Ultraviolet-A Radiation (UVA) as a Stress and the Influence of Provenance and Leaf Age on the Expression of Phenolic Compounds by Eucalyptus camaldulensis ssp. camaldulensis
Abstract
1. Introduction
2. Results
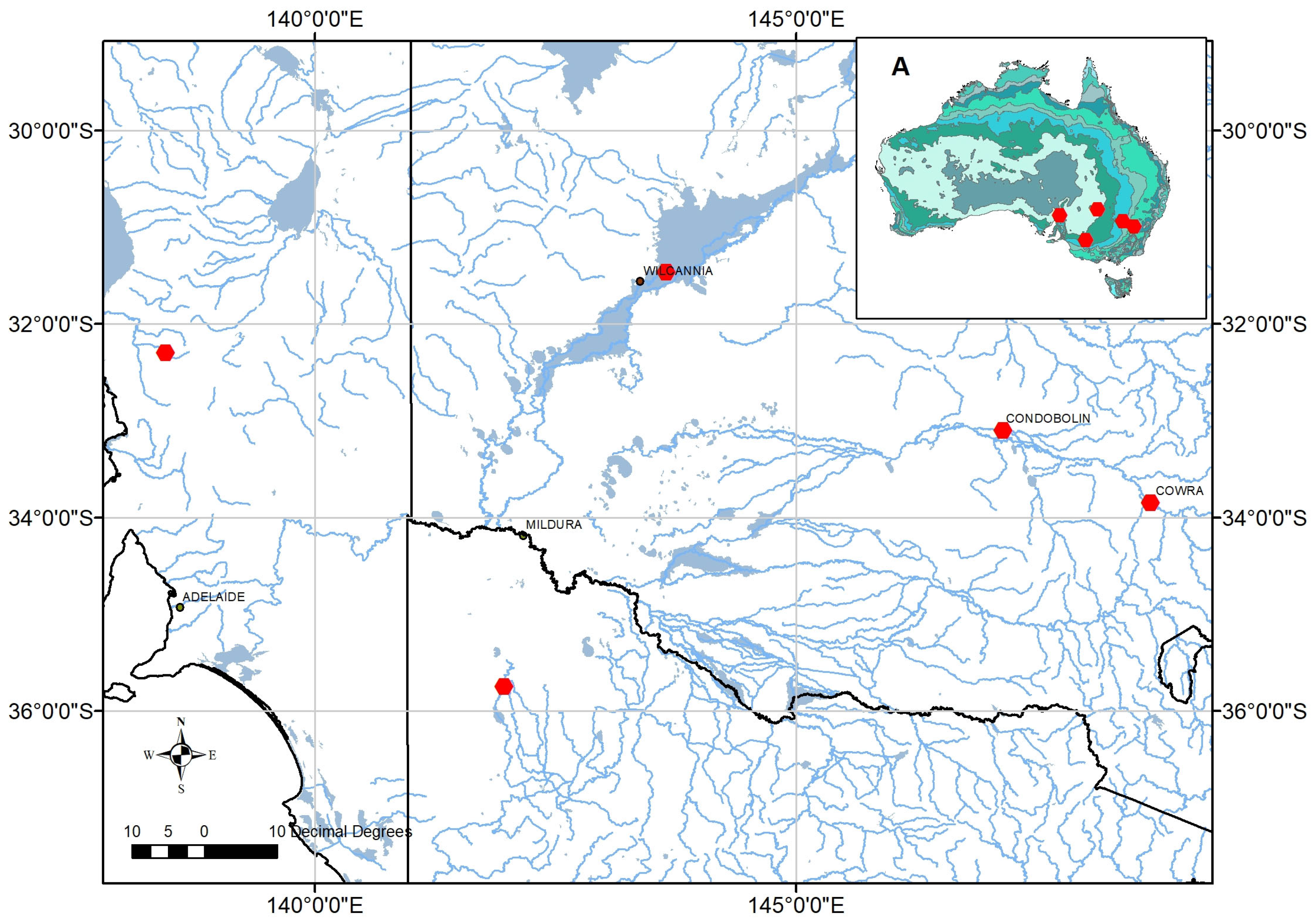
2.1. Effect of Provenance on Phenolic Composition
2.2. Effect of UVA on Phenolic Composition
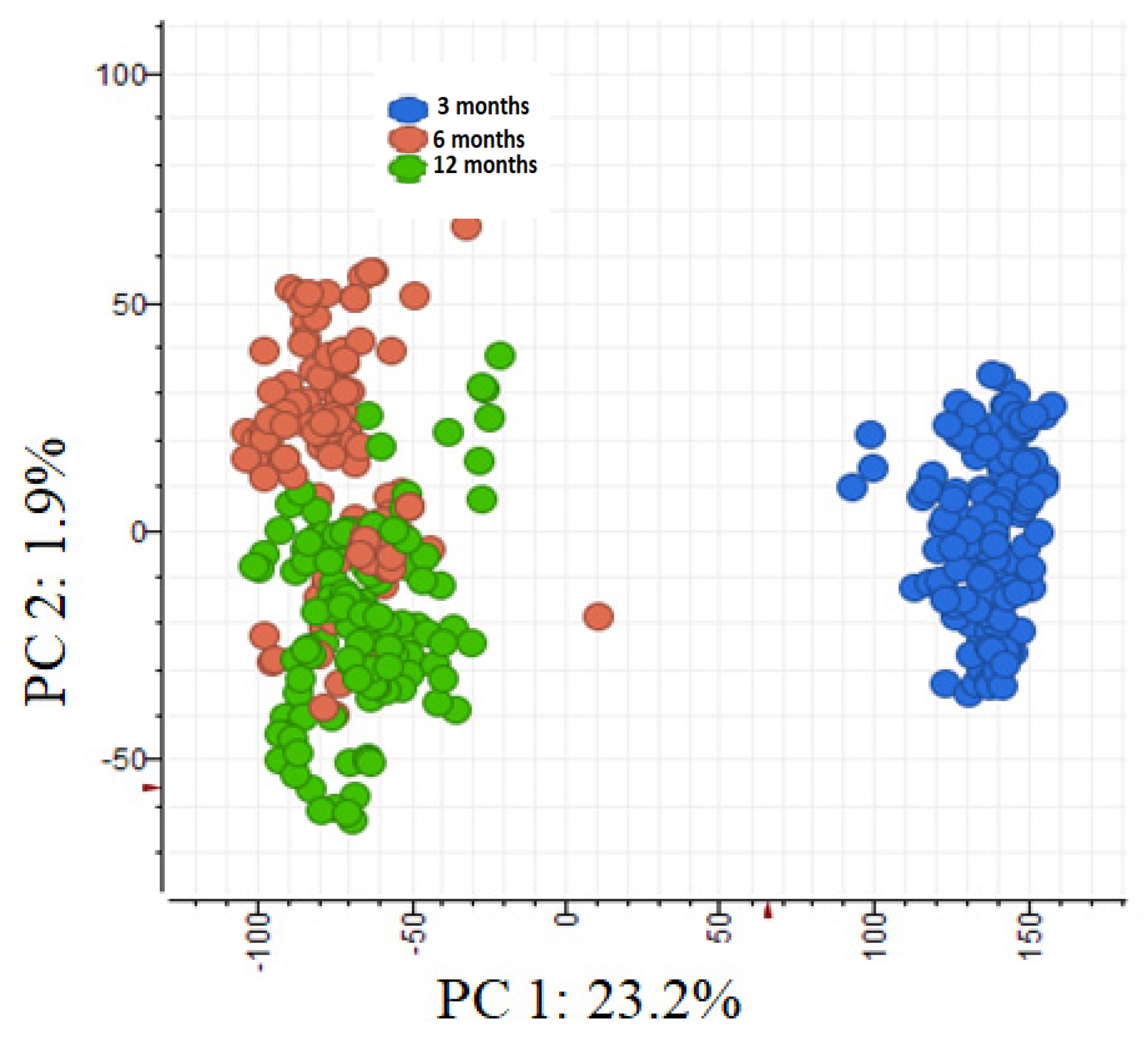
2.3. Effect of Leaf Age on Phenolic Composition
3. Discussion
3.1. Effect of Provenance on Phenolic Composition
3.2. Effect of UVA on Phenolic Composition
3.3. Effect of Leaf Age on Phenolic Composition
3.4. Synthesis and Application
4. Materials and Methods
4.1. Study Species and Growing Conditions
4.2. Experimental Treatments, Leaf Tagging and Harvesting
4.3. LED Lighting
4.4. Phenolics Extraction
4.5. Chromatographic and Mass Spectrometric Analysis
4.6. Data Processing
4.7. Data Analysis and Compound Identification
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merchant, A.; Tausz, M.; Arndt, S.K.; Adams, M.A. Cyclitols and carbohydrates in leaves and roots of 13 Eucalyptus species suggest contrasting physiological responses to water deficit. Plant Cell Environ. 2006, 29, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Merchant, A.; Ladiges, P.Y.; Adams, M.A. Quercitol links the physiology, taxonomy and evolution of 279 eucalypt species. Glob. Ecol. Biogeogr. 2007, 16, 810–819. [Google Scholar] [CrossRef]
- Tharayil, N.; Suseela, V.; Triebwasser, D.J.; Preston, C.M.; Gerard, P.D.; Dukes, J.S. Changes in the structural composition and reactivity of Acer rubrum leaf litter tannins exposed to warming and altered precipitation: Climatic stress-induced tannins are more reactive. New Phytol. 2011, 191, 132–145. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, A.B.; Hovenden, M.J.; Brodribb, T.J.; Potts, B.M.; Davies, N.W.; O’Reilly-Wapstra, J.M. Effect of limited water availability on foliar plant secondary metabolites of two Eucalyptus species. Environ. Exp. Bot. 2014, 105, 55–64. [Google Scholar] [CrossRef]
- McKiernan, A.B.; Potts, B.M.; Brodribb, T.J.; Hovenden, M.J.; Davies, N.W.; McAdam, S.A.M.; Ross, J.J.; Rodemann, T.; O’Reilly-Wapstra, J.M. Responses to mild water deficit and rewatering differ among secondary metabolites but are similar among provenances within Eucalyptus species. Tree Physiol. 2016, 36, 133–147. [Google Scholar] [CrossRef]
- Gourlay, G.; Constabel, C.P. Condensed tannins are inducible antioxidants and protect hybrid poplar against oxidative stress. Tree Physiol. 2019, 39, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Marsal, S.; Julià, A. Analytical methods in untargeted metabolomics: State of the art in 2015. Front. Bioeng. Biotechnol. 2015, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Cañal, M.J.; Escandón, M.; Meijón, M.; Weckwerth, W.; Valledor, L. Integrated physiological, proteomic, and metabolomic analysis of ultra violet (UV) stress responses and adaptation mechanisms in Pinus Radiata. Mol. Cell. Proteom. 2017, 16, 485–501. [Google Scholar] [CrossRef]
- Appel, H.M. Phenolics in ecological interactions: The importance of oxidation. J. Chem. Ecol. 1993, 19, 1521–1552. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.; Jones, C.P.; Hagerman, A.E.; Karonen, M.; Salminen, J.-P. Ellagitannins have greater oxidative activities than condensed tannins and galloyl glucoses at high pH: Potential impact on caterpillars. J. Chem. Ecol. 2006, 32, 2253–2267. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.-P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Marsh, K.J.; Wallis, I.R.; Kulheim, C.; Clark, R.; Nicolle, D.; Foley, W.J.; Salminen, J.-P. New approaches to tannin analysis of leaves can be used to explain in vitro biological activities associated with herbivore defence. New Phytol. 2020, 225, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Ariga, T.; Hamano, M. Radical scavenging action and its mode in procyanidins B-1 and B-3 from azuki beans to peroxyl radicals. Agric. Biol. Chem. 1990, 54, 2499–2504. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Close, D.C.; McArthur, C. Rethinking the role of many plant phenolics—Protection from photodamage not herbivores? Oikos 2002, 99, 166–172. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Björn, L.O.; Bornman, J.F.; Flint, S.D.; Kulandaivelu, G.; Teramura, A.H.; Tevini, M. Effects of increased solar ultraviolet radiation on terrestrial ecosystems. J. Photochem. Photobiol. B Biol. 1998, 46, 40–52. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Jha, M.; Tuteja, N. DNA damage and repair in plants under ultraviolet and ionizing radiations. Sci. World J. 2015, 2015, 250158. [Google Scholar] [CrossRef]
- Mao, K.; Yuan, Z.; Zuo, Z.; Xu, T.; Shen, X.; Gao, C. Changes in global cloud cover based on remote sensing data from 2003 to 2012. Chin. Geogr. Sci. 2019, 29, 306–315. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Caldwell, M.M.; Flint, S.D.; Robinson, S.A.; Bornman, J.F. Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2011, 10, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Bornman, J.F.; Barnes, P.W.; Robinson, S.A.; Ballaré, C.L.; Flint, S.D.; Caldwell, M.M. Solar ultraviolet radiation and ozone depletion-driven climate change: Effects on terrestrial ecosystems. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2015, 14, 88–107. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.L.; du Toit, W. Spectrophotometric analysis of phenolic compounds in grapes and wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Iwai, M.; Ohta, M.; Tsuchiya, H.; Suzuki, T. Enhanced accumulation of caffeic acid, rosmarinic acid and luteolin-glucoside in red perilla cultivated under red diode laser and blue LED illumination followed by UV-A irradiation. J. Funct. Foods 2010, 2, 66–70. [Google Scholar] [CrossRef]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. 2016, 198, 277–283. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Henbest RG, C.; Davis, F.J.; Battey, N.H.; Hadley, P.; Wagstaffe, A. UV irradiance as a major influence on growth, development and secondary products of commercial importance in Lollo Rosso lettuce ‘Revolution’ grown under polyethylene films. Environ. Exp. Bot. 2008, 63, 232–239. [Google Scholar] [CrossRef]
- Otieno, B.A. Genetic and Environmental Influences on Utilisation of Eucalyptus Camaldulensis (Dehnh.) by Leptocybe Invasa Fisher & LaSalle (Hymenoptera: Eulophidae). PhD Thesis, La Trobe University, Melbourne, Australia, 2017. [Google Scholar] [CrossRef]
- Otieno, B.A.; Salminen, J.-P.; Steinbauer, M.J. Resistance of subspecies of Eucalyptus camaldulensis to galling by Leptocybe invasa: Could quinic acid derivatives be responsible for leaf abscission and reduced galling? Agric. For. Entomol. 2022, 24, 167–177. [Google Scholar] [CrossRef]
- Pinkard, E.; Gill, W.; Mohammed, C. Physiology and anatomy of lenticel-like structures on leaves of Eucalyptus nitens and Eucalyptus globulus seedlings. Tree Physiol. 2006, 26, 989–999. [Google Scholar] [CrossRef]
- Close, D.C.; McArthur, C.; Hagerman, A.E.; Davies, N.W.; Beadle, C.L. Phenolic acclimation to ultraviolet-A irradiation in Eucalyptus nitens seedlings raised across a nutrient environment gradient. Photosynthetica 2007, 45, 36–42. [Google Scholar] [CrossRef]
- Abdelwahed, A.; Bouhlel, I.; Skandrani, I.; Valenti, K.; Kadri, M.; Guiraud, P.; Steiman, R.; Mariotte, A.M.; Ghedira, K.; Laporte, F.; et al. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucoes from Pistacia lentiscus: Confirmation by microarray expression profiling. Chem. Biol. Interact. 2007, 165, 1–13. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Mori, T.; Saito, K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e29518. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Close, D.C.; Davies, N.W.; Beadle, C.L. Temporal variation of tannins (galloylglucoses), flavonols and anthocyanins in leaves of Eucalyptus nitens seedlings: Implications for light attenuation and antioxidant activities. Aust. J. Plant Physiol. 2001, 28, 269–278. [Google Scholar] [CrossRef]
- Tian, L.-W.; Xu, M.; Li, Y.; Li, X.-Y.; Wang, D.; Zhu, H.-T.; Yang, C.-R.; Zhang, Y.-J. Phenolic compounds from the branches of Eucalyptus maideni. Chem. Biodivers. 2012, 9, 123–130. [Google Scholar] [CrossRef]
- Ryan, K.G.; Swinny, E.E.; Markham, K.R.; Winefield, C. Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry 2002, 59, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Shourie, A.; Tomar, P.; Srivastava, D.; Chauhan, R. Enhanced biosynthesis of quercetin occurs as a photoprotective measure in Lycopersicon esculentum Mill. under acute UV-B exposure. Braz. Arch. Biol. Technol. 2014, 57, 317–325. [Google Scholar] [CrossRef]
- Agati, G.; Matteini, P.; Goti, A.; Tattini, M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007, 174, 77–89. [Google Scholar] [CrossRef]
- Bielczynski, L.W.; Łącki, M.K.; Hoefnagels, I.; Gambin, A.; Croce, R. Leaf and plant age affect photosynthetic performance and photoprotective capacity. Plant Physiol. 2017, 175, 1634–1648. [Google Scholar] [CrossRef]
- Farnier, K.; Steinbauer, M.J. Elevated anthocyanins protect young Eucalyptus leaves from high irradiance but also indicate foliar nutritional quality to visually attuned psyllids. Ecol. Entomol. 2016, 41, 168–181. [Google Scholar] [CrossRef]
- Sharma, P.J.; Crowden, R.K. Anthocyanins in some Eucalyptus species. Aust. J. Bot. 1974, 22, 623–627. [Google Scholar] [CrossRef]
- McArthur, C.; Loney, P.E.; Davies, N.W.; Jordan, G.J. Early ontogenetic trajectories vary among defence chemicals in seedlings of a fast-growing eucalypt. Austral Ecol. 2010, 35, 157–166. [Google Scholar] [CrossRef]
- Goodger JQ, D.; Heskes, A.M.; Woodrow, I.E. Contrasting ontogenetic trajectories for phenolic and terpenoid defences in Eucalyptus froggattii. Ann. Bot. 2013, 112, 651–659. [Google Scholar] [CrossRef]
- Close, D.; McArthur, C.; Paterson, S.; Fitzgerald, H.; Walsh, A.; Kincade, T. Photoinhibition: A link between effects of the environment on eucalypt leaf chemistry and herbivory. Ecology 2003, 84, 2952–2966. [Google Scholar] [CrossRef]
- Luo, H.; Li, W.; Zhang, X.; Deng, S.; Xu, Q.; Hou, T.; Pang, X.; Zhang, Z.; Zhang, X. In planta high levels of hydrolysable tannins inhibit peroxidase mediated anthocyanin degradation and maintain abaxially red leaves of Excoecaria cochinchinensis. BMC Plant Biol. 2019, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Wam, H.K.; Stolter, C.; Nybakken, L. Compositional changes in foliage phenolics with plant age, a natural experiment in boreal forests. J. Chem. Ecol. 2017, 43, 920–928. [Google Scholar] [CrossRef]
- Holly, C.; Laughlin, G.P.; Ball, M.C. Cold-induced photoinhibition and design of shelters for establishment of eucalypts in pastures. Aust. J. Bot. 1994, 42, 139–147. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Burns, A.E.; Hall, A.; Riegler, M.; Taylor, G.S. Nutritional enhancement of leaves by a psyllid through senescence-like processes: Insect manipulation or plant defence? Oecologia 2014, 176, 1061–1074. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Salminen, J.-P.; Watson, S.J. Yellow, red, dead: The nutritional consequences for Cardiaspina densitexta (Hemiptera: Aphalaridae) nymphs of inducing senescence in old Eucalyptus fasciculosa leaves. Austral Entomol. 2018, 57, 265–278. [Google Scholar] [CrossRef]
- McDonald, M.W.; Brooker MI, H.; Butcher, P.A. A taxonomic revision of Eucalyptus camaldulensis (Myrtaceae). Aust. Syst. Bot. 2009, 22, 257–285. [Google Scholar] [CrossRef]
- James, S.A.; Bell, D.T. Morphology and anatomy of leaves of Eucalyptus camaldulensis clones: Variation between geographically separated locations. Aust. J. Bot. 1995, 43, 415–433. [Google Scholar] [CrossRef]
- Dillon, S.; McEvoy, R.; Baldwin, D.S.; Rees, G.N.; Parsons, Y.; Southerton, S. Characterisation of adaptive genetic diversity in environmentally contrasted populations of Eucalyptus camaldulensis Dehnh. (River Red Gum). PLoS ONE 2014, 9, e103515. [Google Scholar] [CrossRef]
- Ohmart, C.P.; Thomas, J.R.; Stewart, L.G. Differential defoliation by insects among provenances of Eucalyptus delegatensis. Aust. J. Entomol. 1984, 23, 105–111. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Matsuki, M. Suitability of Eucalyptus and Corymbia for Mnesampela privata (Guenée) (Lepidoptera: Geometridae) larvae. Agric. For. Entomol. 2004, 6, 323–332. [Google Scholar] [CrossRef]
- Henery, M.L.; Henson, M.; Wallis, I.R.; Stone, C.; Foley, W.J. Predicting crown damage to Eucalyptus grandis by Paropsis atomaria with direct and indirect measures of leaf composition. For. Ecol. Manag. 2008, 255, 3642–3651. [Google Scholar] [CrossRef]
- Östrand, F.; Wallis, I.R.; Davies, N.W.; Matsuki, M.; Steinbauer, M.J. Causes and consequences of host expansion by Mnesampela privata. J. Chem. Ecol. 2008, 34, 153–167. [Google Scholar] [CrossRef]
- Khanal, S. Use of Metabolomics to Understand Ontogenetic and Environmental Influences on the Abundance and Composition of Foliar Phenolic Compounds in Myrtaceae. Ph.D. Thesis, La Trobe University, Melbourne, Australia, 2020. [Google Scholar] [CrossRef]



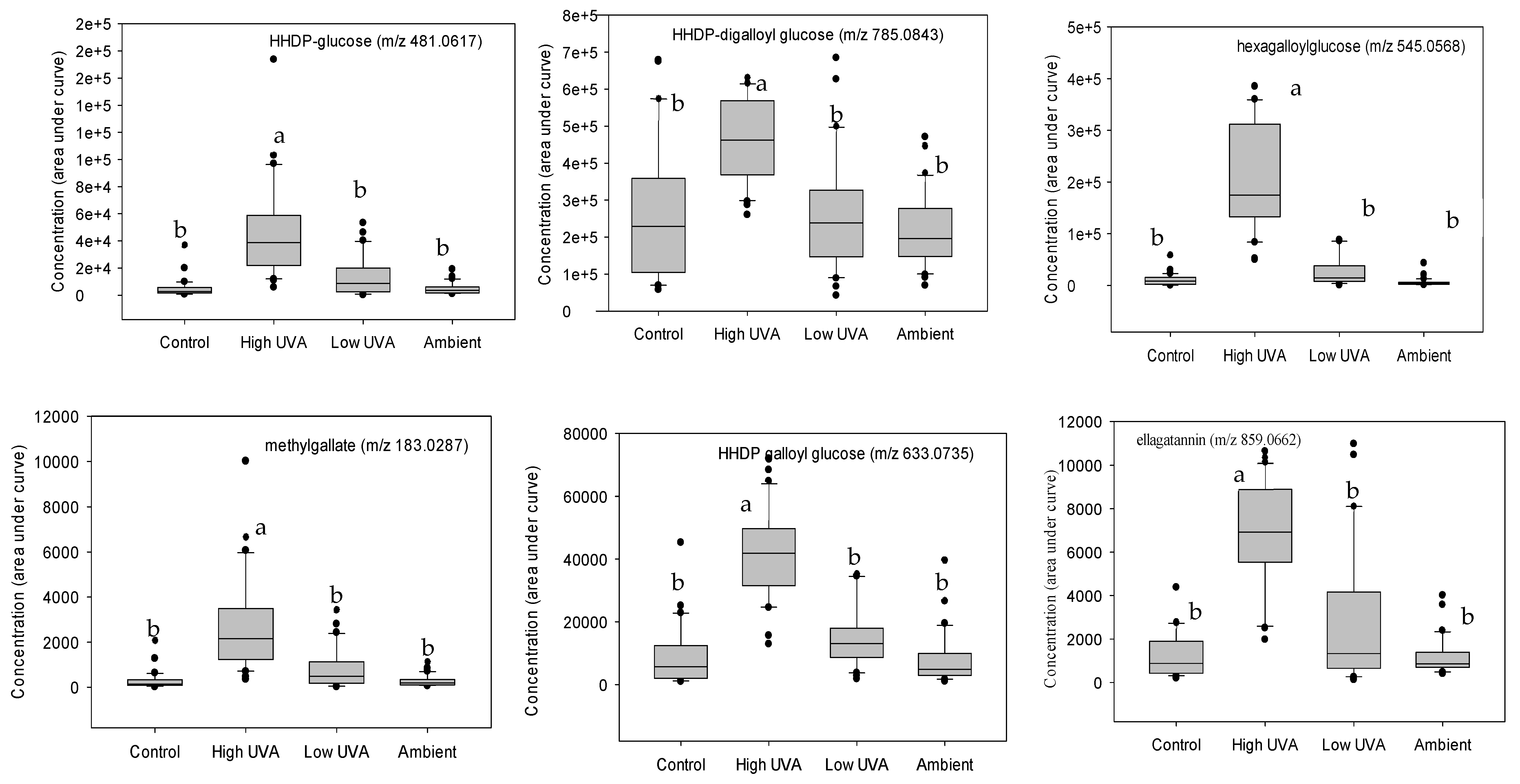
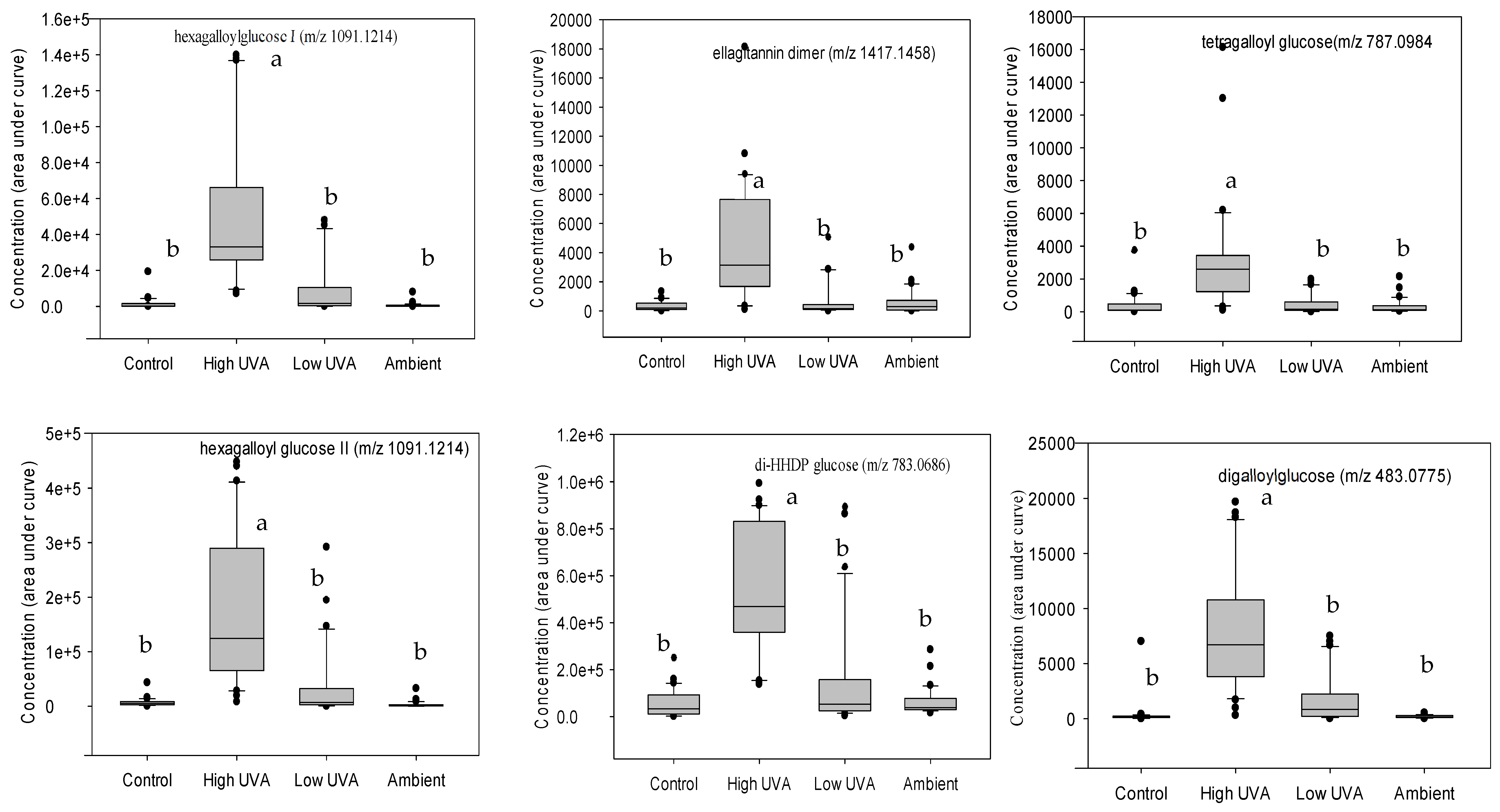


| m/z [M-H]− | Retention Time (min) | Fragments | Tentative Identification |
|---|---|---|---|
| 1091.1214 | 5.85 | 939.1103, 769.0907, 617.0801, 465.0781, 169.0132 | hexagalloylglucose I |
| 1091.1214 | 5.94 | 939.1103, 769.0907, 617.0801, 465.0781, 169.0132 | hexagalloylglucose II |
| 481.0617 | 2.06 | 421.0407, 300.9986, 275.0194 | HHDP-glucose |
| 1417.1458 | 3.82 | 765.0638, 633.0735, 300.9983,275.0201 | ellagitannin dimer |
| 545.0568 | 5.91 | 939.1103, 769.0907, 617.0801, 465.0781, 169.0132 | hexagalloylglucose |
| 785.0843 | 5.42 | 633.0736, 300.9991, 275.0198, 249.0399, 169.0133 | HHDP-digalloylglucose |
| 104.0131 | 5.47 | ~ | unknown |
| 331.0669 | 5.8 | 271.0459, 169.0132, 151.0012, 125.0234 | galloylglucose |
| 783.0686 | 3.37 | 481.0619, 300.9984, 275.0196 | di-HHDP-glucose |
| 787.0984 | 4.63 | 635.0892, 465.0701, 300.9982, 249.0401, 169.0131 | tetragalloylglucose |
| 183.0287 | 5.26 | 168.0053, 124.0153 | methylgallate |
| 433.1725 | 5.21 | ~ | unknown |
| 785.0843 | 4.04 | 633.0736, 300.9991, 275.0198, 249.0399, 169.0133 | HHDP-digalloylglucose |
| 633.0735 | 5.45 | 463.0533, 300.9985, 275.0201, 169.0135 | HHDP-galloylglucose |
| 185.0377 | 4.15 | ~ | unknown |
| 124.0147 | 4.17 | ~ | unknown |
| 859.0662 | 4.33 | 300.9985, 275.0201, 169.0135 | ellagitannin |
| 860.0802 | 4.34 | 300.9985, 275.0201, 169.0135 | ellagitannin |
| 787.0984 | 4.41 | 635.0892, 465.0701, 300.9982, 249.0401, 169.0131 | tetragalloylglucose |
| 483.0775 | 4.13 | 331.0666, 271.0457, 169.0132, 125.0232 | digalloylglucose |
| m/z [M-H]− | Retention Time (min) | Tentative Identification | Young Leaves | Old Leaves |
|---|---|---|---|---|
| 383.1865 | 15.33 | unknown | ↑ | |
| 433.0464 | 5.26 | ellagic acid glycoside | ↑ | |
| 271.082 | 3.65 | glycosylated hydroquinone | ↑ | |
| 463.0511 | 4.72 | ellagic acid hexoside | ↑ | |
| 433.0462 | 5.27 | ellagic acid glycoside | ↑ | |
| 599.1039 | 5.49 | kaempferol derivative | ↑ | |
| 315.0734 | 3.98 | phenolic glycoside | ↑ | |
| 197.0812 | 3.65 | unknown | ↑ | |
| 499.1602 | 14.21 | unknown | ↑ | |
| 783.0693 | 3.30 | pedunculagin | ↑ | |
| 461.0720 | 5.85 | flavonoid glycoside | ↑ | |
| 935.0795 | 5.51 | di-HHDP galloylglucose | ↑ | |
| 789.4441 | 16.02 | unknown | ↑ | |
| 471.2746 | 13.32 | unknown | ↑ | |
| 399.1814 | 15.01 | unknown | ↑ | |
| 609.1459 | 5.10 | quercetin derivative | ↑ |
| ATSC Seedlot Number | Code | GPS Coordinates | Location (State) | Average Annual Rainfall (mm) |
|---|---|---|---|---|
| 20440 | G1 | −31.46 S, 143.65 E | 30 km ENE of Wilcannia (New South Wales) | 264 |
| 20561 | G2 | −35.75 S, 141.96 E | Lake Albacutya (Victoria) | 363 |
| 20437 | G3 | −32.30 S, 138.45 E | Boolcunda Creek (South Australia) | 306 |
| 20430 | G4 | −33.85 S, 148.70 E | Cowra (New South Wales) | 598 |
| 20429 | G5 | −33.10 S, 147.15 E | Condobolin (New South Wales) | 421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanal, S.; Rochfort, S.J.; Steinbauer, M.J. Ultraviolet-A Radiation (UVA) as a Stress and the Influence of Provenance and Leaf Age on the Expression of Phenolic Compounds by Eucalyptus camaldulensis ssp. camaldulensis. Plants 2025, 14, 493. https://doi.org/10.3390/plants14030493
Khanal S, Rochfort SJ, Steinbauer MJ. Ultraviolet-A Radiation (UVA) as a Stress and the Influence of Provenance and Leaf Age on the Expression of Phenolic Compounds by Eucalyptus camaldulensis ssp. camaldulensis. Plants. 2025; 14(3):493. https://doi.org/10.3390/plants14030493
Chicago/Turabian StyleKhanal, Santosh, Simone J. Rochfort, and Martin J. Steinbauer. 2025. "Ultraviolet-A Radiation (UVA) as a Stress and the Influence of Provenance and Leaf Age on the Expression of Phenolic Compounds by Eucalyptus camaldulensis ssp. camaldulensis" Plants 14, no. 3: 493. https://doi.org/10.3390/plants14030493
APA StyleKhanal, S., Rochfort, S. J., & Steinbauer, M. J. (2025). Ultraviolet-A Radiation (UVA) as a Stress and the Influence of Provenance and Leaf Age on the Expression of Phenolic Compounds by Eucalyptus camaldulensis ssp. camaldulensis. Plants, 14(3), 493. https://doi.org/10.3390/plants14030493






