Casimiro, B., Cordeiro, D., Canhoto, J., & Correia, S. (2025). Mediated Transformation of Tamarillo (Solanum betaceum) Callus Cell Suspension Cultures: A Novel Platform for Biotechnological Applications. Plants, 14(7), 1028. https://doi.org/10.3390/plants14071028










