Auxin Controls Root Gravitropic Response by Affecting Starch Granule Accumulation and Cell Wall Modification in Tomato
Abstract
1. Introduction
2. Results
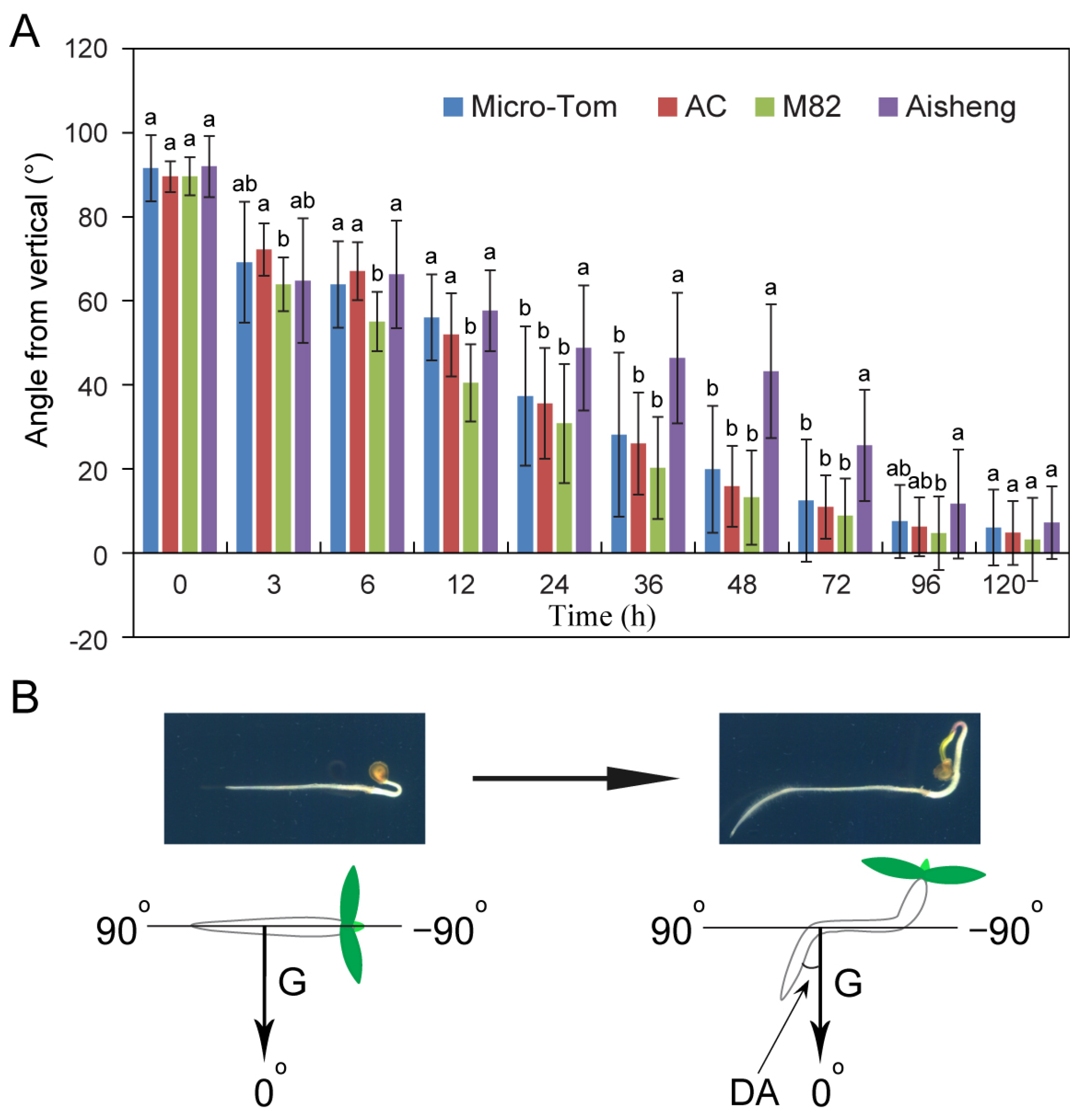
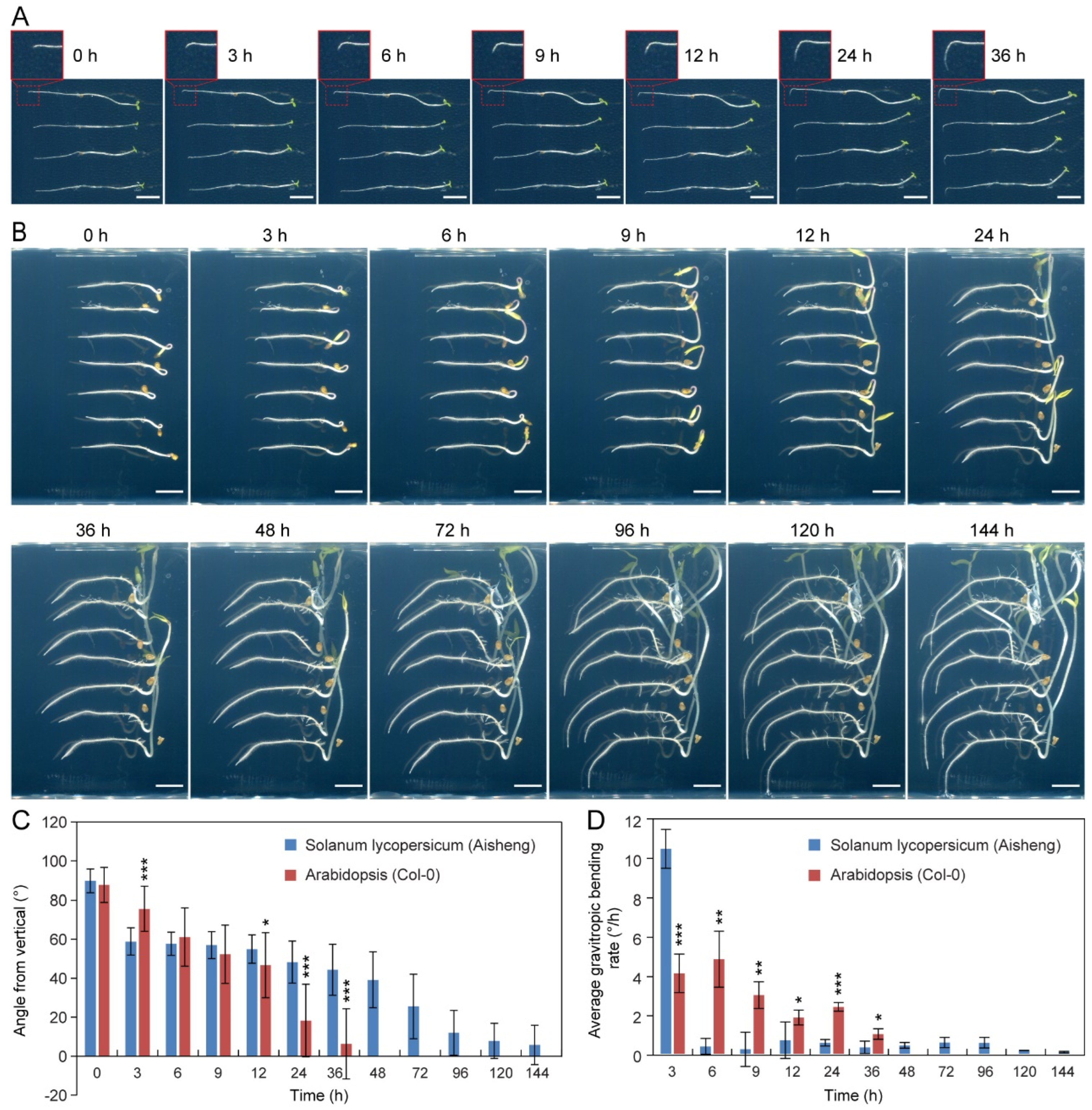
2.1. Lagging Root Gravitropic Response in the Cultivated Cherry Tomatoes (Aisheng)
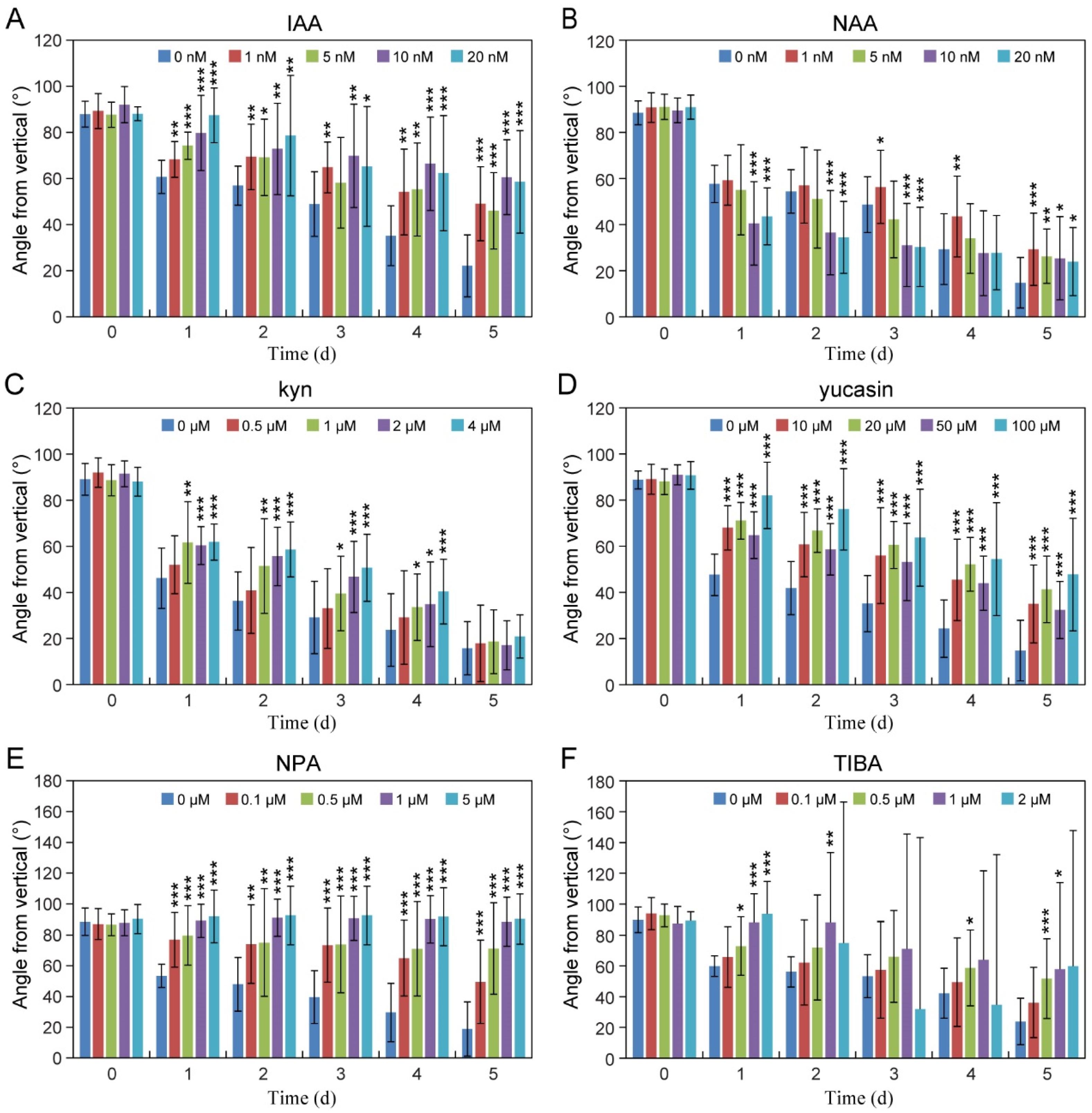
2.2. Auxin-Driven Regulation of Gravitropic Root Growth in Tomato
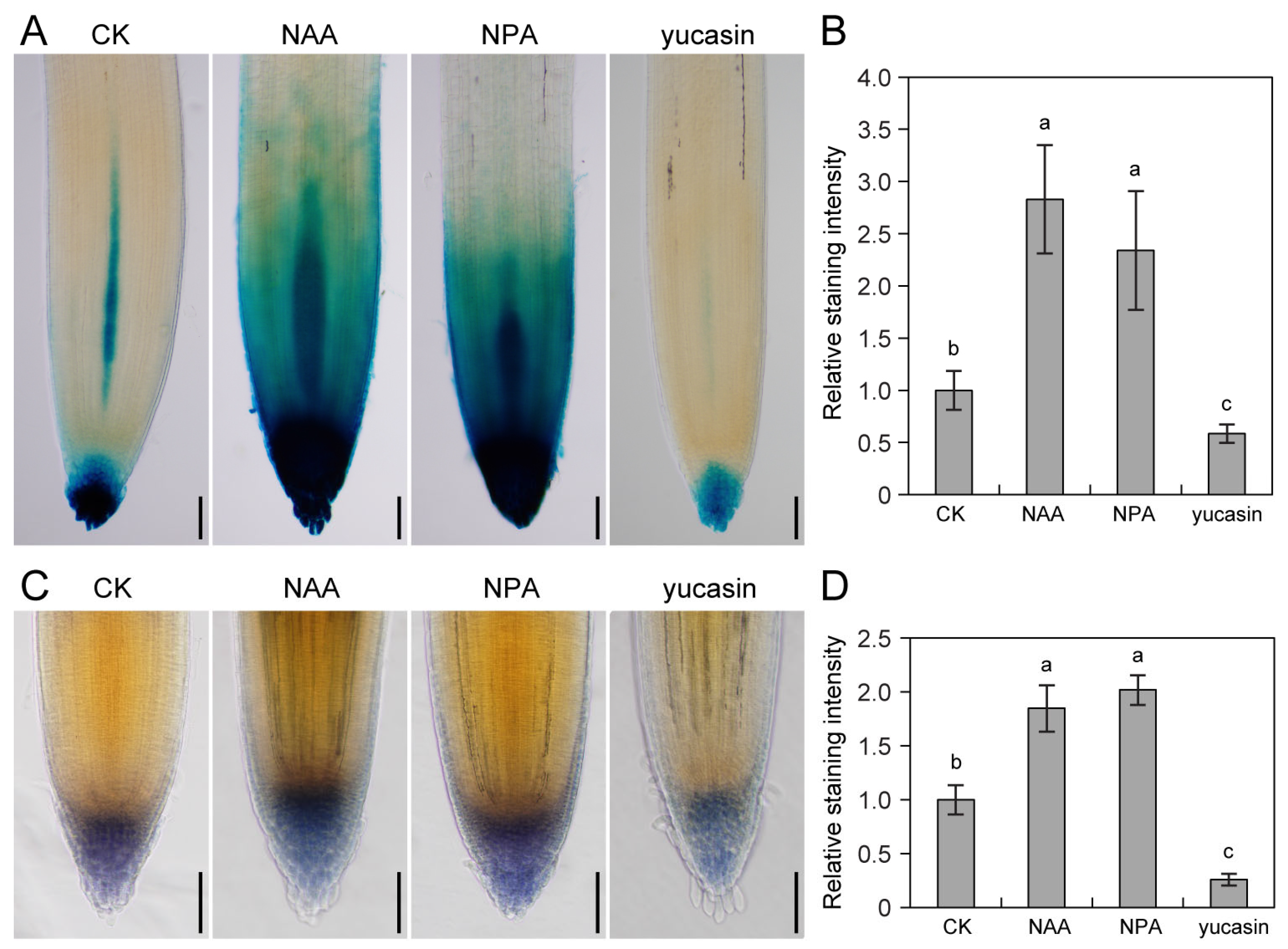
2.3. Auxin Biosynthesis and Transport Are Required for Root Gravitropic Growth
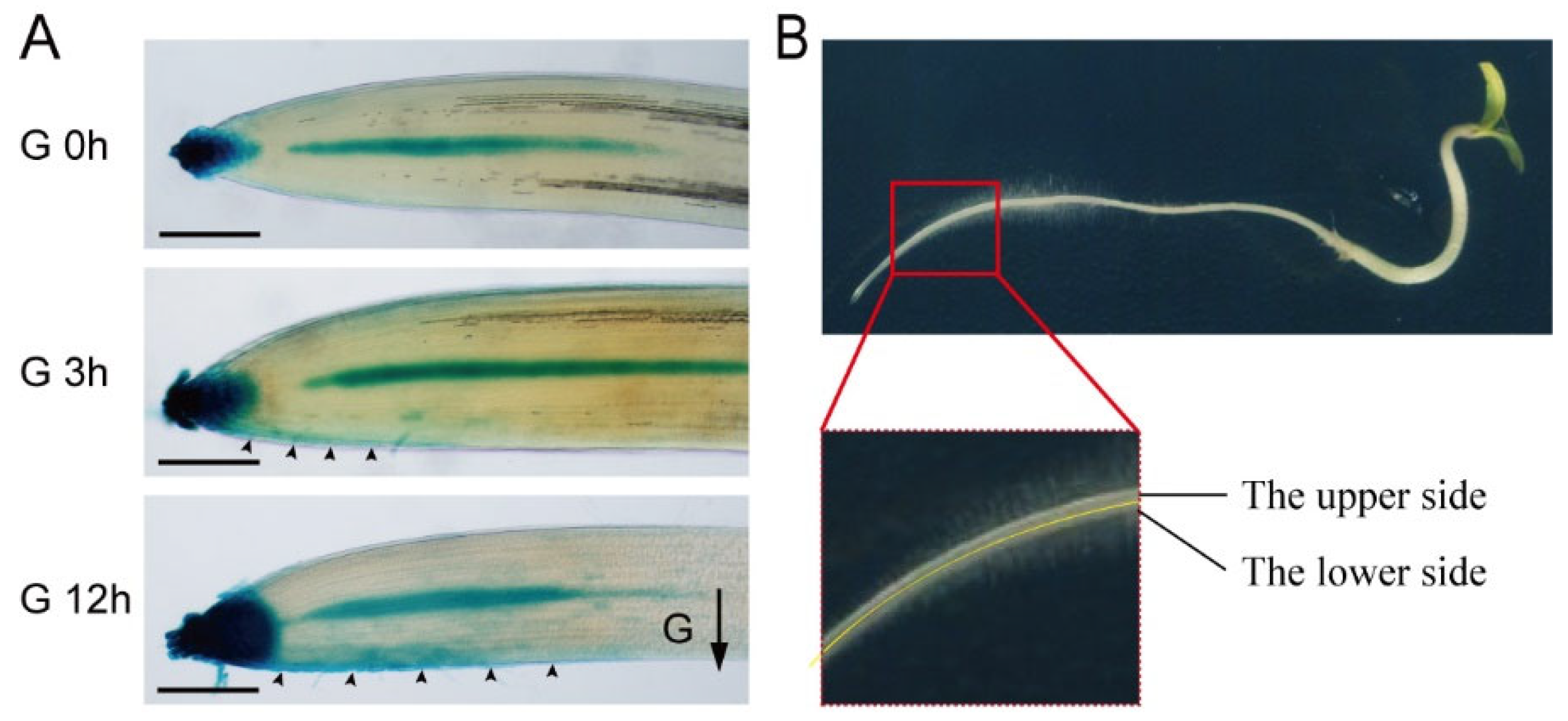
2.4. Auxin Positively Regulates Starch Accumulation in Tomato Root Tips
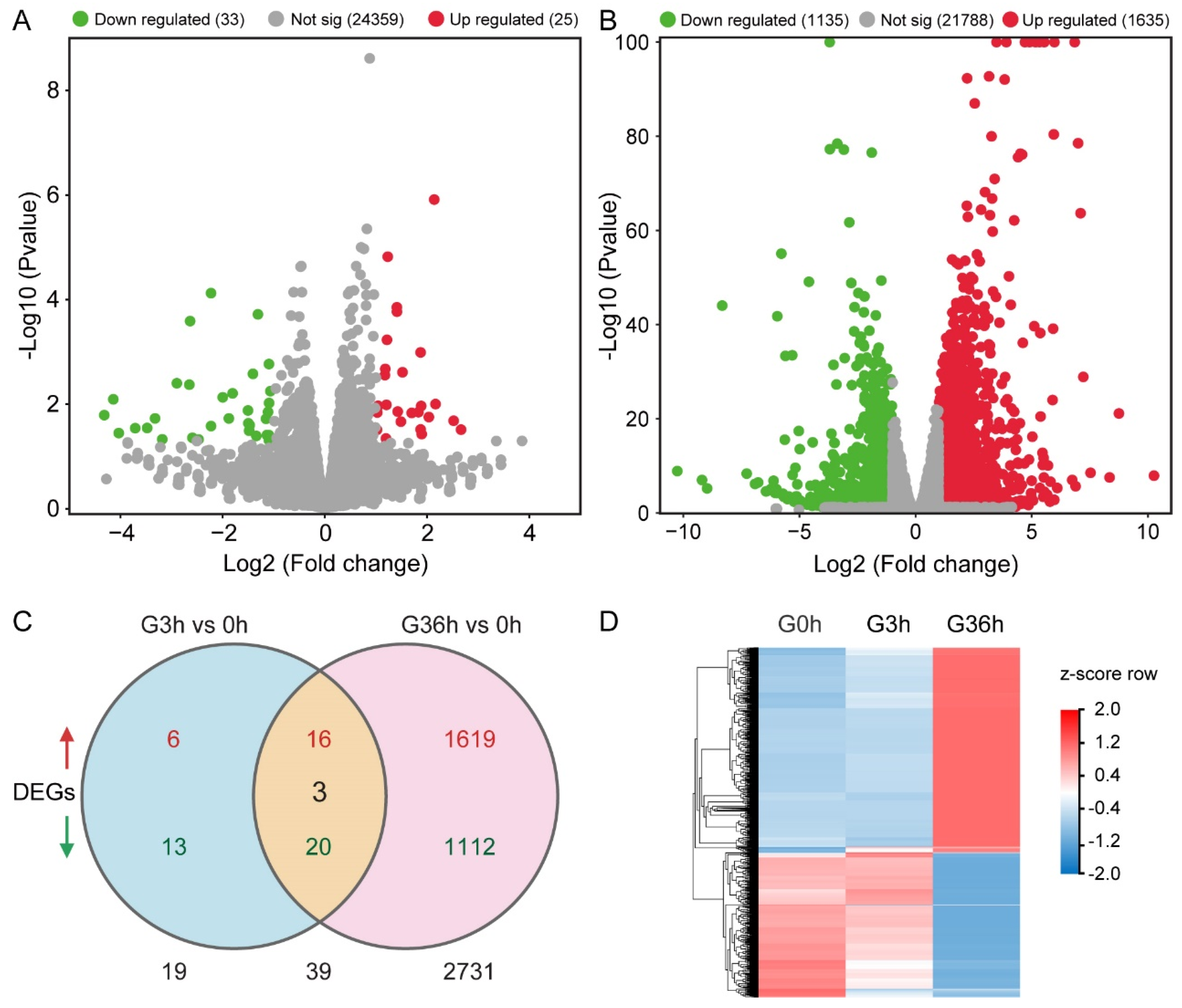
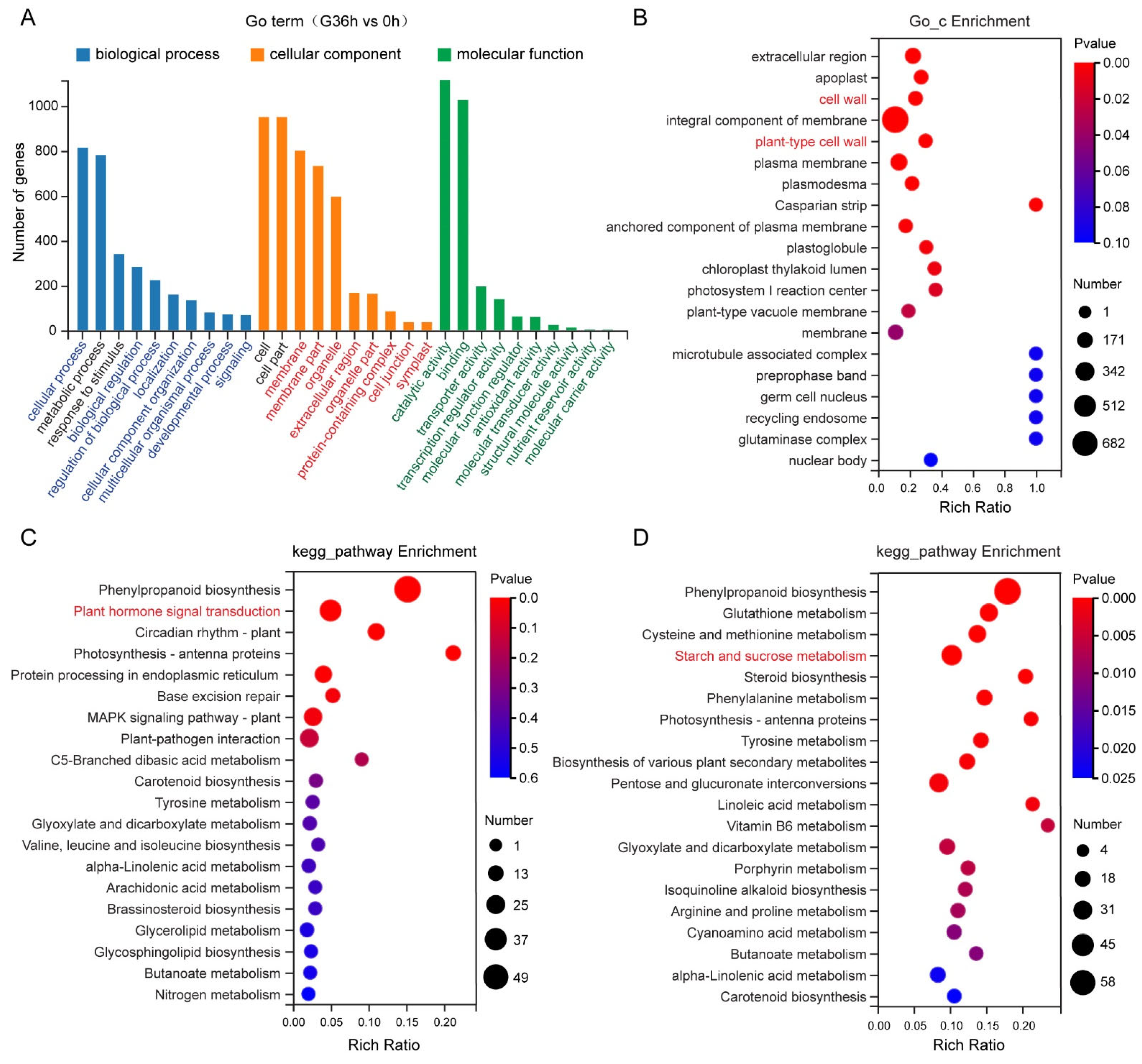
2.5. Transcriptome Analysis Revealed Insights into Tomato Root Gravitropism
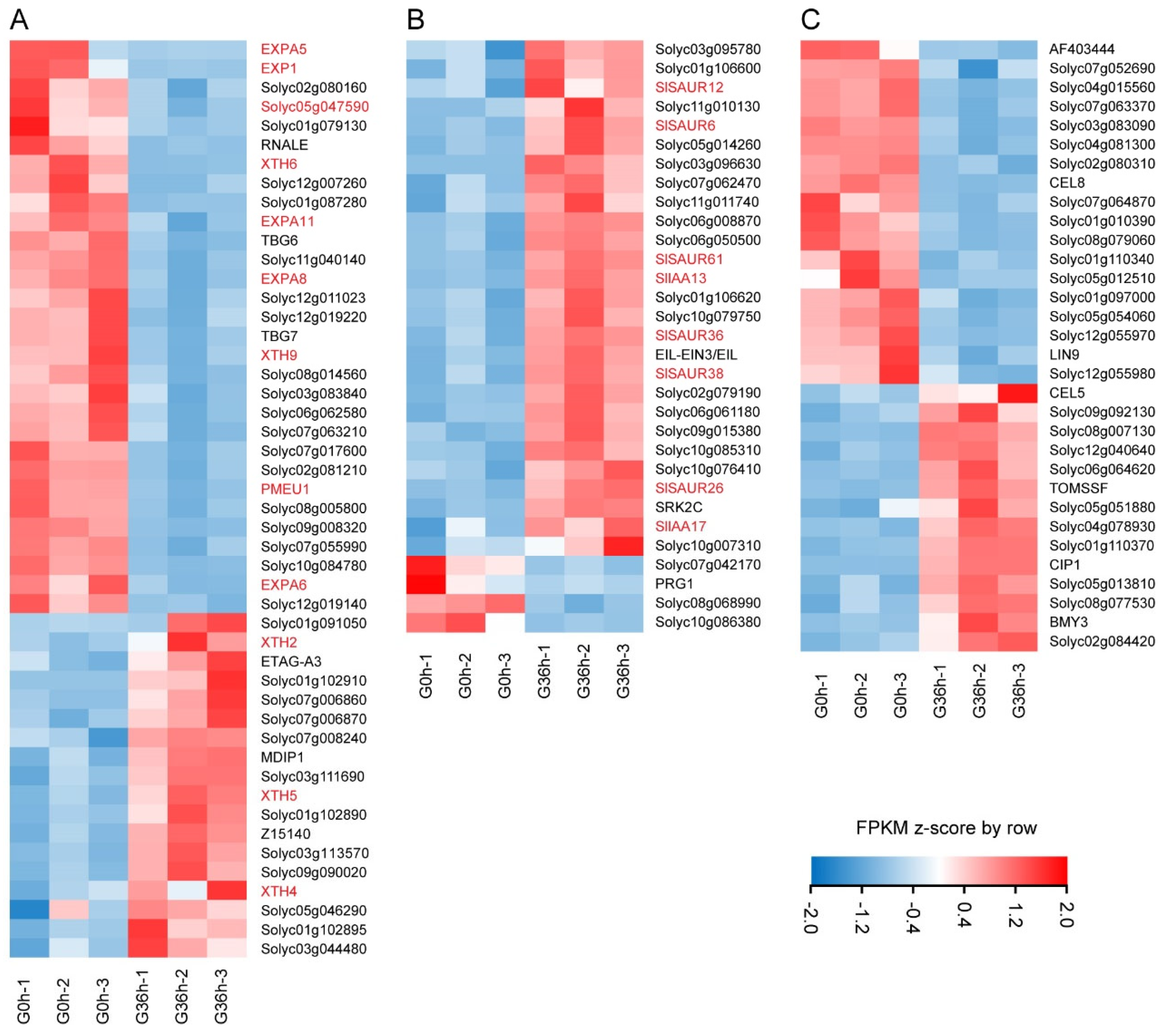
2.6. Starch and Sucrose Metabolism, Auxin Signaling, and Cell Wall Modification Are Important Pathways That Regulate the Response of Tomato Roots to Gravity Signals
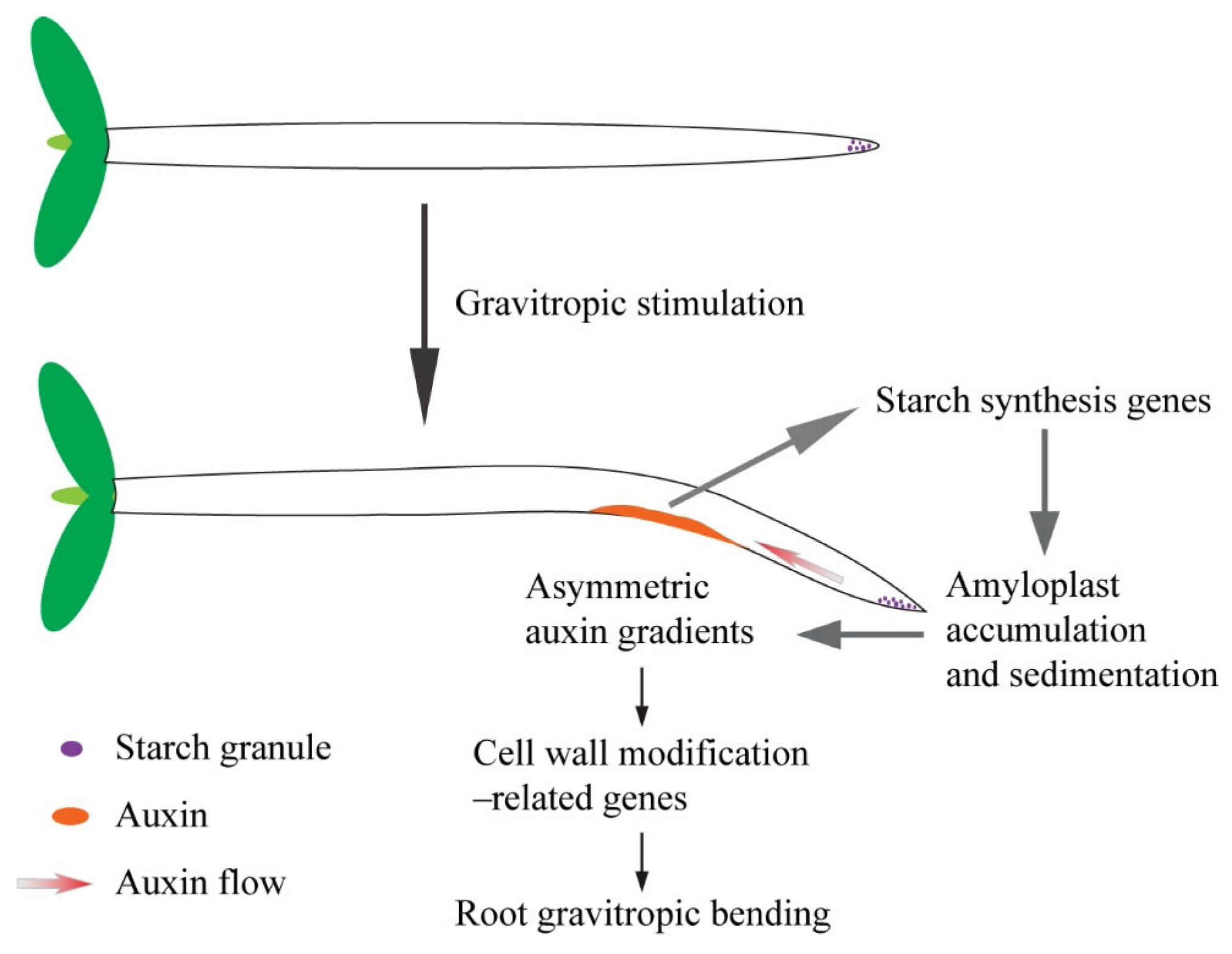
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Root Gravitropism Assays
4.3. Hormone or Inhibitor Treatments
4.4. GUS and Lugol’s Staining
4.5. RNA-Seq Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bailey, P.H.; Currey, J.D.; Fitter, A.H. The role of root system architecture and root hairs in promoting anchorage against uprooting forces in Allium cepa and root mutants of Arabidopsis thaliana. J. Exp. Bot. 2002, 53, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.W.; Kim, J. The RGI1-BAK1 module acts as the main receptor-coreceptor pair for regulating primary root gravitropism and meristem activity in response to RGF1 peptide in Arabidopsis. Plant Signal Behav. 2023, 18, 2229957. [Google Scholar] [CrossRef] [PubMed]
- Moulia, B.; Fournier, M. The power and control of gravitropic movements in plants: A biomechanical and systems biology view. J. Exp. Bot. 2009, 60, 461–486. [Google Scholar] [CrossRef] [PubMed]
- Ottenschläger, I.; Wolff, P.; Wolverton, C.; Bhalerao, R.P.; Sandberg, G.; Ishikawa, H.; Evans, M.; Palme, K. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2987–2991. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, Y.; Lee, H.; Kim, S.; Kang, J.; Kadam, U.S.; Park, S.J.; Sik Chung, W.; Hong, J.C. Cellular and physiological functions of SGR family in gravitropic response in higher plants. J. Adv. Res. 2025, 67, 43–60. [Google Scholar] [CrossRef]
- Nishimura, T.; Mori, S.; Shikata, H.; Nakamura, M.; Hashiguchi, Y.; Abe, Y.; Hagihara, T.; Yoshikawa, H.Y.; Toyota, M.; Higaki, T.; et al. Cell polarity linked to gravity sensing is generated by LZY translocation from statoliths to the plasma membrane. Science 2023, 381, 1006–1010. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Kepinski, S. Things fall into place: How plants sense and respond to gravity. Nature 2024, 631, 745–747. [Google Scholar] [CrossRef]
- Leitz, G.; Kang, B.H.; Schoenwaelder, M.E.; Staehelin, L.A. Statolith sedimentation kinetics and force transduction to the cortical endoplasmic reticulum in gravity-sensing Arabidopsis columella cells. Plant Cell 2009, 21, 843–860. [Google Scholar] [CrossRef]
- Singh, M.; Gupta, A.; Laxmi, A. Striking the right chord: Signaling enigma during root gravitropism. Front. Plant Sci. 2017, 8, 1304. [Google Scholar] [CrossRef]
- Boonsirichai, K.; Guan, C.; Chen, R.; Masson, P.H. Root gravitropism: An experimental tool to investigate basic cellular and molecular processes underlying mechanosensing and signal transmission in plants. Annu. Rev. Plant Biol. 2002, 53, 421–447. [Google Scholar] [CrossRef]
- Baldwin, K.L.; Strohm, A.K.; Masson, P.H. Gravity sensing and signal transduction in vascular plant primary roots. Am. J. Bot. 2013, 100, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Spalding, E.P. LAZY genes mediate the effects of gravity on auxin gradients and plant architecture. Plant Physiol. 2017, 175, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Wolverton, C.; Paya, A.M.; Toska, J. Root cap angle and gravitropic response rate are uncoupled in the Arabidopsis pgm-1 mutant. Physiol. Plant. 2011, 141, 373–382. [Google Scholar] [CrossRef]
- Li, X.; Zhao, R.; Liu, J.; Li, Z.; Chen, A.; Xu, S.; Sheng, X. Dynamic changes in calcium signals during root gravitropism. Plant Physiol. Biochem. 2024, 208, 108481. [Google Scholar] [CrossRef]
- Chen, J.; Yu, R.; Li, N.; Deng, Z.; Zhang, X.; Zhao, Y.; Qu, C.; Yuan, Y.; Pan, Z.; Zhou, Y.; et al. Amyloplast sedimentation repolarizes LAZYs to achieve gravity sensing in plants. Cell 2023, 186, 4788–4802.e15. [Google Scholar] [CrossRef]
- Taniguchi, M.; Furutani, M.; Nishimura, T.; Nakamura, M.; Fushita, T.; Iijima, K.; Baba, K.; Tanaka, H.; Toyota, M.; Tasaka, M.; et al. The Arabidopsis LAZY1 family plays a key role in gravity signaling within statocytes and in branch angle control of roots and shoots. Plant Cell 2017, 29, 1984–1999. [Google Scholar] [CrossRef]
- Swarup, R.; Kargul, J.; Marchant, A.; Zadik, D.; Rahman, A.; Mills, R.; Yemm, A.; May, S.; Williams, L.; Millner, P.; et al. Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 2004, 16, 3069–3083. [Google Scholar] [CrossRef]
- Swarup, R.; Kramer, E.M.; Perry, P.; Knox, K.; Leyser, H.M.; Haseloff, J.; Beemster, G.T.; Bhalerao, R.; Bennett, M.J. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat. Cell Biol. 2005, 7, 1057–1065. [Google Scholar] [CrossRef]
- Grones, P.; Abas, M.; Hajný, J.; Jones, A.; Waidmann, S.; Kleine-Vehn, J.; Friml, J. PID/WAG-mediated phosphorylation of the Arabidopsis PIN3 auxin transporter mediates polarity switches during gravitropism. Sci. Rep. 2018, 8, 10279. [Google Scholar] [CrossRef]
- Kleine-Vehn, J.; Ding, Z.; Jones, A.R.; Tasaka, M.; Morita, M.T.; Friml, J. Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc. Natl. Acad. Sci. USA 2010, 107, 22344–22349. [Google Scholar] [CrossRef]
- Abas, L.; Benjamins, R.; Malenica, N.; Paciorek, T.; Wiśniewska, J.; Moulinier-Anzola, J.C.; Sieberer, T.; Friml, J.; Luschnig, C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 2006, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Takahashi, M.; Shibasaki, K.; Wu, S.; Inaba, T.; Tsurumi, S.; Baskin, T.I. Gravitropism of Arabidopsis thaliana roots requires the polarization of PIN2 toward the root tip in meristematic cortical cells. Plant Cell 2010, 22, 1762–1776. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Friml, J.; Marchant, A.; Ljung, K.; Sandberg, G.; Palme, K.; Bennett, M. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Gene Dev. 2001, 15, 2648–2653. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Verstraeten, I.; Roosjen, M.; Takahashi, K.; Rodriguez, L.; Merrin, J.; Chen, J.; Shabala, L.; Smet, W.; Ren, H.; et al. Cell surface and intracellular auxin signalling for H+ fluxes in root growth. Nature 2021, 599, 273–277. [Google Scholar] [CrossRef]
- Chen, Q.; Dai, X.; De-Paoli, H.; Cheng, Y.; Takebayashi, Y.; Kasahara, H.; Kamiya, Y.; Zhao, Y. Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Physiol. 2014, 55, 1072–1079. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Li, Z.; Chen, A.; Zhao, R.; Xu, S.; Sheng, X. Emerging Arabidopsis roots exhibit hypersensitive gravitropism associated with distinctive auxin synthesis and polar transport within the elongation zone. Plant Physiol. Biochem. 2024, 217, 109257. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Tanaka, K.; Sakai, T.; Sugawara, S.; Kawaide, H.; Natsume, M.; Hanada, A.; Yaeno, T.; Shirasu, K.; Yao, H.; et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18512–18517. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Robertson-Hoyt, J.; Yun, J.; Benavente, L.M.; Xie, D.Y.; Dolezal, K.; Schlereth, A.; Jürgens, G.; Alonso, J.M. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 2008, 133, 177–191. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Zhang, C.G.; Wu, L.; Zhang, C.G.; Chai, J.; Wang, M.; Jha, A.; Jia, P.F.; Cui, S.J.; Yang, M.; et al. Functional characterization of the CKRC1/TAA1 gene and dissection of hormonal actions in the Arabidopsis root. Plant J. 2011, 66, 516–527. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Ma, X.; Yang, Z.; Pang, C.; Yu, J.; Wang, G.; Friml, J.; Xiao, G. Auxin-mediated statolith production for root gravitropism. New Phytol. 2019, 224, 761–774. [Google Scholar] [CrossRef]
- Liu, H.; Liu, B.; Chen, X.; Zhu, H.; Zou, C.; Men, S. AUX1 acts upstream of PIN2 in regulating root gravitropism. Biochem. Biophys. Res. Commun. 2018, 507, 433–436. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Brumos, J.; Li, H.; Ji, Y.; Ke, M.; Gong, X.; Zeng, Q.; Li, W.; Zhang, X.; An, F.; et al. A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 2011, 23, 3944–3960. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hayashi, K.; Suzuki, H.; Gyohda, A.; Takaoka, C.; Sakaguchi, Y.; Matsumoto, S.; Kasahara, H.; Sakai, T.; Kato, J.; et al. Yucasin is a potent inhibitor of YUCCA, a key enzyme in auxin biosynthesis. Plant J. 2014, 77, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.J.; Marchant, A.; Green, H.G.; May, S.T.; Ward, S.P.; Millner, P.A.; Walker, A.R.; Schulz, B.; Feldmann, K.A. Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 1996, 273, 948–950. [Google Scholar] [CrossRef]
- Okushima, Y.; Overvoorde, P.J.; Arima, K.; Alonso, J.M.; Chan, A.; Chang, C.; Ecker, J.R.; Hughes, B.; Lui, A.; Nguyen, D.; et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: Unique and overlapping functions of ARF7 and ARF19. Plant Cell 2005, 17, 444–463. [Google Scholar] [CrossRef]
- Abas, L.; Kolb, M.; Stadlmann, J.; Janacek, D.P.; Lukic, K.; Schwechheimer, C.; Sazanov, L.A.; Mach, L.; Friml, J.; Hammes, U.Z. Naphthylphthalamic acid associates with and inhibits PIN auxin transporters. Proc. Natl. Acad. Sci. USA 2021, 118, e2020857118. [Google Scholar] [CrossRef]
- Ung, K.L.; Winkler, M.; Schulz, L.; Kolb, M.; Janacek, D.P.; Dedic, E.; Stokes, D.L.; Hammes, U.Z.; Pedersen, B.P. Structures and mechanism of the plant PIN-FORMED auxin transporter. Nature 2022, 609, 605–610. [Google Scholar] [CrossRef]
- Band, L.R.; Wells, D.M.; Larrieu, A.; Sun, J.; Middleton, A.M.; French, A.P.; Brunoud, G.; Sato, E.M.; Wilson, M.H.; Péret, B.; et al. Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc. Natl. Acad. Sci. USA 2012, 109, 4668–4673. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Ribeyre, Z.; Dauzat, M.; Reyt, G.; Hidalgo-Shrestha, C.; Diehl, P.; Frenger, M.; Simonneau, T.; Muller, B.; Salt, D.E.; et al. Physiological roles of Casparian strips and suberin in the transport of water and solutes. New Phytol. 2021, 232, 2295–2307. [Google Scholar] [CrossRef]
- Cantó-Pastor, A.; Kajala, K.; Shaar-Moshe, L.; Manzano, C.; Timilsena, P.; De Bellis, D.; Gray, S.; Holbein, J.; Yang, H.; Mohammad, S.; et al. A suberized exodermis is required for tomato drought tolerance. Nat. Plants 2024, 10, 118–130. [Google Scholar] [CrossRef]
- Furutani, M.; Hirano, Y.; Nishimura, T.; Nakamura, M.; Taniguchi, M.; Suzuki, K.; Oshida, R.; Kondo, C.; Sun, S.; Kato, K.; et al. Polar recruitment of RLD by LAZY1-like protein during gravity signaling in root branch angle control. Nat. Commun. 2020, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choi, D.; Kende, H. Expansins: Ever-expanding numbers and functions. Curr. Opin. Plant Biol. 2001, 4, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Spalding, E.P.; Gray, W.M. Rapid auxin-mediated cell expansion. Annu. Rev. Plant Biol. 2020, 71, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, R.; Wang, J.; Lin, Z.; Han, X.; Deng, Z.; Fan, L.; He, H.; Deng, X.W.; Chen, H. The asymmetric expression of SAUR genes mediated by ARF7/19 promotes the gravitropism and phototropism of plant hypocotyls. Cell Rep. 2020, 31, 107529. [Google Scholar] [CrossRef]
- Dong, X.; Ma, C.; Xu, T.; Reid, M.S.; Jiang, C.Z.; Li, T. Auxin response and transport during induction of pedicel abscission in tomato. Hortic. Res. 2021, 8, 192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wu, Y.; Cai, J.; Xu, L.; Zhou, C.; Wang, C. Auxin Controls Root Gravitropic Response by Affecting Starch Granule Accumulation and Cell Wall Modification in Tomato. Plants 2025, 14, 1020. https://doi.org/10.3390/plants14071020
Liu H, Wu Y, Cai J, Xu L, Zhou C, Wang C. Auxin Controls Root Gravitropic Response by Affecting Starch Granule Accumulation and Cell Wall Modification in Tomato. Plants. 2025; 14(7):1020. https://doi.org/10.3390/plants14071020
Chicago/Turabian StyleLiu, Huabin, Yue Wu, Jiahui Cai, Lele Xu, Cheng Zhou, and Chengliang Wang. 2025. "Auxin Controls Root Gravitropic Response by Affecting Starch Granule Accumulation and Cell Wall Modification in Tomato" Plants 14, no. 7: 1020. https://doi.org/10.3390/plants14071020
APA StyleLiu, H., Wu, Y., Cai, J., Xu, L., Zhou, C., & Wang, C. (2025). Auxin Controls Root Gravitropic Response by Affecting Starch Granule Accumulation and Cell Wall Modification in Tomato. Plants, 14(7), 1020. https://doi.org/10.3390/plants14071020





