1. Introduction
M. Savatieri (Monochasma savatieri Franch. ex Maxim.) is a semi-parasitic perennial herb belonging to the Scrophulariaceae family. It is distributed across multiple provinces in China, including Zhejiang, Jiangxi, Fujian, Anhui, Jiangsu, Hunan, and Guangdong, commonly found along forest roadsides and in thickets. This species is also reported in Japan.
M. savatieri, a traditional Chinese medicinal herb, is used holistically to treat conditions such as respiratory infections, inflammatory disorders, and gynecological ailments [
1]. Its key bioactive components—flavonoids, alkaloids, saponins, and polysaccharides [
2,
3,
4]—contribute to broad pharmacological activities, including antimicrobial, antioxidant, and anti-inflammatory effects [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. Recent studies emphasize its anticancer potential [
15].
In recent years, the decline in wild resources of M. savatieri and the challenges in procuring raw materials for the pharmaceutical industry have necessitated a shift toward its artificial cultivation. The author’s research team has played a key role in transitioning M. savatieri from a wild to a domesticated species, successfully addressing challenges related to seedling development, transplantation, and large-scale cultivation. A base for the artificial cultivation of M. savatieri and a germplasm resource nursery has also been established.
However, production challenges remain, including significant individual variability, inconsistent yield and quality, and notable differences in disease resistance. Understanding the reproductive biology of
M. savatieri is essential for variety selection and the development of effective cultivation techniques. Unveiling the biological aspects of flowers, such as floral characteristics, the lifespan of flowering organs, and the extent of self-crossing affinity, is critical for breeding improvement. In the study of the Scrophulariaceae family, the reproductive system of
Scrophularia ningpoensis Hemsl is partially self-compatible, requiring pollinators for outcrossing with a natural outcrossing rate of 72.15%, classifying it as a typical outcrossing plant [
16]. Previous research has explored the flowering traits of
M. savatieri in natural habitats [
17], but limited studies have examined its flowering dynamics and pollination characteristics under cultivated conditions. Additionally, research addressing the effects of different artificial cultivation environments is scarce.
This study conducted an in-depth investigation into the flowering dynamics, pollination characteristics, stigma development and longevity, and self-incompatibility of cultivated M. savatieri grown from seed. The findings hold significant value for the selection and breeding of M. savatieri.
2. Results
2.1. Observations on the Appearance and Flowering Dynamics of M. savatieri Flowers
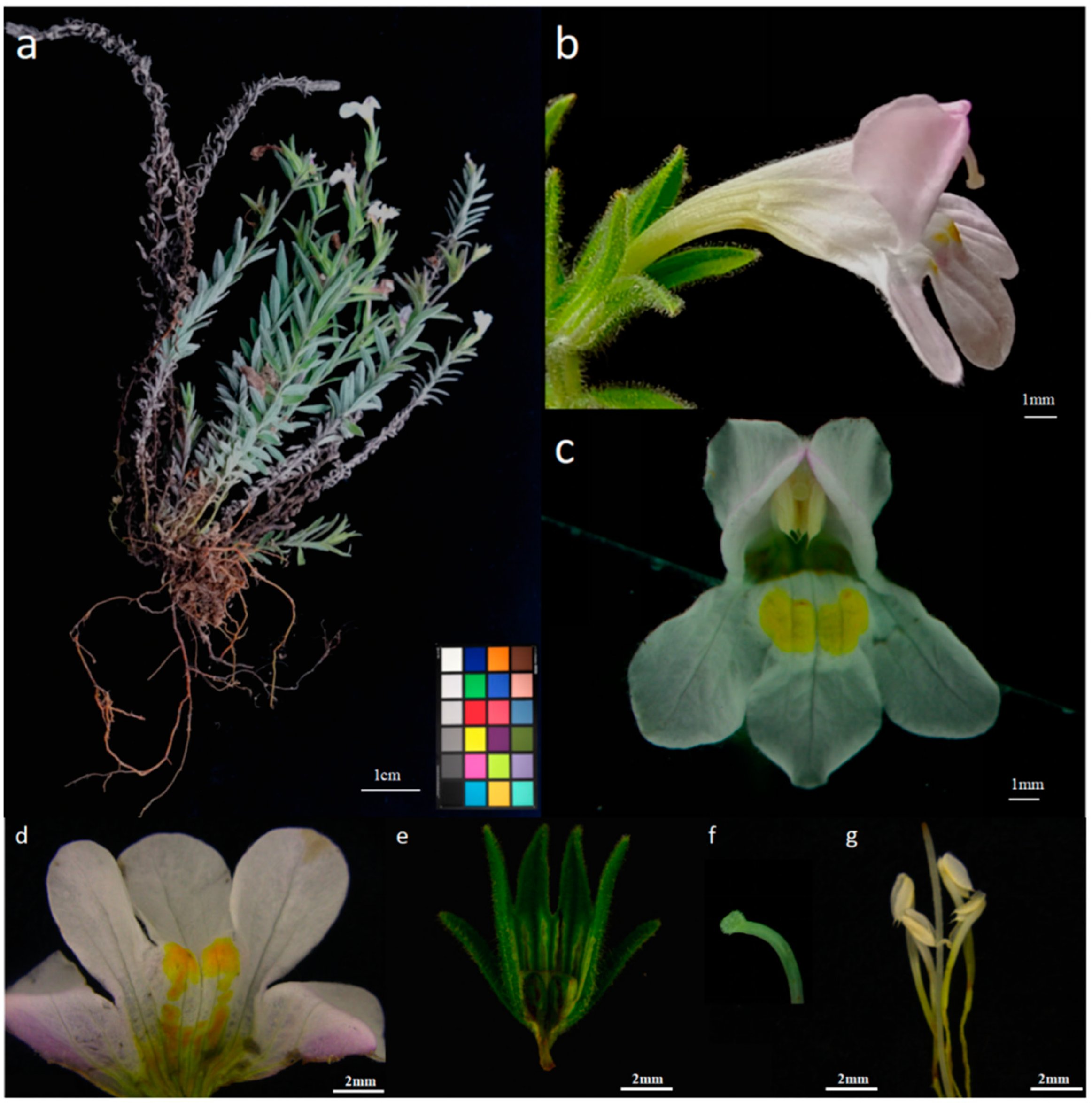
2.1.1. Appearance and Morphology of M. savatieri Flowers
In the Flora of China [
18], detailed records have been made concerning the morphology and size of each part of
M. savatieri flowers, while the color of the floral organs has been less documented. In this experiment, the size and color of each floral part were recorded by observing traits in indoor cultivated specimens.
M. savatieri produces terminal racemes with branches that are glandular-hairy and possess wooly hairs near the flowers. The flowers are sparsely distributed, typically solitary in the leaf axils, and are borne on short pedicels measuring 0.15 to 0.55 cm in length. Each flower has two leafy bracteoles measuring 0.69 to 1.21 cm in length and 0.15 to 0.25 cm in width, located at the base of the calyx tube. The calyx tube is membranous and wooly in texture, occasionally interspersed with glandular hairs. It measures 0.90 to 1.95 cm in length and displays nine prominent ribs, with four extending into the calyx teeth (
Figure 1e). The calyx teeth, numbering four, are herbaceous, linear to linear-lanceolate with an acuminate apex; they are about equal in length to the calyx tube, measuring 0.10 to 0.30 cm in width (
Figure 1b).
The corolla primarily exhibits a white or light pink coloration, with darker pink sections. The upper lip is two-merous, while the lower lip is three-merous and lighter in color (
Figure 1c). The exterior of the floral tube has a deeper hue, and the corolla progressively darkens as the flower matures and blooms.
The basal section of the floral tube features a yellowish band extending to the opening of the lower lip, forming a two-pointed yellow region highlighted by a few orange spots (
Figure 1d). In flowers with darker pink corollas, the band and spots appear orange-red. The exterior of the corolla exhibits subtle pilosity. The floral tube is slender, expanding near the throat, and measures about twice the length of the calyx, ranging from 0.90 to 1.95 cm in length, 1.15 to 1.92 cm in height, and 1.20 to 2.20 cm in width. The limb is bilabiate, with the upper lip being slightly galeate and bifurcated, and both petals are reflexed. The lower lip is trilobate, with the middle lobe slightly larger, both obovate, rounded at the terminus, and marginally spreading (
Figure 1b).
The morphology of
M. savatieri flowers includes four stamens, of which two are more prominent and inserted on the floral tube. The anterior pair reaches a length of about 7 mm, while the posterior pair measures about 6 mm. The anthers are yellowish, dorsifixed, and slightly exposed within the corolla’s throat. They are two-loculed, parallel, and equal, measuring 0.15–0.30 cm in length and 0.06–0.08 cm in width, and are distinctly separated. The anthers are long and ovate, tapering at the lower section, with a small convex tip and a longitudinal cleft (
Figure 1g).
The ovary is long and ovate with a slender, elongated style that has a curved tip. The stigma is oblong and covered with white tomentum (
Figure 1f). The growth of
M. savatieri outdoors is more influenced by rainfall, leading to a smaller overall plant size compared to indoor-grown specimens; however, the single-flower morphology remains consistent between the two environments.
2.1.2. Dynamics of Flowering in M. savatieri
The study observed no significant differences in the blooming dynamics of individual flowers and inflorescences of M. savatieri under greenhouse and outdoor field cultivation. Differences in the flowering period, flower count, and fruiting rate were, however, noted between whole plants and populations under varying cultivation practices.
The racemes of
M. savatieri are terminal, with single flowers arranged oppositely. The number of flowers per flowering branch ranged from 2 to 10, depending on branch length and plant growth conditions, with an average of 6.33 ± 2.36 flowers. The flowering period lasted about 20.89 ± 7.03 days. Inflorescences bloomed sequentially from the base upwards, with typically one to two flowers opening simultaneously per inflorescence (
Figure 2). Additionally, branches sprouting between March and April were capable of flowering within the same year.
The floral morphology of
M. savatieri exhibited notable changes during its developmental stages, which were categorized into four phases: the bud stage (1–3 days), bud appearance (4–8 days), pollination stage (9–14 days), and fruiting stage (15–35 days), as shown in
Figure 3. Flower development progressed from bud to bloom in about five days. Three days after blooming, the petals began to darken and crumple, with petals wilting within five to six days. By the ninth day, ovary expansion began, and fruit dehiscence occurred around the twentieth day. The flowering of
M. savatieri predominantly occurred in the early morning hours, specifically between 4:00 and 6:00 AM (
Figure 4). Under greenhouse cultivation, flowering began in late February. The blooming phase commenced in late March, with most plants completing their flowering cycle by mid-May. The overall flowering period concluded in early June (
Figure 5). The number of flowers per plant ranged from 17 to 241, with an average of 60.63 ± 39.94 flowers and a fruiting rate of 87.10 ± 8.67%. Each capsule contained 72.57 ± 16.54 seeds. The duration from the first flower opening to the full bloom of the entire plant was about 7 days. The full bloom period lasted about 7 days, followed by the sequential opening of a few flowers under favorable conditions. The flowering period for the whole plant ranged from 30 to 60 days.
In outdoor field cultivation,
M. savatieri initiated flowering in late March. The blooming phase extended from late March to late April, concluding by the end of April (
Figure 6). Under optimal conditions characterized by sunny weather and minimal rainfall, the number of flowers per plant varied between 26 and 139, averaging 58.32 ± 29.92 flowers, with a fruiting rate of 78.27 ± 10.01%. Under rainy conditions, the average flower count per plant was 48.00 ± 19.61, with a fruiting rate of 56.67 ± 16.37%. The time from the first bloom to the full flowering of the plant spanned about 3 to 4 days, with the full bloom phase lasting about 7 days. A limited number of flowers continued to open sequentially under favorable conditions. The overall flowering duration for the entire plant ranged from 20 to 30 days.
2.1.3. M. savatieri Loose Powder Dynamics
The dynamics of stamen development were consistent under both indoor and outdoor cultivation conditions for
M. savatieri. Anther development progressed in parallel with flower bud growth. At the initial stage, when flower buds emerged, stamens were clustered together, and the anthers displayed a two-locular structure. About three days before anthesis, flower buds slightly enlarged, with the anthers retaining their two-locular form. One day prior to anthesis, a clear separation into two chambers was observed, and the anthers became fully developed (
Figure 7(c3)).
Within one hour after corolla blooming, the anthers initiated dehiscence and pollen dispersal (
Figure 7(d3)), with the anterior pair of stamens dehiscing first. After blooming, the anthers adhered to the upper lip of the corolla and slightly protruded at the corolla’s throat. The pollen appeared white, retained its color over time, and was completely dispersed upon anther dehiscence.
The pollen dispersal of
M. savatieri began about two hours after flowering, with the stamens releasing pollen sequentially. Under sunny conditions, pollen dispersal was concentrated between 7:00 and 11:00 AM, with the majority of pollen released in the morning (refer to
Figure 7(e2)). Elevated air humidity inhibited pollen dispersal, leading to a prolonged release on overcast days. In such cases, pollen occasionally persisted for 2 to 3 days after anther dehiscence.
2.1.4. The Development of Stigma in the M. savatieri
The initial blooming phase of
M. savatieri is marked by the position of the stigma near the upper lip, with its tip extending beyond the corolla and curving downward. Within two days, the stigma adjusts its position to match the flower’s depth (
Figure 8). This dynamic movement of the pistil promotes cross-pollination during the early flowering phase, while self-pollination occurs if external pollen is unavailable.
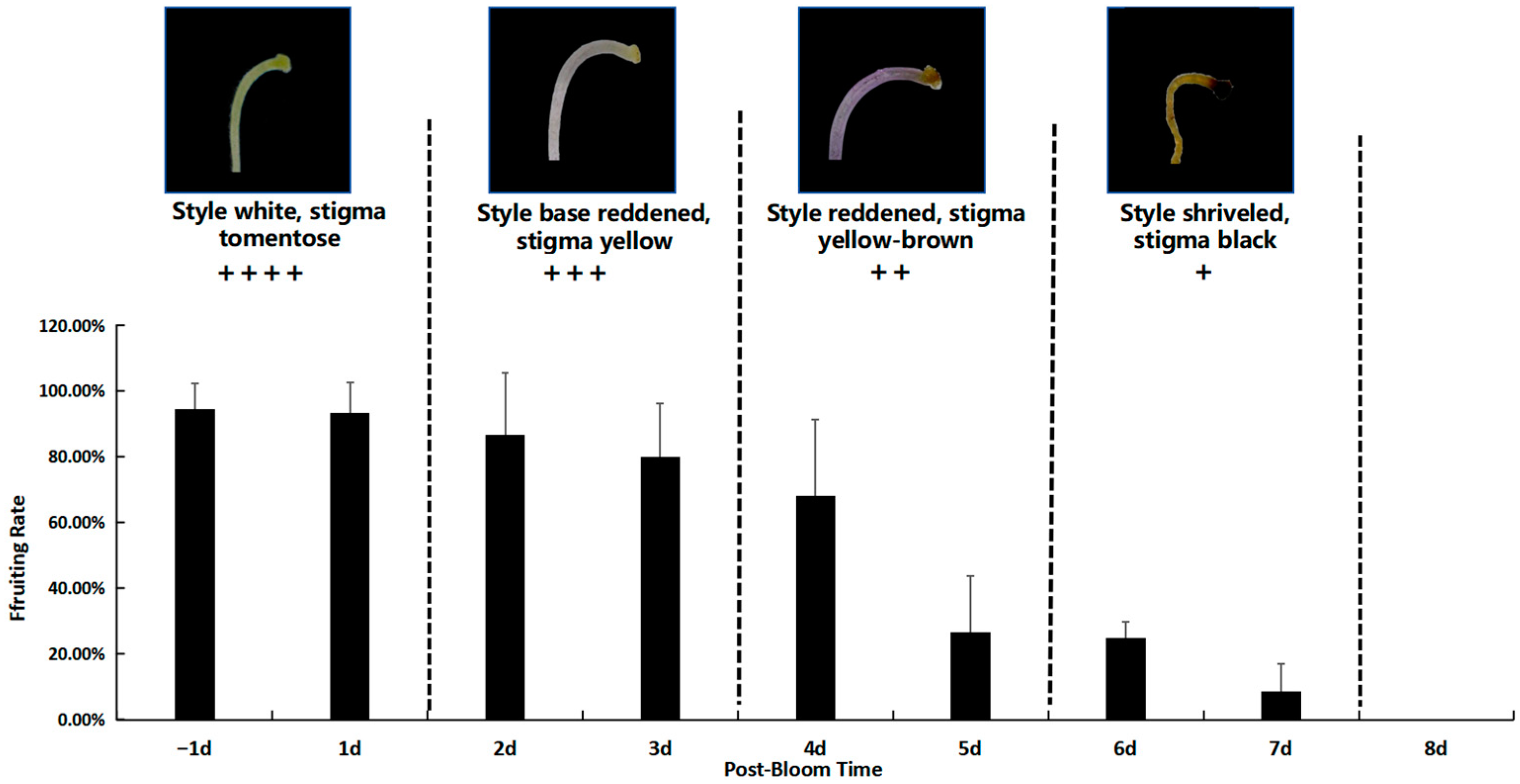
Prior to pollination, the stigma appeared white and was covered with downy hairs. It was capable of receiving pollen immediately following anthesis. The stigma retained its white and downy appearance one day before and after corolla dehiscence, a phase during which its activity peaked. During this period, the pollination and fruiting rate reached 93.30 ± 9.43%.
The stigma maintained optimal activity for 2 to 3 days post-anthesis. During this phase, the style began to redden from the base, and the stigma turned yellow, resulting in a pollination and fruiting rate of 80.00 ± 16.33%. By 4 to 5 days post-anthesis, the style had fully reddened, and the stigma transitioned to a yellow-brown color. Stigma activity significantly declined at this stage, leading to a reduced fruiting rate of 25.00 ± 5.00%. By 5 to 7 days post-anthesis, the stigma had desiccated and become inactive (
Figure 9).
Manual pollination of newly opened flowers revealed differences in stigma morphology between pollinated and unpollinated flowers. Contact with pollen caused the downy hairs on the stigma to decrease rapidly, and the stigma turned yellow within one hour (
Figure 10).
2.2. The Self-Compatibility of M. savatieri
The study demonstrated that M. savatieri, when subjected to artificial emasculation and bagging, failed to produce fruit, resulting in a D/A ratio of 0. This result indicates that M. savatieri is unable to produce seeds through apomixis and relies on pollination for reproduction.
The fruiting rate of indoor
M. savatieri subjected to artificial emasculation without bagging (pure crossbreeding) was observed to be 32.50%, whereas the rate under outdoor conditions was lower at 15.63%. In contrast, the natural fruiting rate of indoor
M. savatieri natural pollination (control group, CK) was 88.57%, while outdoor conditions yielded a lower rate of 56.67%. Additionally, artificial pollination experiments revealed that artificial self-pollination resulted in a fruiting rate of 85.29%, while artificial cross-pollination achieved a higher fruiting rate of 90.00% (
Table 1). These findings suggest that the pollination success rate in outdoor
M. savatieri is reduced, primarily due to the overlap of the flowering period with the rainy season in its native region, which results in increased rainfall during this critical phase.
Analysis of the outcrossing rate revealed that indoor-cultivated M. savatieri achieved a rate of 28.80%, whereas outdoor-cultivated plants exhibited a lower rate of 9.60%. This indicates a higher occurrence of heterogamy under indoor conditions compared to outdoor environments. The outcrossing rate for both conditions ranged between 5% and 50%, suggesting that M. savatieri is typically an often cross-pollinated plant, capable of both self-pollination and outcrossing pollination.
Floral structures of M. savatieri exhibited monoecy with herkogamy (stigma–anther separation) and synchronized anther dehiscence with stigma receptivity. An outcrossing index (OCI) of 3 (Dafni’s criterion) indicated morphological adaptations favoring cross-pollination, despite the retained self-compatibility.
The A1 group underwent natural pollination in a greenhouse environment (CK1), while the A2 group experienced natural pollination in a field environment (CK2). The B group was directly bagged. The C1 group involved emasculation without bagging in the greenhouse, and the C2 group involved emasculation without bagging in the field. The D group featured emasculation followed by bagging. The E group focused on artificial geitonogamy (self-pollination within the same flower), and the F group engaged in artificial xenogamy.
2.3. Determination of Pollen Viability of M. savatieri
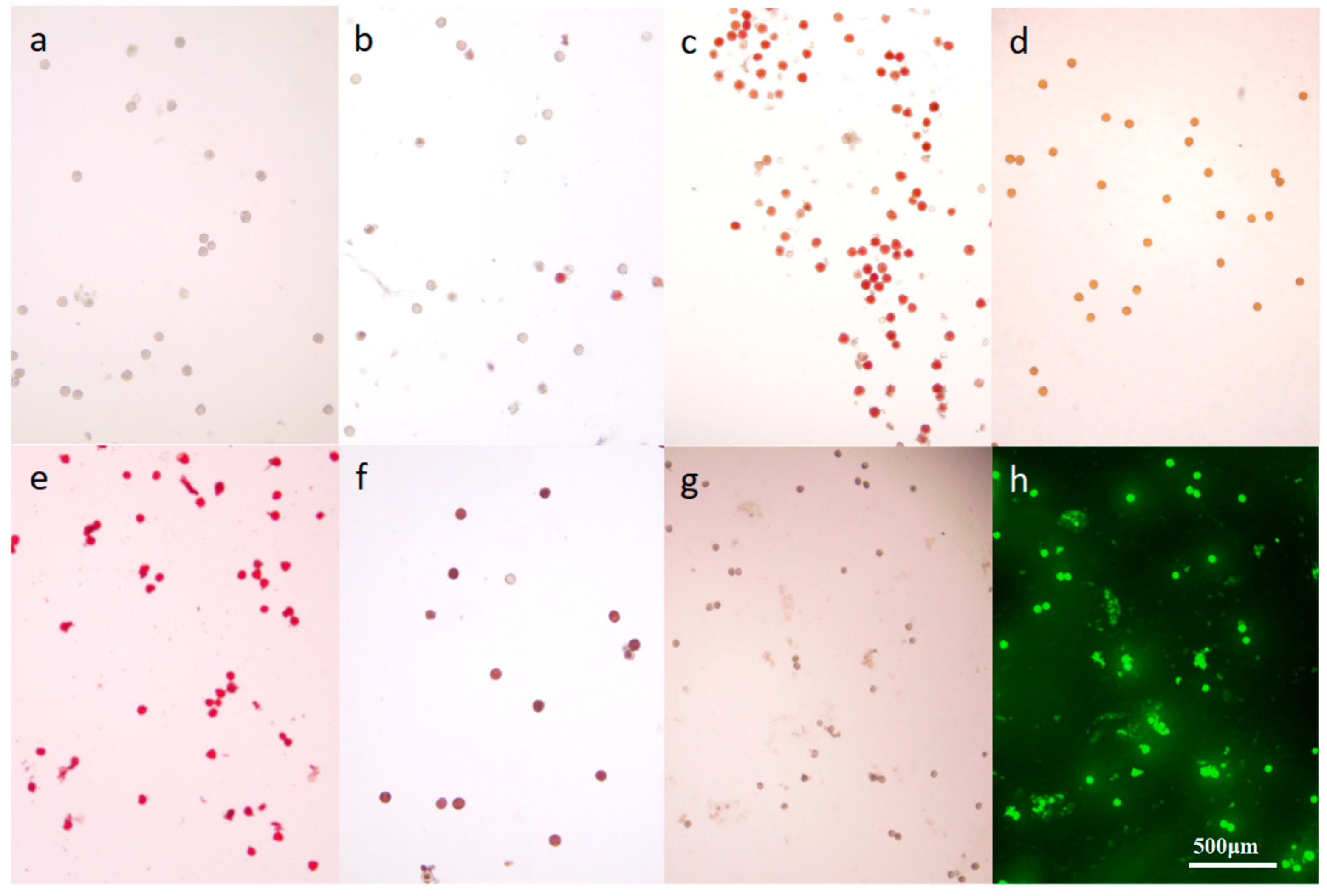
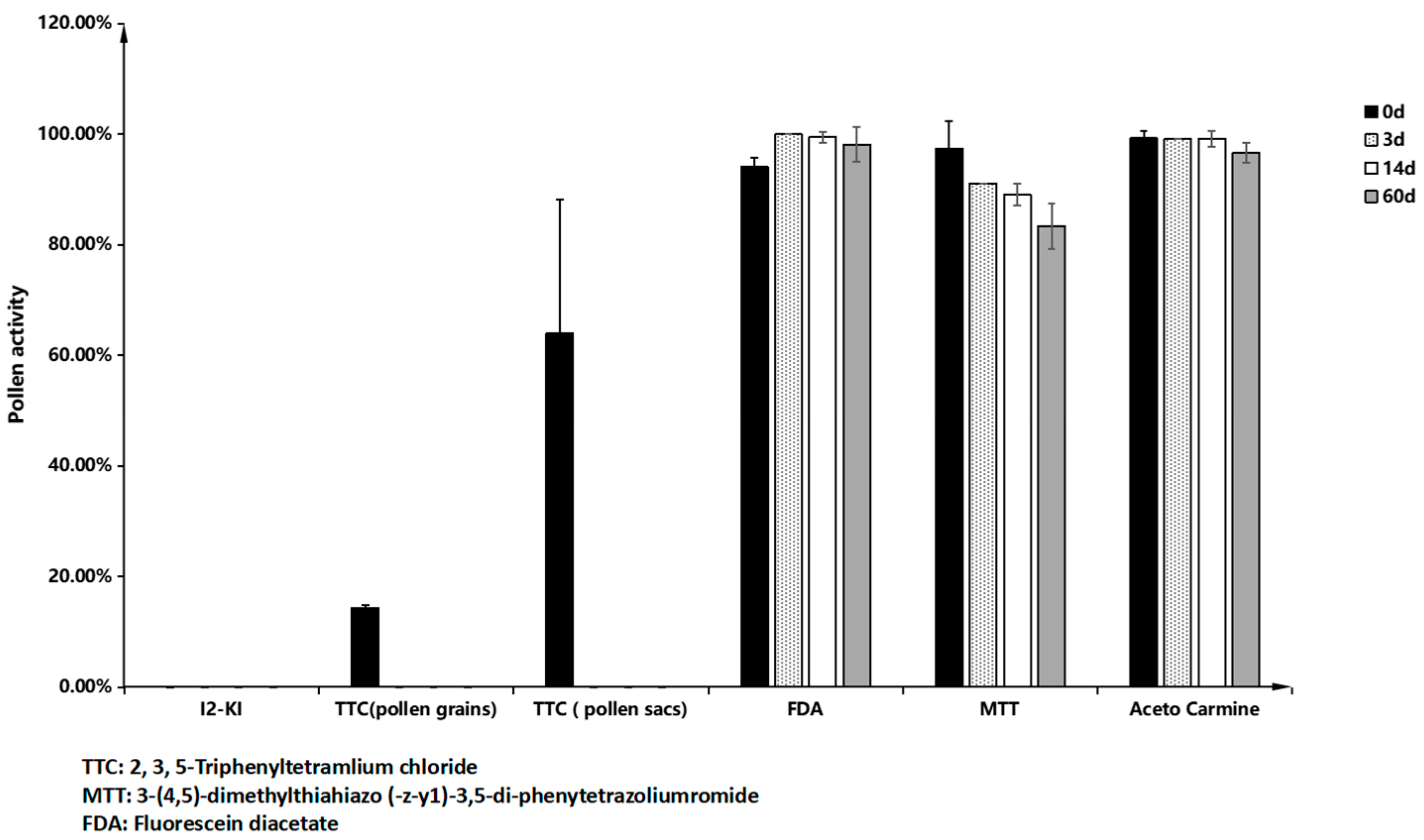
The analysis of various staining techniques for
M. savatieri pollen revealed that scattered pollen remained colorless when treated with TTC (2, 3, 5-Triphenyltetramlium chloride) solution. However, superior staining results were obtained when entire pollen sacs were immersed in the solution (
Figure 11b,c). This suggests that the respiration activity of
M. savatieri pollen is relatively weak, and the TTC solution undergoes a detectable color change only when densely packed pollen is involved in respiration. The I
2-KI method was found unsuitable for assessing pollen viability in
M. savatieri due to the pollen’s yellow-brown color and the absence of the expected blue coloration (
Figure 11d). Acetocarmine effectively stained the nuclei of viable
M. savatieri pollen cells red (
Figure 11e). The MTT (3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide) assay produced a blue-violet crystalline substance through the action of dehydrogenase enzymes in viable pollen grains, staining viable pollen blue, while non-viable pollen displayed a distinct yellowish-brown color (
Figure 11f). The FDA (fluorescein diacetate) staining method revealed fluorescence in viable
M. savatieri pollen under blue light (
Figure 11h). Three staining methods—acetocarmine, MTT, and FDA—were employed to assess the viability of antler grass pollen post-dispersal. The acetocarmine method demonstrated that pollen viability exceeded 95% within three days of dispersal and maintained high activity over an extended duration. The MTT method indicated a viability of 92.76% ± 2.98% after three days, with pollen activity recorded at 83.37% ± 4.12% after 60 days. The FDA method further confirmed that pollen viability remained above 95% within the initial three days, with sustained high activity over time (
Figure 12).
3. Discussion
3.1. Stigma Dynamics and Pollination Characteristics of M. savatieri
The floral and pollination characteristics, along with breeding systems, demonstrate mutual adaptation in plants. M. savatieri is characterized by a predominantly white to light pink corolla, with occasional dark pink variations. The corolla diameter exceeds 1 cm, and the lower labellum features a bright yellow color block, which effectively attracts pollinators.
Approach herkogamy, the most common form of herkogamy, involves pollinators entering the flower and first contacting the highly protruding stigma, which may also intercept pollen carried by wind [
19]. The style of
M. savatieri extends beyond the corolla, while the stamens remain close to the upper lip, consistent with stigma-probing androgynous ectopia.
Delayed self-fertilization is a widely recognized reproductive adaptation that ensures seed production in the absence of pollinators while prioritizing outcrossing when pollinators are abundant. The curling of the style, which brings the stigma surface into contact with the anthers or fallen pollen on non-stigma regions during late anthesis, is a typical floral movement facilitating delayed self-pollination [
20].
Similar floral behavior is observed in mallow plants, such as
Hibiscus trionum L., where flowers feature prominent stigmas capable of bending in the opposite direction shortly after opening. This movement enables the stigma to contact anthers, promoting self-pollination. If stigmas receive pollen before or during the bending process, self-pollination is inhibited, as the stigma either remains in or reverts to an upright position [
21]. In
Viola pubescens, delayed self-fertilization occurs when the stigma moves downward to contact pollen that has fallen onto anterior petals from the anthers [
22]. In
M. savatieri, the style’s downward bend allows the stigma to make contact with pollen on the lower lip or that remaining in the anthers, promoting delayed self-fertilization.
Reproductive mechanisms represent the primary link between progenitors and their descendants and among descendants within advanced plants and animals. Unlike animals, reproductive strategies in advanced plants exhibit considerable variation across taxa, as well as among groups and individuals within a single species. Understanding the reproductive system is essential for investigating gene transfer within plant populations.
The outcrossing rate is a key characteristic of the mating system. Heterogamous plants typically exhibit an outcrossing rate exceeding 50%, while normally heterogamous plants demonstrate an outcrossing rate between 5% and 50%. In contrast, self-pollinated plants generally show an outcrossing rate below 5%. Factors such as plant traits and cultivation conditions influence the natural hybridization rate. For example, increasing the distance between Setaria plantings reduces the likelihood of outcrossing, whereas strong winds during the flowering period, particularly in the wind’s direction, enhance the probability of natural hybridization.
The experimental results confirmed that M. savatieri functions as a normally heterogamous pollinator under both indoor and outdoor cultivation conditions. Observations of the floral structures of M. savatieri revealed its monoecious characteristics, with stamens and pistils of unequal lengths. The synchronization of anther pollination with peak stigma activity, combined with an outcrossing index (OCI) of 3 based on Dafni’s criterion, suggests that M. savatieri exhibits traits characteristic of consistently heterogamous plants.
3.2. Flowering Time, Stigma Activity, and Suitable Conditions for Artificial Pollination of M. savatieri
Among flowering plants, hybridization is a key strategy employed by breeders to develop progeny with advantageous agronomic traits or enhanced resistance to abiotic and biotic stresses. This process is typically facilitated through artificial pollination techniques.
The survival duration of pollen and the acceptance period of stigmas are closely associated with species-specific characteristics. M. savatieri exhibits a raceme inflorescence, with floral development divided into four distinct phases: the bud stage (1–3 days), bud appearance (4–8 days), pollination stage (9–14 days), and fruiting stage (15–35 days). A single flowering branch typically bears 2 to 10 flowers, with an average bloom count of 6.33 ± 2.36. The complete flowering duration of a branch is about 20.89 ± 7.03 days. Under indoor cultivation, M. savatieri demonstrated an extended flowering period from late February to mid-May, with individual plants flowering for 30 to 60 days. In contrast, outdoor cultivation resulted in a shorter flowering period from late March to mid-May, with individual plants flowering for 20 to 30 days.
M. savatieri exhibits strong pollen vitality one day prior to anthesis. Under natural conditions, anthers begin to open one hour after flower blooming, with pollen dispersal completed within five hours under sunny conditions. In cloudy or rainy weather, the pollen dispersal period extends to 2–3 days. Notably, pollen vitality remains high for up to 60 days post-dispersal.
The findings indicate that optimal pollination in M. savatieri occurs within specific time windows relative to flower opening. One day prior to anthesis, stigma activity peaks, achieving a pollination and fruiting rate of 93.30 ± 9.43%. Stigma activity remains high during the first bloom. Between 2 and 3 days post-flowering, the stigma remains favorable for pollination, with the style beginning to redden from the base and the stigma turning yellow. During this phase, the pollination and fruiting rate is 80.00 ± 16.33%. By 4 to 5 days post-flowering, the style becomes fully red, and the stigma transitions to a yellowish-brown color, with activity significantly reduced, leading to a fruiting rate of 25.00 ± 5.00%. After 5 to 7 days, the stigma desiccates and becomes inactive. Based on these observations, it is recommended that artificial pollination be conducted within the first 1 to 2 days following flower opening to maximize pollination success and fruit set.
3.3. Staining Methods for the Detection of Suitable Viability of Pollen from M. savatieri
During the experimental period, no significant changes were observed in the appearance of pollen, indicating that evaluating pollen viability based solely on its physical appearance is not feasible. The primary methods for assessing pollen viability include in vitro germination, pollination, and staining. The in vitro germination method, although accurate, is complex, time-consuming, and requires optimization of conditions, making it impractical for rapid viability detection. The pollination method, on the other hand, is highly influenced by environmental factors and has been employed less frequently in recent years. Staining techniques are more widely used due to their simplicity, rapidity, and effectiveness.
The I
2-KI staining method is based on the accumulation of starch in pollen, with blue-stained pollen considered viable [
23]. In the present study, the pollen of
M. savatieri was stained yellowish-brown with I
2-KI, indicating that this method is unsuitable for assessing pollen viability, likely due to the low starch content in its pollen.
The TTC (2, 3, 5-Triphenyltetramlium chloride) method, which induces a red coloration through a reaction with succinate dehydrogenase in viable cells, is widely used for pollen viability assessment. In the case of M. savatieri, dispersed pollen remained colorless after treatment with the TTC solution. However, better staining results were achieved by immersing the entire pollen sac in the TTC solution. This may be attributed to the weak respiration of M. savatieri pollen, as the dense aggregation of pollen is required to generate sufficient respiration to induce a detectable color change in TTC.
Acetocarmine staining targets the nuclei of pollen cells and has demonstrated clear and distinguishable results in species. In the case of M. savatieri, ace to carmine staining resulted in rapid and distinct staining, but the viability results remained consistently above 95% over extended durations, suggesting potential overestimation of viability.
MTT staining functions by utilizing the dehydrogenase enzyme in active pollen, which reduces MTT to a water-insoluble blue-violet precipitate. M. savatieri pollen exhibited significant color differentiation with the MTT staining solution, making it an effective technique for assessing pollen viability.
The FDA fluorescence staining method relies on fluorescein diacetate (FDA) reacting with pollen grain esterases to produce fluorescein, thereby enabling staining and viability assessment. However, it has a tendency to overestimate pollen viability. M. savatieri pollen, stained using the FDA method, emitted strong green fluorescence, with results remaining above 95% for extended periods, indicating possible overestimation when applying this technique for viability assessment.
Among the five pollen staining methods evaluated in this study, I2-KI and TTC staining proved ineffective for assessing M. savatieri pollen viability. Acetocarmine and FDA staining methods produced reliable yet potentially elevated results. MTT staining emerged as the most suitable and accurate method for assessing M. savatieri pollen viability due to its clear and consistent differentiation between viable and non-viable pollen. The pollen viability of M. savatieri exceeded 85% in both indoor and outdoor populations, as determined by MTT staining. This high viability ensures a sufficient supply of functional pollen grains, even under outdoor environmental stressors such as heavy rainfall.
5. Conclusions
M. savatieri is classified as a cross-pollinated post-flower species with monoecious flowers. Indoor cultivation is recommended for this plant, as environmental factors such as rainfall during flowering significantly reduce fruiting rates and challenge centralized harvesting in outdoor environments due to sequential fruit ripening and water-induced capsule dehiscence.
For pollen viability assessment, the MTT staining method was found to be more suitable than I2-KI or TTC staining. As a frequently heterogamous pollinated plant, systematic crossbreeding strategies should be implemented. However, to enhance breeding outcomes, parental plants must undergo rigorous self-crossing selection to eliminate inferior individuals and retain superior pure lines as hybrid parents.