Ecological Relationships Between Woody Species Diversity and Propagation Strategies of Aulonemia queko
Abstract
1. Introduction
2. Results
2.1. Floristic Composition
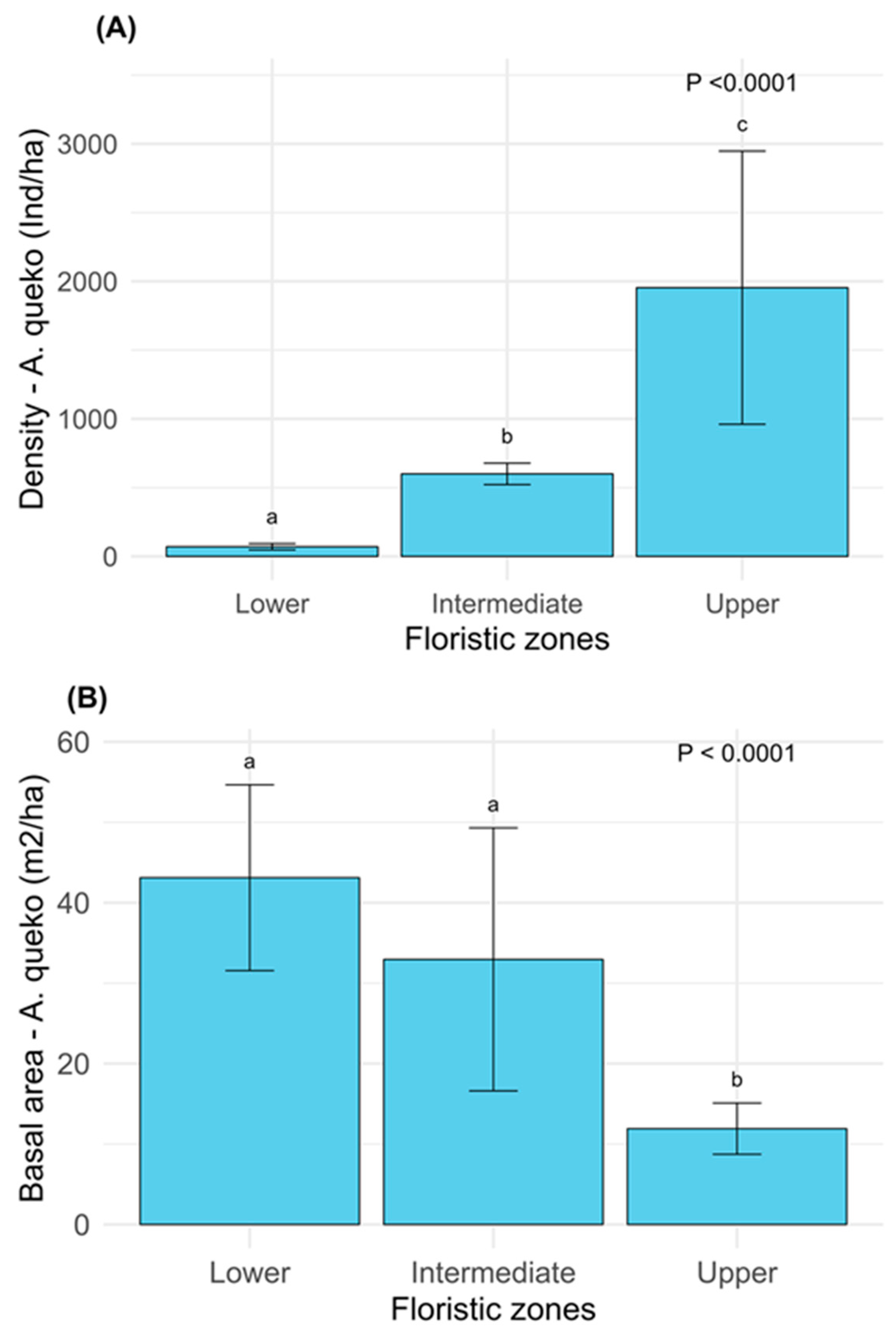
2.2. Composition, Diversity, Structure of Woody Species, and Dominance of A. queko
2.3. Relationships Between Ecological Parameters of Woody Species and A. queko
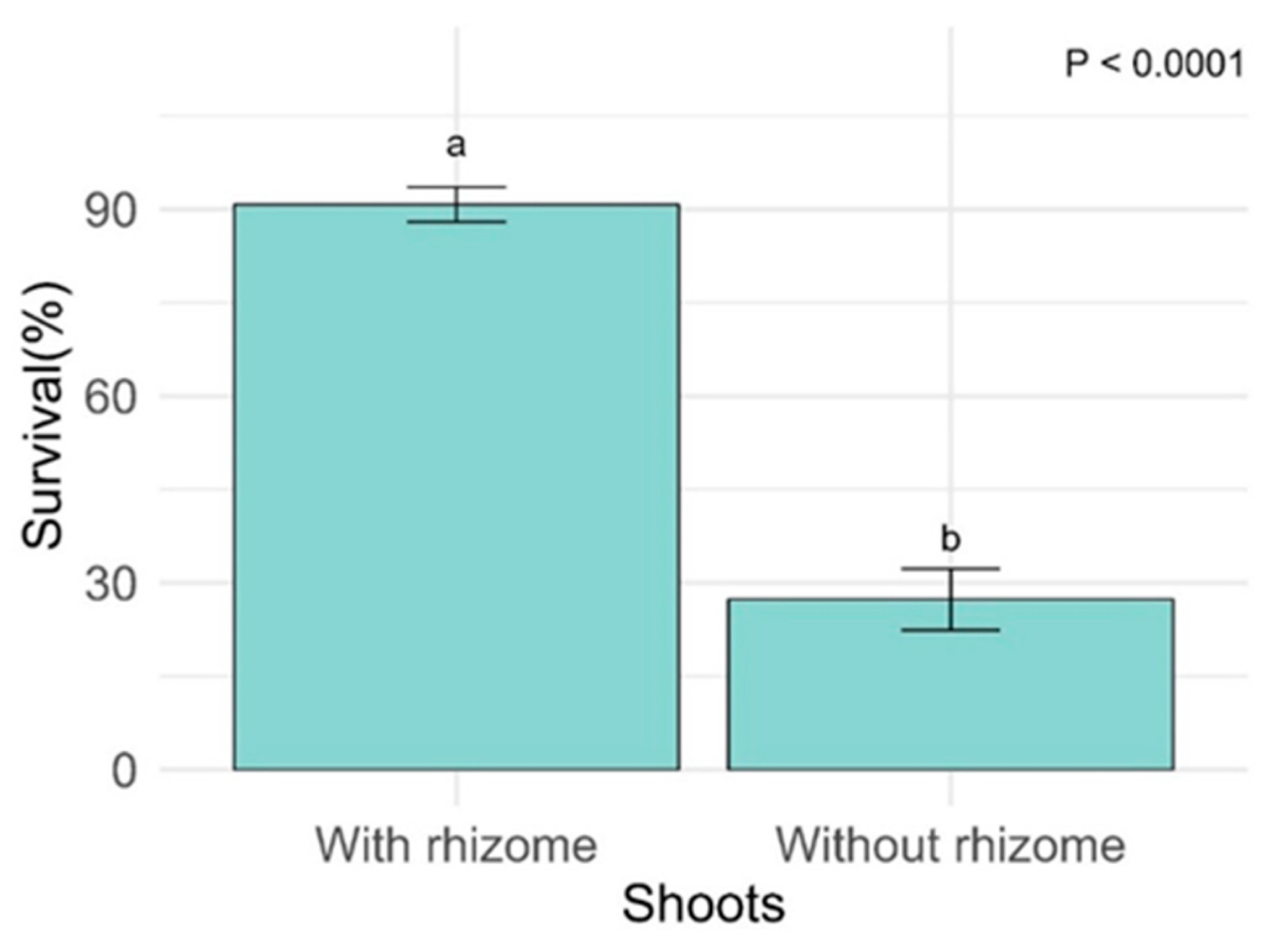
2.4. Survival of A. queko
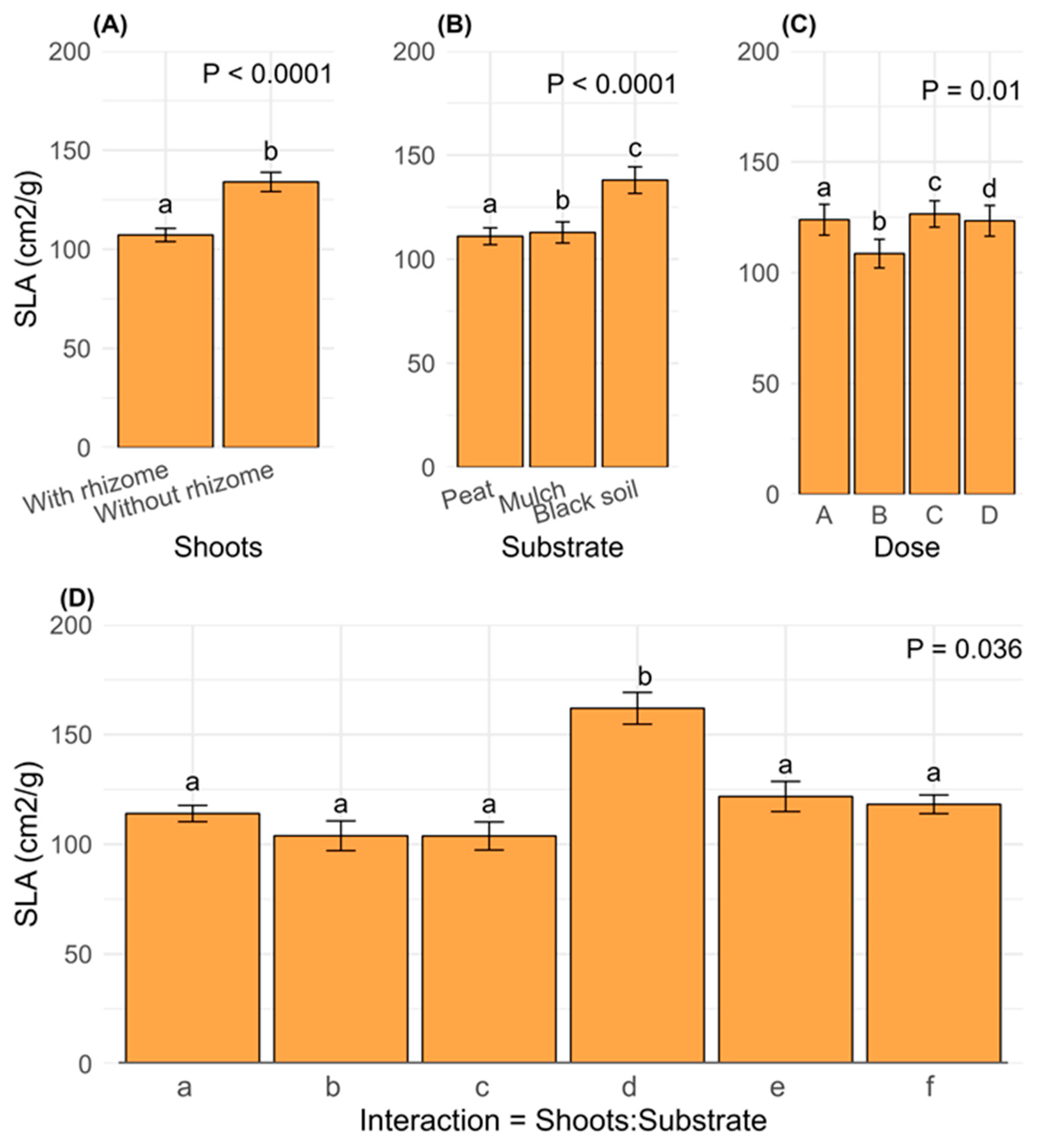
2.5. Growth in Diameter
3. Discussion
3.1. Variation in Composition, Diversity, and Structure Along the Elevation Gradient
3.2. Influence of A. queko on Woody Vegetation Composition, Diversity, and Structure
3.3. Survival and Influence of Rhizomes
3.4. Diameter Growth and Interaction with Substrate and Hormone Doses
3.5. Root Abundance and Interaction Effects
3.6. Specific Leaf Area (SLA) and Growth Parameters
4. Materials and Methods
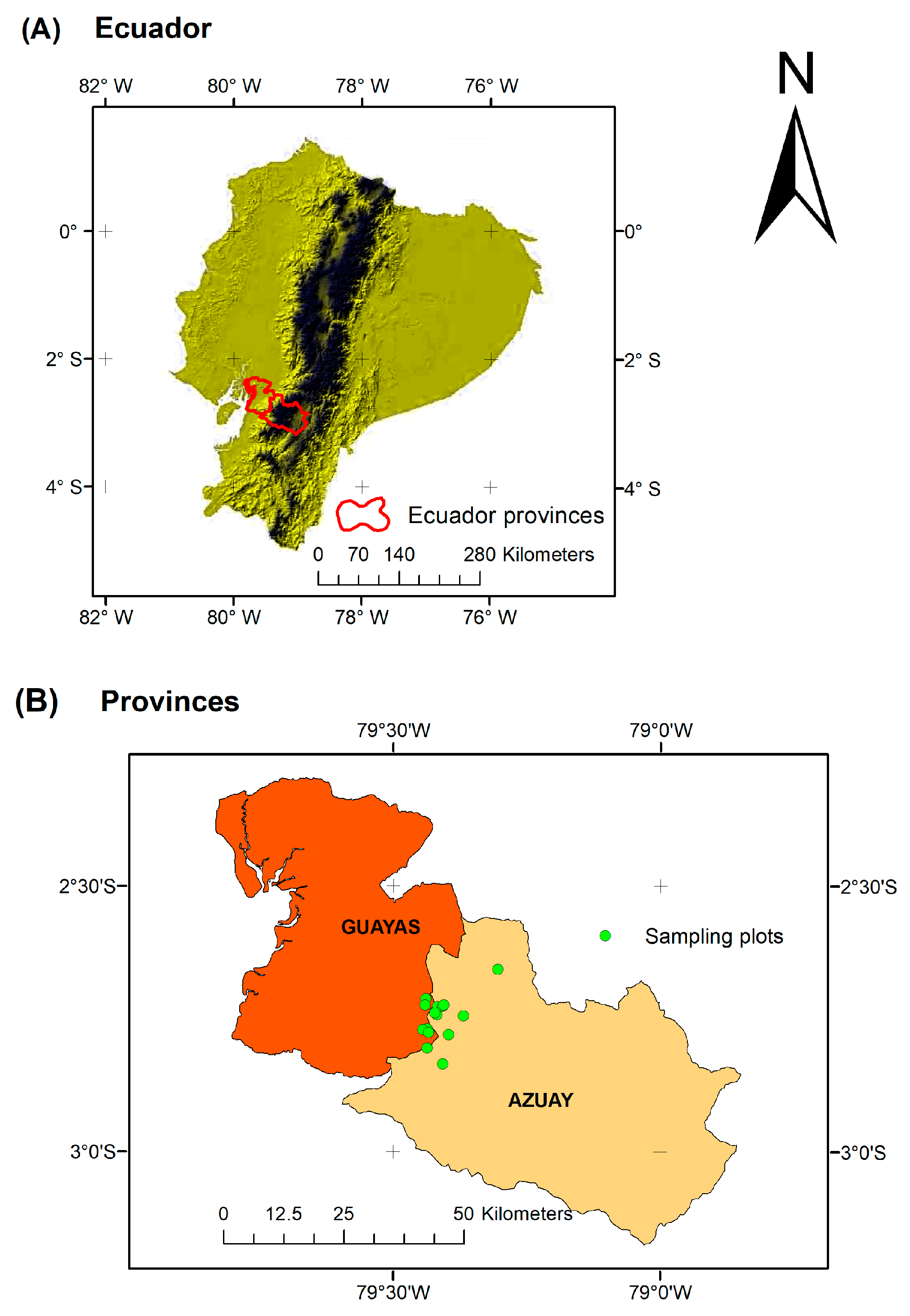
4.1. Study Area
4.2. Research Area and Experimental Design for the Propagation of A. queko
4.3. Preparation of Propagation Material (First Factor)
4.4. Preparation of Substrate (Second Factor)
4.5. Application of Indole-3-Butyric Acid (IBA) (Third Factor)
4.6. Data Collection
- DBH: Diameter at breast height.
- Lp: living plants.
- Dp: dead plants.
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NMDS | Non-metric Multidimensional Scaling |
| IBA | Indole-3-Butyric Acid |
| SLA | Specific Leaf Area |
| LA | Leaf Area |
| GLM | Generalized Linear Models |
| CRD | Completely Randomized Design |
References
- Bravo Velásquez, E. La Biodiversidad en el Ecuador; Abya-Yala/UPS: Quito, Ecuador, 2014; Available online: https://dspace.ups.edu.ec/bitstream/123456789/6788/1/La%20Biodiversidad.pdf (accessed on 21 August 2024).
- Mathez-Stiefel, S.-L.; von Dach, S.W.; Zimmermann, A.; Breu, T.; Molden, D. Focus issue: The role of culture in transformation towards sustainable development in mountains. Mt. Res. Dev. 2019, 39, 1–2. [Google Scholar] [CrossRef]
- De la Torre, L.; Navarrete, H.; Muriel, P.; Macía, M.J.; Balslev, H. Enciclopedia de las Plantas Útiles del Ecuador (con extracto de datos); Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador: Quito, Ecuador; Herbario AAU del Departamento de Ciencias Biológicas de la Universidad de Aarhus: Aarhus, Ecuador, 2008; Available online: https://bit.ly/4iyy3Mq (accessed on 11 October 2024).
- Hazzi, N.A.; Moreno, J.S.; Ortiz-Movliav, C.; Palacio, R.D. Biogeographic regions and events of isolation and diversification of the endemic biota of the tropical Andes. Proc. Natl. Acad. Sci. USA 2018, 115, 7985–7990. [Google Scholar] [CrossRef]
- Curtis, P.G.; Slay, C.M.; Harris, N.L.; Tyukavina, A.; Hansen, M.C. Classifying drivers of global forest loss. Science 2018, 361, 1108–1111. [Google Scholar] [CrossRef]
- Laurance, W.F.; Goosem, M.; Laurance, S.G. Impacts of roads and linear clearings on tropical forests. Trends Ecol. Evol. 2009, 24, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Montti, L.; Campanello, P.I.; Gatti, M.G.; Blundo, C.; Austin, A.T.; Sala, O.E.; Goldstein, G. Understory bamboo flowering provides a very narrow light window of opportunity for canopy-tree recruitment in a neotropical forest of Misiones, Argentina. For. Ecol. Manag. 2011, 262, 1360–1369. [Google Scholar] [CrossRef]
- Griscom, B.W.; Daly, D.C.; Ashton, M.S. Floristics of bamboo-dominated stands in lowland terra-firma forests of southwestern Amazonia1. J. Torrey Bot. Soc. 2007, 134, 108–125. [Google Scholar] [CrossRef]
- Griscom, B.W.; Ashton, P.M.S. A self-perpetuating bamboo disturbance cycle in a neotropical forest. J. Trop. Ecol. 2006, 22, 587–597. [Google Scholar] [CrossRef]
- Fadrique, B.; Gann, D.; Nelson, B.W.; Saatchi, S.; Feeley, K.J. Bamboo phenology and life cycle drive seasonal and long-term functioning of Amazonian bamboo-dominated forests. J. Ecol. 2021, 109, 860–876. [Google Scholar] [CrossRef]
- Grombone Guaratini, M.T.; Gomes, E.P.C.; Alves, L.F. Impacts of bamboo dominance and die-off on seedling dynamics in a tropical secondary forest (Brazil). Austral Ecol. 2024, 49, e13479. [Google Scholar] [CrossRef]
- Guerreiro, C. Flowering cycles of woody bamboos native to southern South America. J. Plant Res. 2014, 127, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Larpkern, P.; Moe, S.R.; Totland, Ø. Bamboo dominance reduces tree regeneration in a disturbed tropical forest. Oecologia 2011, 165, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Vinha, D.; Alves, L.F.; Zaidan, L.B.P.; Grombone-Guaratini, M.T. Influência da superabundância por Aulonemia aristulata (Bambuseae) sobre o banco de sementes transitório em um fragmento de Floresta Atlântica. Rodriguesia 2017, 68, 1177–1186. [Google Scholar] [CrossRef]
- Fadrique, B.; Baraloto, C.; Bravo-Avila, C.H.; Feeley, K.J. Bamboo climatic tolerances are decoupled from leaf functional traits across an Andean elevation gradient. Oikos 2022, 2022, e09229. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Shi, W.; Kronzucker, H.J.; Hou, L.; Yang, H.; Song, Q.; Liu, J.; Shi, J.; Yang, Q. Coordination of nitrogen uptake and assimilation favours the growth and competitiveness of moso bamboo over native tree species in high-NH4+ environments. J. Plant Physiol. 2021, 266, 153508. [Google Scholar] [CrossRef] [PubMed]
- Mensah, S.; Kakaï, R.G.; Seifert, T. Patterns of biomass allocation between foliage and woody structure: The effects of tree size and specific functional traits. Ann. For. Res. 2016, 59, 1–12. [Google Scholar] [CrossRef]
- Cusack, D.F.; Addo-Danso, S.D.; Agee, E.A.; Andersen, K.M.; Arnaud, M.; Batterman, S.A.; Brearley, F.Q.; Ciochina, M.I.; Cordeiro, A.L.; Dallstream, C. Tradeoffs and synergies in tropical forest root traits and dynamics for nutrient and water acquisition: Field and modeling advances. Front. For. Glob. Change 2021, 4, 704469. [Google Scholar] [CrossRef]
- Ferdous, J.; Islam, M.; Rahman, M. The role of tree size, wood anatomical and leaf stomatal traits in shaping tree hydraulic efficiency and safety in a South Asian tropical moist forest. Glob. Ecol. Conserv. 2023, 43, e02453. [Google Scholar] [CrossRef]
- Royal Botanic Gardens, K. Aulonemia queko Goudot. Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:26916-2#higher-classification (accessed on 14 February 2025).
- Judziewicz, E.J.; Clark, L.G.; Londono, X.; Stern, M. American Bamboos; Smithsonian: Washington, DC, USA, 1999. [Google Scholar]
- Liese, W.; Welling, J.; Tang, T.K.H. Utilization of bamboo. In Bamboo: The Plant and Its Uses; Springer: Berlin/Heidelberg, Germany, 2015; pp. 299–346. [Google Scholar] [CrossRef]
- Londoño, X. Distribución, Morfología, Taxonomía, Anatomía, Silvicultura y usos de los Bambúes del Nuevo Mundo. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2002. Available online: http://www.maderinsa.com/guadua/taller.html (accessed on 10 November 2024).
- Añazco, M. Estudio de vulnerabilidad al cambio climático orientado a la caña guadua; INBAR: Quito, Ecuador, 2013; Available online: https://bit.ly/3zNzFjd (accessed on 14 June 2024).
- Bystriakova, N.; Kapos, V.; Lysenko, I. Bamboo Biodiversity: Africa, Madagascar and the Americas; UNEP/Earthprint: Nairobi, Kenya, 2004; Available online: https://bit.ly/40mQUBv (accessed on 26 August 2024).
- Giraldo-Cañas, D. Gramíneas (Poaceae) ornamentales y usadas en artesanías en Colombia. Polibotánica 2010, 163–191. Available online: https://bit.ly/4deUFOw (accessed on 11 November 2024).
- Peña, L.A.; Muñoz-García, J.A.; Pabón, F.A.; Becerra-Galvis, B.; Carvajal-Suárez, F.A. Nuevos registros de la Tortolita chusquera (Columbidae: Paraclaravis mondetoura) para el Departamento de Norte de Santander, Colombia. Ornitología Colombiana 2022, 52–56. Available online: https://bit.ly/3ENUkq6 (accessed on 14 October 2024). [CrossRef]
- Hernández-Mena, L.E.; Lopes e Lima, A.; Fracarolli, J.A.; Bizzo, W.A. Neotropical woody bamboo for sustainable biobased products and bioenergy: A review. Int. J. Sustain. Eng. 2024, 17, 1021–1040. [Google Scholar] [CrossRef]
- Nfornkah, B.N.; Nath, A.J.; Kaam, R.; Chimi, C.D.; Mezafack, K.L. Bamboo-Based Forest Landscape Restoration: Practical Lessons and Initiatives to Upscale in Africa. In Bamboo Science and Technology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 329–356. [Google Scholar] [CrossRef]
- Singh, L.; Thul, S.T.; Manu, T.M. Development of bamboo biodiversity on mining degraded lands: A sustainable solution for climate change mitigation. In Phytorestoration of Abandoned Mining and Oil Drilling Sites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 439–451. [Google Scholar] [CrossRef]
- Aguirre, Z. Manual de Prácticas Agroecológicas de Los Andes Ecuatorianos; Editorial Abya Yala: Quito, Ecuador, 1996; Available online: https://bit.ly/3zPj8va (accessed on 11 December 2024).
- .Guerreiro, C.I.; Vega, A.S. Muerte por reproducción sexual, el caso (sin resolver) de la floración de los bambúes. Ecol. Austral 2022, 33, 1–313. [Google Scholar] [CrossRef]
- Ramírez-Narváez, P.N.; Velasco-Linares, P. Características de la floración en poblaciones de Chusquea scandens Kunth-Bogotá, DC (Colombia). Caldasia 2016, 38, 137–147. [Google Scholar] [CrossRef][Green Version]
- Ray, S.S.; Ali, M.N. Factors affecting macropropagation of bamboo with special reference to culm cuttings: A review update. N. Z. J. For. Sci. 2017, 47, 17. [Google Scholar] [CrossRef]
- Suwal, M.M.; Lamichhane, J.; Gauchan, D.P. Regeneration technique of bamboo species through nodal segments: A review. Nepal J. Biotechnol. 2020, 8, 54–68. [Google Scholar] [CrossRef]
- Hossain, M.; Kumar, S.; Seca, G.; Maheran, A.; Nor-Aini, A. Mass propagation of Dendrocalamus asper by branch cutting. J. Trop. For. Sci. 2018, 30, 82–88. [Google Scholar] [CrossRef]
- Banerjee, M.; Gantait, S.; Pramanik, B.R. A two step method for accelerated mass propagation of Dendrocalamus asper and their evaluation in field. Physiol. Mol. Biol. Plants 2011, 17, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, J.; Cao, Y.; Lu, X.; Duan, N.; Ren, P.; Chen, K. In vitro callus induction and plant regeneration from mature seed embryo and young shoots in a giant sympodial bamboo, Dendrocalamus farinosus (Keng et Keng f.) Chia et HL Fung. Afr. J. Biotechnol. 2011, 10, 3210–3215. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, J.; Sonkar, M.; Mishra, Y.; Shirin, F. Relationship of season and cuttings’ diameter with rooting ability of culm-branch cuttings in Bambusa tulda and Bambusa nutans. J. Plant Growth Regul. 2022, 41, 2491–2498. [Google Scholar] [CrossRef]
- .Körner, C. The use of ‘altitude’in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- Grubb, P.J. The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol. Rev. 1977, 52, 107–145. Available online: https://bit.ly/49CQaN3 (accessed on 9 September 2024). [CrossRef]
- Griscom, B.W.; Ashton, P.M.S. Bamboo control of forest succession: Guadua sarcocarpa in Southeastern Peru. For. Ecol. Manag. 2003, 175, 445–454. [Google Scholar] [CrossRef]
- Hartmann, H.T. Hartmann & Kester's Plant Propagation: Principles and Practices; Pearson: London, UK, 2014; Available online: https://bit.ly/4gEXaLu (accessed on 11 October 2024).
- Li, L.; Li, J.; Wang, X.; Wang, W.; Leung, F.; Liu, X.; Wang, C. Growth reduction and alteration of nonstructural carbohydrate (NSC) allocation in a sympodial bamboo (Indocalamus decorus) under atmospheric O3 enrichment. Sci. Total Environ. 2022, 826, 154096. [Google Scholar] [CrossRef] [PubMed]
- Nivot, N.; Olivier, A.; Lapointe, L. Vegetative propagation of five northern forest understory plant species from either rhizome or stem sections. HortScience 2008, 43, 1531–1537. [Google Scholar] [CrossRef]
- Frick, E.M.; Strader, L.C. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 2018, 69, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, P.; Bandyopadhyay, K.; Bhaduri, D.; Bhattacharyya, R.; Aggarwal, P. Effect of mulch on soil thermal regimes-a review. Int. J. Agric. Environ. Biotechnol. 2015, 8, 645–658. [Google Scholar] [CrossRef]
- Galatowitsch, S. Seedling establishment in restored ecosystems. In Seedling Ecology and Evolution; Leck, M., Parker, T., Simpson, R., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 307–331. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Basit, A.; Mohamed, H.I.; Ali, I.; Ullah, S.; Kamel, E.A.; Shalaby, T.A.; Ramadan, K.M.; Alkhateeb, A.A.; Ghazzawy, H.S. Mulching as a sustainable water and soil saving practice in agriculture: A review. Agronomy 2022, 12, 1881. [Google Scholar] [CrossRef]
- Biswas, P.; Kumari, A.; Modi, A.; Kumar, N. Improvement and regulation of steviol glycoside biosynthesis in Stevia rebaudiana Bertoni. Gene 2024, 891, 147809. [Google Scholar] [CrossRef] [PubMed]
- Kalanzi, F.; Mwanja, C.K. Effect of nodal cutting position and plant growth regulator on bud sprouting of Dendrocalamus giganteus Wall. Ex Munro in Uganda. Adv. Bamboo Sci. 2023, 2, 100016. [Google Scholar] [CrossRef]
- Davies, P.J. The plant hormones: Their nature, occurrence, and functions. In Plant Hormones: Physiology, Biochemistry and Molecular Biology; Springer: Berlin/Heidelberg, Germany, 1995; pp. 1–12. [Google Scholar] [CrossRef]
- Ramírez, Y.G.; Freire-Seijo, M.; Mederos, B.R.P.; Rivalta, O.H. Efecto del AIB y el TDZ en el enraizamiento in vitro de plantas de Bambusa vulgaris var vulgaris Schrad. ex Wendl. Rev. Colomb. De Biotecnol. 2012, 14, 200–207. Available online: https://www.redalyc.org/articulo.oa?id=77624081018 (accessed on 17 November 2024).
- Lesmes-Vesga, R.A.; Chaparro, J.X.; Sarkhosh, A.; Ritenour, M.A.; Cano, L.M.; Rossi, L. Effect of propagation systems and indole-3-butyric acid potassium salt (K-IBA) concentrations on the propagation of peach rootstocks by stem cuttings. Plants 2021, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Zahid, N.A.; Jaafar, H.Z.; Hakiman, M. Alterations in microrhizome induction, shoot multiplication and rooting of ginger (Zingiber officinale Roscoe) var. Bentong with regards to sucrose and plant growth regulators application. Agronomy 2021, 11, 320. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, Y.; Zhao, J.; Zhou, B.; Ge, X.; Li, Q.; Li, M. Root response of moso bamboo (Phyllostachys edulis (Carrière) J. Houz.) seedlings to drought with different intensities and durations. Forests 2020, 12, 50. [Google Scholar] [CrossRef]
- McSteen, P. Auxin and monocot development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001479. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Song, C.; Zheng, L.; Rong, X.; Jia, L.; Luo, L.; Zhang, C.; Qu, X.; Xuan, W. A comparison of lateral root patterning among dicot and monocot plants. Plant Sci. 2018, 274, 201–211. [Google Scholar] [CrossRef]
- Lambers, H.; Oliveira, R.S.; Lambers, H.; Oliveira, R.S. Growth and Allocation; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Torres, C.D.; Magnin, A.; Sabatier, S.; Puntieri, J.G.; Caraglio, Y. Assessing coordinated intra-specific variation in root/shoot traits in two herbaceous species based on architecture and ontogeny. Folia Geobot. 2022, 57, 167–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Sha, Z.; Ma, W.; Dayananda, B.; Fu, B.; Li, S.; Lyu, R. Morphological and physiological responses of critically endangered Acer catalpifolium to nitrogen deposition levels. Glob. Ecol. Conserv. 2023, 43, e02431. [Google Scholar] [CrossRef]
- Eden, M.; Gerke, H.H.; Houot, S. Organic waste recycling in agriculture and related effects on soil water retention and plant available water: A review. Agron. Sustain. Dev. 2017, 37, 11. [Google Scholar] [CrossRef]
- Gomes, G.; Scortecci, K. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 2000, 32, 219–230. [Google Scholar] [CrossRef]
- Rademacher, W. Plant growth regulators: Backgrounds and uses in plant production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Arellano, G.; Cala, V.; Fuentes, A.; Cayola, L.; Jørgensen, P.; Macía, M. A standard protocol for woody plant inventories and soil characterisation using temporary 0.1-ha plots in tropical forests. J. Trop. For. Sci. 2016, 28, 508–516. Available online: https://www.jstor.org/stable/43956817 (accessed on 3 July 2024).
- Noboa Sanchez, F.; Rojas, C. Productos Forestales No Maderables: Guia de Apoyo Para el Desarrollo de Actividades Empresariales; Desarrollo Forestal Comunal: Quito, Ecuador, 2000. [Google Scholar]
- Clewell, A.F.; Aronson, J. Ecological Restoration: Principles, Values, and Structure of an Emerging Profession; Island Press: Washington, DC, USA, 2012; Available online: https://bit.ly/40siwpc (accessed on 9 October 2024).
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–15. [Google Scholar] [CrossRef]
- De Cáceres, M.; Jansen, F. Indicspecies: Functions to Assess the Strength and Significance of Relationship of Species Site Group Associations. R Package Version; 2011; Volume 1. Available online: https://cran.r-project.org/web/packages/indicspecies (accessed on 10 October 2024).
- Kuhn, M. Caret: Classification and Regression Training; Astrophysics Source Code Library. 2015; ascl: 1505.1003. Available online: https://ui.adsabs.harvard.edu/abs/2015ascl.soft05003K (accessed on 10 October 2024).




| NMDS1 | NMDS2 | r2 | Pr (>r) | p-Value |
|---|---|---|---|---|
| Density A. queko | 0.57 | 0.82 | 0.31 | 0.118 |
| Basal area A. queko | −0.94 | −0.34 | 0.43 | 0.037 * |
| Elevation | 0.99 | 0.12 | 0.66 | 0.001 *** |
| Code Interaction (X Axis—Figure 4 and Figure 5) | Shoots | Substrate | Dose | Code Interaction (X Axis—Figure 4 and Figure 5) | Shoots | Substrate | Dose |
|---|---|---|---|---|---|---|---|
| a | With rhizome | Mulch | A | m | Without rhizome | Mulch | A |
| b | With rhizome | Mulch | B | n | Without rhizome | Mulch | B |
| c | With rhizome | Mulch | C | o | Without rhizome | Mulch | C |
| d | With rhizome | Mulch | D | p | Without rhizome | Mulch | D |
| e | With rhizome | Tnoire | A | q | Without rhizome | Tnoire | A |
| f | With rhizome | Tnoire | B | r | Without rhizome | Tnoire | B |
| g | With rhizome | Tnoire | C | s | Without rhizome | Tnoire | C |
| h | With rhizome | Tnoire | D | t | Without rhizome | Tnoire | D |
| i | With rhizome | Peat | A | u | Without rhizome | Peat | A |
| j | With rhizome | Peat | B | v | Without rhizome | Peat | B |
| k | With rhizome | Peat | C | w | Without rhizome | Peat | C |
| l | With rhizome | Peat | D | x | Without rhizome | Peat | D |
| Code Interaction (X Axis—Figure 6) | Shoots | Substrate |
|---|---|---|
| a | With rhizome | Peat |
| b | With rhizome | Mulch |
| c | With rhizome | Black soil |
| d | Without rhizome | Peat |
| e | Without rhizome | Mulch |
| f | Without rhizome | Black soil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedillo, H.; García-Montero, L.G.; Bermúdez, F.; Arciniegas, A.; Rocano, M.; Jadán, O. Ecological Relationships Between Woody Species Diversity and Propagation Strategies of Aulonemia queko. Plants 2025, 14, 744. https://doi.org/10.3390/plants14050744
Cedillo H, García-Montero LG, Bermúdez F, Arciniegas A, Rocano M, Jadán O. Ecological Relationships Between Woody Species Diversity and Propagation Strategies of Aulonemia queko. Plants. 2025; 14(5):744. https://doi.org/10.3390/plants14050744
Chicago/Turabian StyleCedillo, Hugo, Luis G. García-Montero, Fernando Bermúdez, Andrés Arciniegas, Mélida Rocano, and Oswaldo Jadán. 2025. "Ecological Relationships Between Woody Species Diversity and Propagation Strategies of Aulonemia queko" Plants 14, no. 5: 744. https://doi.org/10.3390/plants14050744
APA StyleCedillo, H., García-Montero, L. G., Bermúdez, F., Arciniegas, A., Rocano, M., & Jadán, O. (2025). Ecological Relationships Between Woody Species Diversity and Propagation Strategies of Aulonemia queko. Plants, 14(5), 744. https://doi.org/10.3390/plants14050744






