Factors Influencing Orchid Species Richness in the Central Balkans: The Importance of Belowground Organ Types
Abstract
1. Introduction
2. Materials and Methods
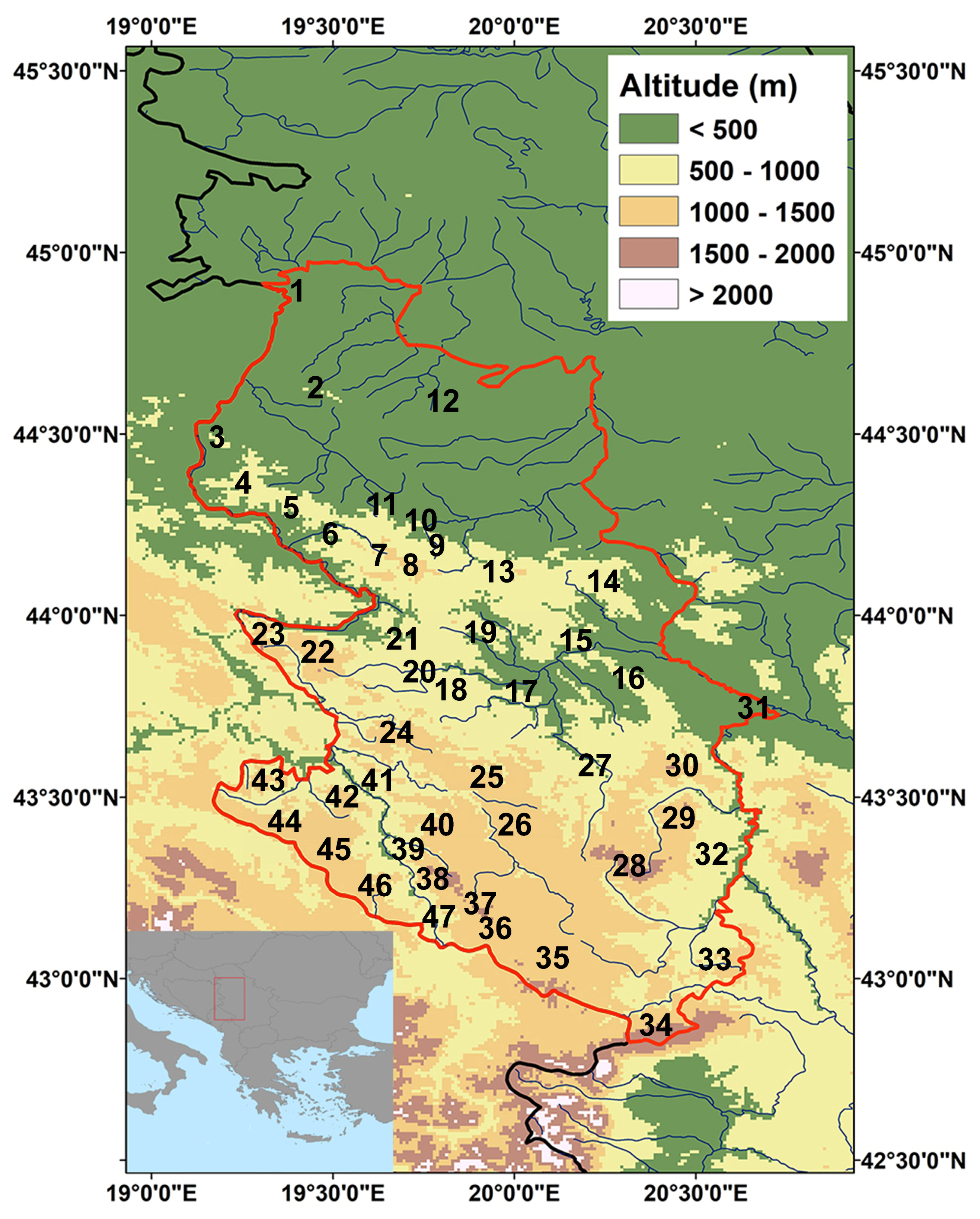
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
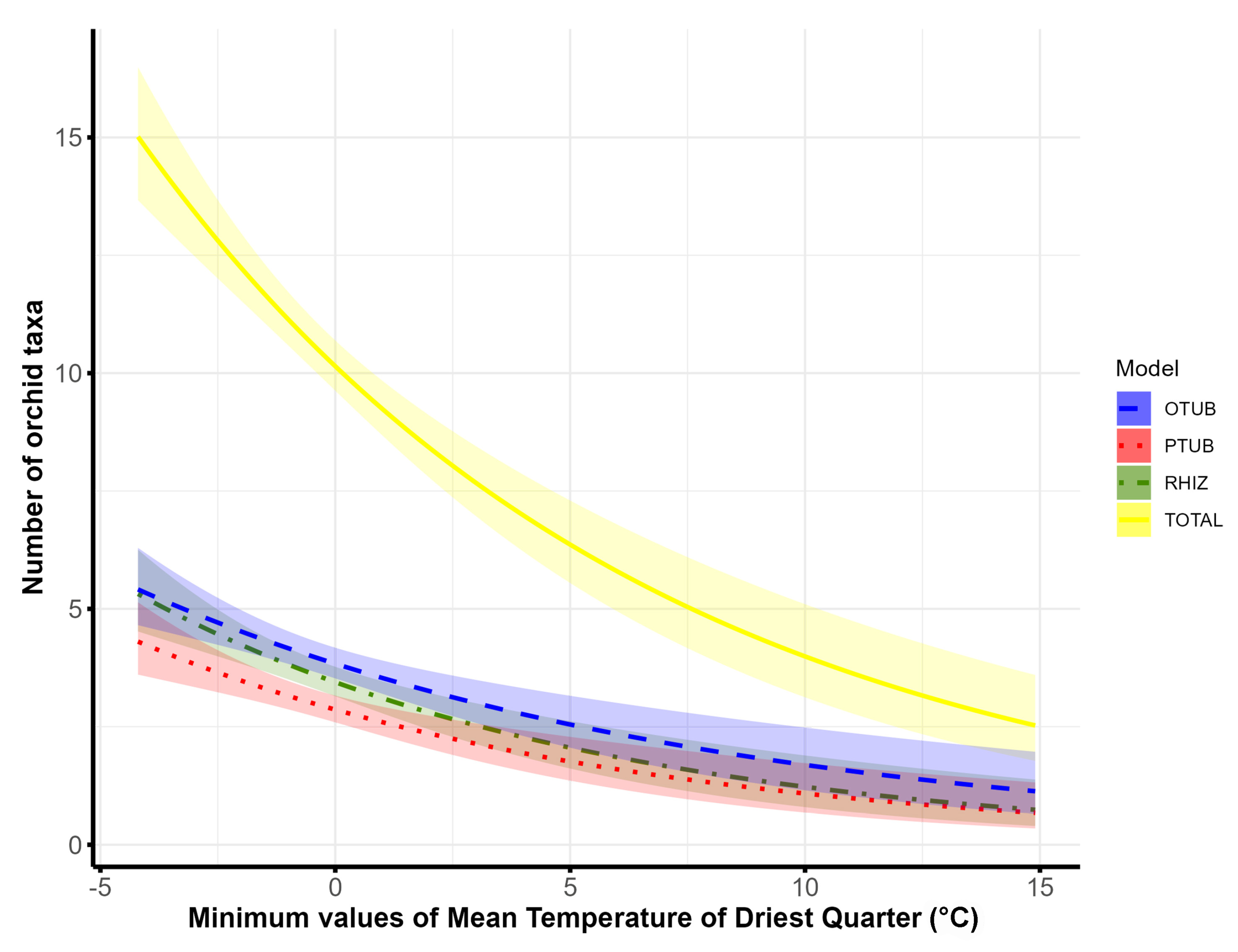
3. Results
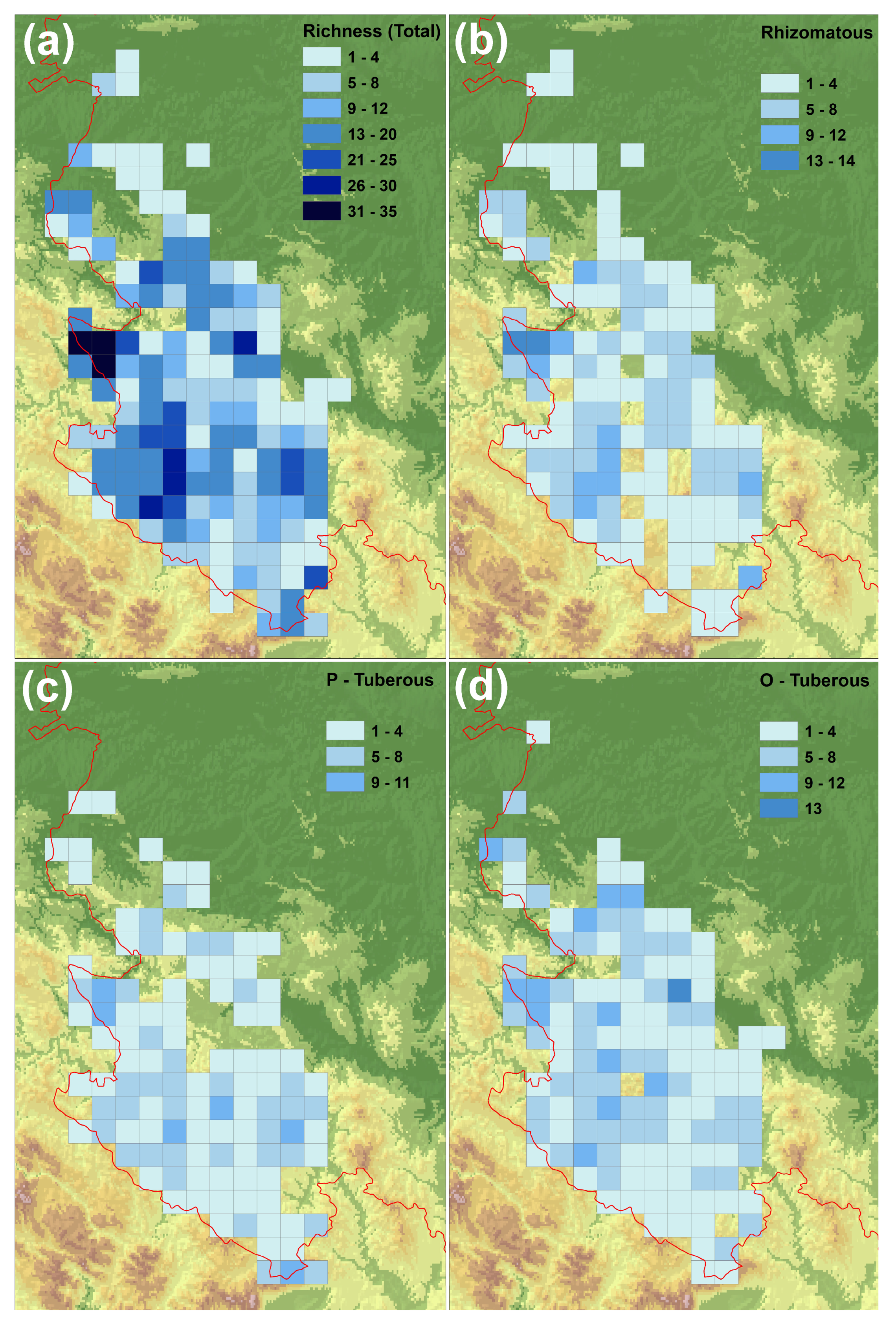
3.1. Distribution of Orchid Species Richness in the Central Balkans
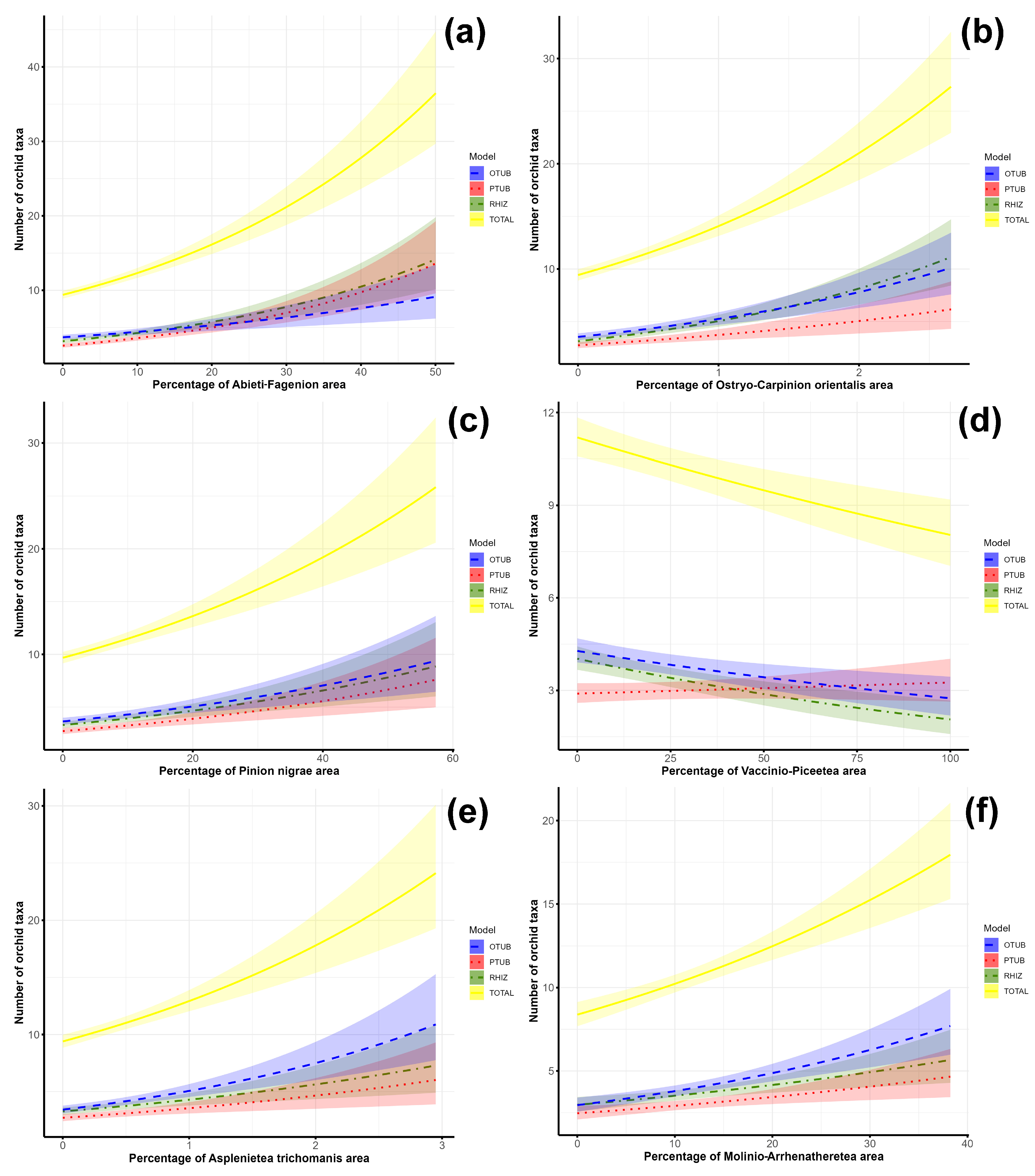
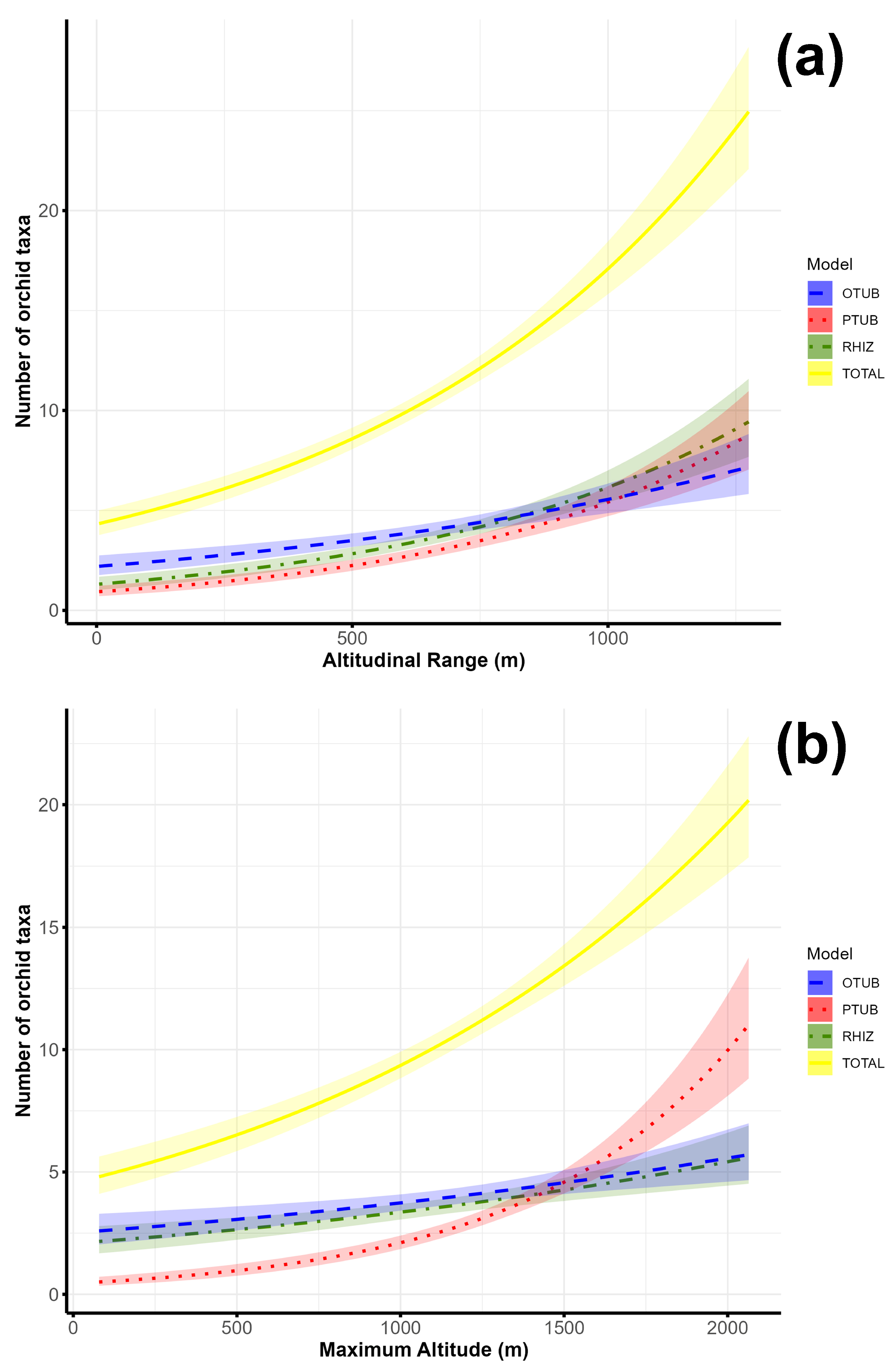
3.2. Factors Determining Orchid Species Richness in the Central Balkans
4. Discussion
4.1. Distribution of Orchid Species Richness in the Central Balkans
4.2. Factors Determining Orchid Species Richness in the Central Balkans
4.2.1. Factors Determining the Total Orchid Species Richness in the Central Balkans
4.2.2. Factors Determining the Richness of Rhizomatous Orchids in the Central Balkans
4.2.3. Factors Determining the Richness of Palmate Tuberous Orchids in the Central Balkans
4.2.4. Factors Determining the Richness of Ovoid Tuberous Orchids in the Central Balkans
4.3. Implications for Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Givnish, T.J.; Spalink, D.; Ames, M.; Lyon, S.P.; Hunter, S.J.; Zuluaga, A.; Doucette, A.; Caro, G.G.; McDaniel, J.; Clements, M.A.; et al. Orchid historical biogeography, diversification, Antarctica and the paradox of orchid dispersal. J. Biogeogr. 2016, 43, 1905–1916. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.A.; Bogarín, D.; Przelomska, N.A.; Ackerman, J.D.; Balbuena, J.A.; Bellot, S.; Bühlmann, R.P.; Cabrera, B.; Cano, J.A.; Charitonidou, M.; et al. The origin and speciation of orchids. New Phytol. 2024, 242, 700–716. [Google Scholar] [CrossRef] [PubMed]
- Lussu, M.; Ancillotto, L.; Labadessa, R.; Di Musciano, M.; Zannini, P.; Testolin, R.; Santi, F.; Dolci, D.; Conti, M.; Marignani, M.; et al. Prioritizing conservation of terrestrial orchids: A gap analysis for Italy. Biol. Conserv. 2024, 289, 110385. [Google Scholar] [CrossRef]
- Vereecken, N.J.; Dafni, A.; Cozzolino, S. Pollination Syndromes in Mediterranean Orchids—Implications for Speciation, Taxonomy and Conservation. Bot. Rev. 2020, 76, 220–240. [Google Scholar] [CrossRef]
- McCormick, M.K.; Jacquemyn, H. What constrains the distribution of orchid populations? New Phytol. 2014, 202, 392–400. [Google Scholar] [CrossRef]
- Li, T.; Wu, S.; Yang, W.; Selosse, M.-A.; Gao, J. How Mycorrhizal Associations Influence Orchid Distribution and Population Dynamics. Front. Plant Sci. 2021, 12, 647114. [Google Scholar] [CrossRef]
- Ackerman, J.D.; Phillips, R.D.; Tremblay, R.L.; Karremans, A.; Reiter, N.; Peter, C.I.; Bogarín, D.; Pérez-Escobar, O.A.; Liu, H. Beyond the various contrivances by which orchids are pollinated: Global patterns in orchid pollination biology. Bot. J. Linn. 2023, 202, 295–324. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, Y.; Tianb, Y.; Lib, J.; Hea, J.-S.; Tanga, Z. Distribution and conservation of orchid species richness in China. Biol. Conserv. 2015, 181, 64–72. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidis, I.; Karagiannakidou, V. Identifying areas of high importance for orchid conservation in east Macedonia (NE Greece). Biodivers. Conserv. 2009, 18, 1765–1780. [Google Scholar] [CrossRef]
- Yudaputra, A.; Munawaroh, E.; Usmadi, D.; Purnomo, D.W.; Astuti, I.P.; Puspitaningtyas, D.M.; Handayani, T.; Garvita, R.V.; Aprilianti, P.; Wawangningrum, H.; et al. Vulnerability of lowland and upland orchids in their spatially response to climate change and land cover change. Ecol. Inform. 2024, 80, 102534. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Honnay, O.; Pailler, T. Range size variation, nestedness and species turnover of orchid species along an altitudinal gradient on Réunion Island: Implications for conservation. Biol. Conserv. 2007, 136, 388–397. [Google Scholar] [CrossRef]
- Acharya, K.P.; Vetaas, O.R.; Birks, H.J.B. Orchid species richness along Himalayan elevational gradients. J. Biogeogr. 2011, 38, 1821–1833. [Google Scholar] [CrossRef]
- Štípková, Z.; Traxmandlová, I.; Kindlmann, P. Determinants of orchid species diversity in Latin America. Lankesteriana 2016, 16, 293–297. [Google Scholar] [CrossRef]
- Schödelbauerová, I.; Roberts, D.L.; Kindlmann, P. Size of protected areas is the main determinant of species diversity in orchids. Biol. Conserv. 2009, 142, 2329–2334. [Google Scholar] [CrossRef]
- Evans, A.; Jacquemyn, H. Range size and niche breadth as predictors of climate-induced habitat change in Epipactis (Orchidaceae). Front. Ecol. Evol. 2022, 10, 894616. [Google Scholar] [CrossRef]
- Evans, A.; de Kort, H.; Brys, R.; Duffy, K.J.; Jersáková, J.; Kull, T.; Selosse, M.-A.; Tsiftsis, S.; Minasiewicz, J.; Jacquemyn, H. Historical biogeography and local adaptation explain population genetic structure in a widespread terrestrial orchid. Ann. Bot. 2023, 131, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.M.; Pulliam, H.R. Hierarchical analysis of species distribution and abundance across environmental gradients. Ecology 2007, 88, 3144–3152. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidis, I.; Karagiannakidou, V.; Alifragis, D. Niche analysis and conservation of the orchids of east Macedonia (NE Greece). Acta Oecol. 2008, 33, 27–35. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidis, I.; Papaioannou, A. Ecology of orchid Goodyera repens in its southern distribution limits. Plant Biosyst. 2012, 146, 857–866. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Djordjević, V. Modelling sexually deceptive orchid species distributions under future climates: The importance of plant–pollinator interactions. Sci. Rep. 2020, 10, 10623. [Google Scholar] [CrossRef] [PubMed]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Stevanović, V. Factors affecting the distribution and abundance of orchids in grasslands and herbaceous wetlands. Syst. Biodivers. 2016, 14, 355–370. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Jakovljević, K.; Stevanović, V. Patterns of distribution, abundance and composition of forest terrestrial orchids. Biodivers. Conserv. 2020, 29, 4111–4134. [Google Scholar] [CrossRef]
- Hrivnák, M.; Slezák, M.; Galvánek, D.; Vlčko, J.; Belanová, E.; Rízová, V.; Senko, D.; Hrivnák, R. Species Richness, Ecology, and Prediction of Orchids in Central Europe: Local-Scale Study. Diversity 2020, 12, 154. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; Canchani-Viruet, A. Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytol. 2018, 219, 1207–1215. [Google Scholar] [CrossRef]
- Kirillova, I.A.; Dubrovskiy, Y.A.; Degteva, S.V.; Novakovskiy, A.B. Ecological and habitat ranges of orchids in the northernmost regions of their distribution areas: A case study from Ural Mountains, Russia. Plant Divers. 2023, 45, 211–218. [Google Scholar] [CrossRef]
- Traxmandlová, I.; Ackerman, J.D.; Tremblay, R.L.; Roberts, D.L.; Štípková, Z.; Kindlmann, P. Determinants of orchid species diversity in world islands. New Phytol. 2018, 217, 12–15. [Google Scholar] [CrossRef]
- Janečková, P.; Wotavová, K.; Schödelbauerová, I.; Jersáková, J.; Kindlmann, P. Relative effects of management and environmental conditions on performance and survival of populations of a terrestrial orchid, Dactylorhiza majalis. Biol. Conserv. 2006, 129, 40–49. [Google Scholar] [CrossRef]
- Phillips, R.D.; Brown, A.P.; Dixon, K.W.; Hopper, S.D. Orchid biogeography and the factors associated with rarity in a biodiversity hotspot: The Southwest Australian Floristic Region. J. Biogeogr. 2011, 38, 487–501. [Google Scholar] [CrossRef]
- Cardelús, L.C.; Colwell, R.K.; Watkins, J.E., Jr. Vascular epiphyte distribution patterns: Explaining the mid-elevation richness peak. J. Ecol. 2006, 94, 144–156. [Google Scholar] [CrossRef]
- Zhang, S.-B.; Chen, W.Y.; Huang, J.L.; Bi, Y.F.; Yang, X.-F. Orchid Species Richness along Elevational and Environmental Gradients in Yunnan, China. PLoS ONE 2015, 10, e0142621. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Štípková, Z.; Kindlmann, P. Role of way of life, latitude, elevation and climate on the richness and distribution of orchid species. Biodivers. Conserv. 2019, 28, 75–96. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidis, I. Temporal and spatial patterns of orchid species distribution in Greece: Implications for conservation. Biodivers. Conserv. 2020, 29, 3461–3489. [Google Scholar] [CrossRef]
- Štípková, Z.; Tsiftsis, S.; Kindlmann, P. Pollination Mechanisms are Driving Orchid Distribution in Space. Sci. Rep. 2020, 10, 850. [Google Scholar] [CrossRef]
- Štípková, Z.; Tsiftsis, S.; Kindlmann, P. Distribution of Orchids with Different Rooting Systems in the Czech Republic. Plants 2021, 10, 632. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S.; Kindlmann, P.; Stevanović, V. Orchid diversity along an altitudinal gradient in the central Balkans. Front. Ecol. Evol. 2022, 10, 929266. [Google Scholar] [CrossRef]
- Lussu, M.; Zannini, P.; Testolin, R.; Dolci, D.; Conti, M.; Martellos, S.; Chiarucci, A. Biogeography of orchids and their pollination syndromes in small Mediterranean islands. J. Biogeogr. 2023, 51, 869–877. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Kindlmann, P. Advances in orchid research in East Macedonia (NE Greece) and the importance of current data in furthering our understanding of the orchids’ altitudinal requirements. J. Nat. Conserv. 2023, 72, 126346. [Google Scholar] [CrossRef]
- Taylor, A.; Keppel, G.; Weigelt, P.; Zotz, G.; Kreft, H. Functional traits are key to understanding orchid diversity on islands. Ecography 2021, 44, 703–714. [Google Scholar] [CrossRef]
- Ai, Y.Y.; Liu, Q.; Hu, H.X.; Shen, T.; Mo, Y.X.; Wu, X.F.; Li, J.L.; Dossa, G.G.O.; Song, L. Terrestrial and epiphytic orchids exhibit different diversity and distribution patterns along an elevation gradient of Mt. Victoria, Myanmar. Glob. Ecol. Conserv. 2023, 42, e02408. [Google Scholar] [CrossRef]
- De Bock, K.; Jacquemyn, H.; Ospina-Calderón, N.H.; Flanagan, N.S.; Ventre-Lespiaucq, A. Variation in root functional traits of Neotropical epiphytic and terrestrial orchids along an elevational gradient. Bot. J. Linn. 2024, boae040. [Google Scholar] [CrossRef]
- Averyanov, L. Averyanov, L. A review of the genus Dactylorhiza. In Orchid Biology—Reviews and Perspectives; Arditti, J., Ed.; V. Timber Press Inc.: Portland, OR, USA, 1990; pp. 159–206. [Google Scholar]
- Tatarenko, I. Growth habits of temperate terrestrial orchids. In Orchid Biology—Reviews and Perspectives, IX; Cameron, K.M., Arditti, J., Kull, T., Eds.; The New York Botanical Garden Press: Bronx, NY, USA, 2007; pp. 91–161. [Google Scholar]
- Dressler, R.L. The Orchids: Natural History and Classification; Harvard University Press: Cambridge, MA, USA, 1981. [Google Scholar]
- Hágsater, E.; Dumont, V. (Eds.) Orchids: Status, Survey and Conservation Action Plan; IUCN: Gland, Switzerland; Cambridge, UK, 1996. [Google Scholar]
- Tsiftsis, S. The complex effect of heterogeneity and isolation in determining alpha and beta orchid diversity on islands in the Aegean archipelago. Syst. Biodivers. 2020, 18, 281–294. [Google Scholar] [CrossRef]
- Djordjević, V.; Aćić, S.; Kabaš, E.; Lazarević, P.; Tsiftsis, S.; Lakušić, D. The Orchids of Wetland Vegetation in the Central Balkans. Diversity 2023, 15, 26. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Štípková, Z.; Rejmánek, M.; Kindlmann, P. Predictions of species distributions based only on models estimating future climate change are not reliable. Sci. Rep. 2024, 14, 25778. [Google Scholar] [CrossRef] [PubMed]
- Chiari, M.; Djerić, N.; Garfagnoli, F.; Hrvatović, H.; Krstić, M.; Levi, N.; Malasoma, A.; Marroni, M.; Menna, F.; Nirta, G.; et al. The geology of the Zlatibor-Maljen area (western Serbia): A geotraverse accross theophiolites of the Dinaric-Hellenic collisional belt. Ofioliti 2011, 36, 139–166. [Google Scholar]
- Gawlick, H.J.; Sudar, M.; Missoni, S.; Suzuki, H.; Lein, R.; Jovanović, D. Triassic-Jurassic geodynamic evolution of the Dinaridic Ophiolite Belt (Inner Dinarides, SW Serbia). J. Alpine Geol. 2017, 55, 1–167. [Google Scholar]
- Djordjević, V.; Tsiftsis, S. Patterns of orchid species richness and composition in relation to geological substrates. Wulfenia 2019, 26, 1–21. [Google Scholar]
- Kojić, M.; Popović, R.; Karadžić, B. Syntaxonomic Review of the Vegetation of Serbia; Institute for Biological Research Siniša Stanković: Belgrade, Serbia, 1998. (In Serbian) [Google Scholar]
- Ilić, T.; Kuzmanović, N.; Vukojičić, S.; Lakušić, D. Phytogeographic Characteristics of Montane Coniferous Forests of the Central Balkan Peninsula (SE Europe). Plants 2022, 11, 3194. [Google Scholar] [CrossRef]
- Sekulić, D.; Karadžić, B.; Kuzmanović, N.; Jarić, S.; Mitrović, M.; Pavlović, P. Diversity of Ostrya carpinifolia Forests in Ravine Habitats of Serbia (S-E Europe). Diversity 2021, 13, 59. [Google Scholar] [CrossRef]
- Baumann, H.; Künkele, S.; Lorenz, R. Die Orchideen Europas; Eugen Ulmer KG, Mit angrenzenden Gebieten: Stuttgart, Germany, 2006. [Google Scholar]
- Delforge, P. Orchids of Europe, North Africa and the Middle East; A. & C. Black: London, UK, 2006. [Google Scholar]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2024. Available online: http://www.plantsoftheworldonline.org/ (accessed on 16 November 2024).
- Djordjević, V.; Niketić, M.; Stevanović, V. Orchids of Serbia: Taxonomy, Life Forms, Pollination Systems, and Phytogeographical Analysis. In Orchidaceae: Characteristics, Distribution and Taxonomy; Djordjević, V., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2021; pp. 57–163. [Google Scholar]
- Lampinen, R. Universal Transverse Mercator (UTM) and Military Grid Reference System (MGRS). 2001. Available online: http://www.luomus.fi/english/botany/afe/map/utm.htm (accessed on 16 November 2024).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Lakušić, D.; Kuzmanović, N.; Kovačević, J. Generalized Habitat Map of Serbia (GKaSS-02) [Generalizovana Karta Staništa Srbije]—Tumač Karte; Verzija 2. Centar za informacije o biodiverzitetu; Univerzitet u Beogradu, Biološki fakultet: Preduzeće za geomatiku MapSoft d.o.o.: Belgrade, Serbia, 2021. [Google Scholar]
- Lakušić, D.; Kuzmanović, N.; Kovačević, J. Generalized habitat map of Serbia. In Proceedings of the 14th Symposium on Flora of Southeastern Serbia and Neighboring Regions, Kladovo, Serbia, 26–29 June 2022; Ranđelović, V., Stojanović-Radić, Z., Nikolić, D., Jenačković Gocić, D., Eds.; Department of Biology and Ecology, Faculty of Sciences and Mathematics, University of Niš: Niš. Institute for Nature Conservation of Serbia: Belgrade, Serbia, 2022; p. 35. [Google Scholar]
- Biau, G.; Scornet, E. A Random Forest Guided Tour. TEST 2016, 25, 197–227. [Google Scholar] [CrossRef]
- Su, Z.; Xu, Z.; Lin, L.; Chen, Y.; Hu, H.; Wei, S.; Luo, S. Exploration of the Contribution of Fire Carbon Emissions to PM2.5 and Their Influencing Factors in Laotian Tropical Rainforests. Remote Sens. 2022, 14, 4052. [Google Scholar] [CrossRef]
- R Core Team 2024. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 10 September 2024).
- ESRI 2020. ArcGIS—ArcMap: ArcInfo (Version 10.8); Environmental Science Research Institute: Redlands, CA, USA, 2020. [Google Scholar]
- Tomović, G.; Niketić, M.; Lakušić, D.; Randjelović, V.; Stevanović, V. Balkan Endemic Plants in Central Serbia and Kosovo Regions: Distribution Patterns, Ecological Characteristics, and Centres of Diversity. Bot. J. Linn. Soc. 2014, 176, 173–202. [Google Scholar] [CrossRef]
- Vukojičić, S.; Jakovljević, K.; Matevski, V.; Randjelović, V.; Niketić, M.; Lakušić, D. Distribution, Diversity and Conservation of Boreo-Montane Plant Species in the Central Part of the Balkan Peninsula and the Southern Part of the Pannonian Plain. Folia Geobot. 2014, 49, 487–505. [Google Scholar] [CrossRef]
- Djordjević, V.; Lazarević, P.; Stanković, V.; Kabaš, E. Epipactis exilis (Orchidaceae), a species new to the flora of Serbia. Phyton—Ann. Rei Bot. 2023, 62–63, 29–35. [Google Scholar]
- Tsiftsis, S.; Antonopoulos, Z. Atlas of the Greek Orchids; Mediterraneo Editions: Rethymno, Greece, 2017; Volume 1. [Google Scholar]
- Antonopoulos, Z.; Tsiftsis, S. Atlas of the Greek Orchids; Mediterraneo Editions: Rethymno, Greece, 2017; Volume 2. [Google Scholar]
- Nikolić, T. (Ed.) Flora Croatica Database; Prirodoslovno-matematički fakultet, Sveučilište u Zagrebu: Botanički zavod: Zagreb, Croatia, 2024; Available online: http://hirc.botanic.hr/fcd (accessed on 10 September 2024).
- Vangjeli, J.; Ruci, B.; Mullaj, A.; Paparisto, K.; Qosja, X. Flora e Shqipërisë Vol. 4. (Flora of Albania 4); Tiranë: Akademia e Shkencave e Republikes se Shqiperise: Tirana, Albania, 2000; 502p. [Google Scholar]
- Stevanović, V.; Jovanović, S.; Lakušić, D.; Niketić, M. Diverzitet vaskularne flore Jugoslavije sa pregledom vrsta od međunarodnog značaja. In Biodiverzitet Jugoslavije sa Pregledom Vrsta od Međunarodnog Značaja; Stevanović, V., Vasić, V., Eds.; Ecolibri & Biološki Fakultet: Beograd, Serbia, 1995; pp. 183–217. ISBN 86-7078-004-6. [Google Scholar]
- Pulević, V. Građa za Vaskularnu Floru Crne Gore. Dopuna Conspectus Florae Montenegrinae J. Rohlene; Republički zavod za zaštitu prirode Crne Gore: Podgorica, Montenegro, 2005. [Google Scholar]
- Šabanović, E.; Djordjević, V.; Milanović, Ð.; Boškailo, A.; Šarić, Š.; Huseinović, S.; Randjelović, V. Checklist of the Orchidaceae of Bosnia and Herzegovina. Phyton-Ann. Rei Bot. 2021, 61, 83–95. [Google Scholar]
- Domozetski, L. New data on the orchids (Orchidaceae) in Bulgaria. Phytol. Balc. 2024, 30, 203–224. [Google Scholar] [CrossRef]
- Dolinar, B. Kukavičke v Sloveniji; Pipinova knjiga: Ljubljana, Slovenia, 2015. (In Slovenian) [Google Scholar]
- Hristovski, S.; Ćušterevska, R.; Trenčeva, M. Traunsteinera globosa (L.) Rchb. (Orchidaceae), a new species for the flora of the Republic of North Macedonia. Acta Mus. Maced. Sci. Nat. 2022, 25, 79–82. [Google Scholar]
- Mišić, V. Klisure i kanjoni kao refugijumi reliktne vegetacije i njihov značaj za nauku i praksu. In Vegetacija SR Srbije 1; Janković, M., Pantić, N., Mišić, V., Diklić, N., Gajić, M., Eds.; Srpska akademija nauka i umetnosti: Beograd, Serbia, 1984; pp. 268–278. (In Serbian) [Google Scholar]
- Večeřa, M.; Divíšek, J.; Lenoir, J.; Jiménez-Alfaro, B.; Biurrun, I.; Knollová, I.; Agrillo, E.; Campos, J.A.; Čarni, A.; Crespo Jiménez, G.; et al. Alpha diversity of vascular plants in European forests. J. Biogeogr. 2019, 46, 1919–1935. [Google Scholar] [CrossRef]
- Wittlinger, L.; Petrikovičová, L. Phytogeographical Analysis and Ecological Factors of the Distribution of Orchidaceae Taxa in the Western Carpathians (Local study). Plants 2021, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, A.M. Litter quality effects of beech and hornbeam on undergrowth species diversity in Luxembourg forests on limestone and decalcified marl. J. Veg. Sci. 2010, 21, 248–261. [Google Scholar] [CrossRef]
- Molnár, A. (Ed.) Atlas of Hungarian Orchids; Kossuth Kiadó: Budapest, Hungary, 2011. (In Hungarian) [Google Scholar]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Forests: Vegetation Ecology of Central Europe; Springer International Publishing: Cham, Switzerland, 2017; Volume I. [Google Scholar]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Stevanović, V. Orchid species richness and composition in relation to vegetation types. Wulfenia 2020, 27, 183–210. [Google Scholar]
- Tsiftsis, S.; Karagiannakidou, V.; Tsiripidis, I. The orchid flora of East Macedonia (NE Greece). J. Eur. Orch. 2007, 39, 489–526. [Google Scholar]
- Landi, M.; Frignani, F.; Lazzeri, C.; Angiolini, C. Abundance of orchids on calcareous grasslands in relation to community species, environmental, and vegetation conditions. Russ. J. Ecol. 2009, 40, 486–494. [Google Scholar] [CrossRef]
- Procházka, A.; Mikita, T.; Jelínek, P. The Relationship between some Forest Stand Properties and the Occurrence of Orchids in the Central Part of the Moravian Karst Protected Landscape Area. Acta Univ. Agric. Silvic. Mendel. Brun. 2017, 65, 919–931. [Google Scholar] [CrossRef]
- Mikavica, I.; Ranđelović, D.; Djordjević, V.; Rakić, T.; Gajić, G.; Mutić, J. Concentration and mobility of trace elements (Li, Ba, Sr, Ag, Hg, B) and macronutrients (Ca, Mg, K) in soil-orchid system on different bedrock types. Environ. Sci. Pollut. Res. 2023, 30, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, K.; Mišljenović, T.; Djordjević, V.; van der Ent, A.; Ćosić, M.; Andrejić, G.; Šinžar-Sekulić, J. Elemental and ecophysiological profiles of orchid Dactylorhiza sambucina show distinct responses to contrasting geological substrates. Flora 2023, 303, 152276. [Google Scholar] [CrossRef]
- Djordjević, V.; Tomović, G.; Lakušić, D. Epipactis purpurata Sm. (Orchidaceae)—A new species in the flora of Serbia. Arch. Biol. Sci. 2010, 62, 1175–1180. [Google Scholar] [CrossRef]
- Djordjević, V.; Jakovljević, K.; Stevanović, V. Three Taxa of Epipactis (Orchidaceae–Epidendroideae) New for the Flora of Serbia. Phyton—Ann. Rei Bot. 2016, 56, 77–89. [Google Scholar]
- Djordjević, V. Epipactis muelleri (Orchidaceae-Neottieae), a Species New to the Flora of Serbia. Phyton—Ann. Rei Bot. 2016, 56, 303–312. [Google Scholar]
- McKendrick, S.L.; Leake, J.R.; Taylor, D.L.; Read, D.J. Symbiotic germination and development of the mycoheterotrophic orchid Neottia nidus-avis in nature and its requirement for locally distributed Sebacina spp. New Phytol. 2002, 154, 233–247. [Google Scholar] [CrossRef]
- Selosse, M.A.; Faccio, A.; Scappaticci, G.; Bonfante, P. Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microb. Ecol. 2004, 47, 416–426. [Google Scholar] [CrossRef]
- Girlanda, M.; Selosse, M.A.; Cafasso, D.; Brilli, F.; Delfine, S.; Fabian, R.; Ghignone, S.; Pinelli, P.; Segreto, R.; Loreto, F.; et al. Inefficient photosynthesis in the Mediterranean orchid Limodorum abortivum is mirrored by specific association to ectomycorrhizal Russulaceae. Mol. Ecol. 2006, 15, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Pillon, Y.; Fay, M.F.; Shipunov, A.B.; Chase, M.W. Species diversity versus phylogenetic diversity: A practical study in the taxonomically difficult genus Dactylorhiza (Orchidaceae). Biol. Conserv. 2006, 129, 4–13. [Google Scholar] [CrossRef]
- Øien, D.-I.; Moen, A. Flowering and survival of Dactylorhiza lapponica and Gymnadenia conopsea in the Solendet Nature Reserve, Central Norway. In Trends and Fluctuations and Underlying Mechanisms in Terrestrial Orchid Populations; Kindlmann, P., Willems, J.H., Whigham, D.F., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2002; pp. 3–22. [Google Scholar]
- Rasmussen, H.N.; Dixon, K.W.; Jersáková, J.; Těšitelová, T. Germination and seedling establishment in orchids: A complex of requirements. Ann. Bot. 2015, 116, 391–402. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Li, J.; Qin, J.; Zhang, W.; Huang, W.; Hu, H. Physiological diversity of orchids. Plant Divers. 2018, 40, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Light, M.H.S.; MacConaill, M. Factors affecting germinable seed yield in Cypripedium calceolus var. pubescens (Willd.) Correll and Epipactis helleborine (L.) Crantz (Orchidaceae). Bot. J. Linn. Soc. 1998, 126, 3–26. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Terrestrial Orchids from Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Blinova, I.V. Populations of orchids at the northern limit of their distribution (Murmansk Oblast): Effect of climate. Russ. J. Ecol. 2008, 39, 26–33. [Google Scholar] [CrossRef]
- Arditti, J.; Ghani, A.K.A. Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef]
- Fekete, R.; Bódis, J.; Fülöp, B.; Süveges, K.; Urgyán, R.; Malkócs, T.; Vincze, O.; Silva, L.; Molnár, V.A. Roadsides provide refuge for orchids: Characteristic of the surrounding landscape. Ecol. Evol. 2020, 10, 13236–13247. [Google Scholar] [CrossRef]
- Ballantyne, M.; Pickering, C. Ecotourism as a threatening process for wild orchids. J. Ecotourism. 2012, 11, 34–47. [Google Scholar] [CrossRef]
- Urban, M.C. Climate change extinctions. Science 2024, 386, 1123–1128. [Google Scholar] [CrossRef]
- Carolli, M.; de Leaniz, C.G.; Jones, J.; Belletti, B.; Huđek, H.; Pusch, M.; Pandakov, P.; Börger, L.; van de Bund, W. Impacts of existing and planned hydropower dams on river fragmentation in the Balkan Region. Sci. Total Environ. 2023, 871, 161940. [Google Scholar] [CrossRef]
- Wang, X.M.; Tang, Y.; Peng, X.F.; Wang, J.; Zhang, S.Q.; Feng, Y.; Peng, P.H. Identifying priorities under highly heterogeneous environments through species distribution models to facilitate orchid conservation. Biodivers. Conserv. 2024, 33, 647–665. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K.W. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef]
| Orchid Taxon | Belowground Organ Types |
|---|---|
| Anacamptis coriophora (L.) R.M.Bateman, Pridgeon & M.W.Chase subsp. coriophora | O-TUB |
| Anacamptis morio (L.) R.M.Bateman, Pridgeon & M.W.Chase subsp. morio | O-TUB |
| Anacamptis morio subsp. caucasica (K.Koch) H.Kretzschmar, Eccarius & H.Dietr. | O-TUB |
| Anacamptis palustris subsp. elegans (Heuff.) R.M.Bateman, Pridgeon & M.W.Chase | O-TUB |
| Anacamptis papilionacea (L.) R.M.Bateman, Pridgeon & M.W.Chase subsp. papilionacea | O-TUB |
| Anacamptis pyramidalis (L.) Rich. | O-TUB |
| Cephalanthera damasonium (Mill.) Druce | R |
| Cephalanthera longifolia (L.) Fritsch | R |
| Cephalanthera rubra (L.) Rich. | R |
| Coeloglossum viride (L.) Hartm. | P-TUB |
| Corallorhiza trifida Châtel. | R |
| Dactylorhiza cordigera (Fr.) Soó subsp. cordigera | P-TUB |
| Dactylorhiza fuchsii (Druce) Soó subsp. fuchsii | P-TUB |
| Dactylorhiza incarnata (L.) Soó subsp. incarnata | P-TUB |
| Dactylorhiza maculata (L.) Soó subsp. maculata | P-TUB |
| Dactylorhiza maculata subsp. transsilvanica (Schur) Soó | P-TUB |
| Dactylorhiza saccifera (Brongn.) Soó subsp. saccifera | P-TUB |
| Dactylorhiza sambucina (L.) Soó | P-TUB |
| Epipactis atrorubens (Hoffm.) Besser | R |
| Epipactis distans Arv.-Touv. | R |
| Epipactis helleborine (L.) Crantz subsp. helleborine | R |
| Epipactis leptochila subsp. neglecta Kümpel | R |
| Epipactis microphylla (Ehrh.) Sw. | R |
| Epipactis muelleri Godfery subsp. muelleri | R |
| Epipactis palustris (L.) Crantz | R |
| Epipactis pontica Taubenheim | R |
| Epipactis purpurata Sm. | R |
| Epipogium aphyllum Sw. | R |
| Goodyera repens (L.) R.Br. | R |
| Gymnadenia conopsea (L.) R.Br. | P-TUB |
| Gymnadenia frivaldii Hampe ex Griseb. | P-TUB |
| Gymnadenia odoratissima (L.) Rich. | P-TUB |
| Himantoglossum calcaratum (Beck) Schltr. subsp. calcaratum | O-TUB |
| Limodorum abortivum (L.) Sw. | R |
| Neotinea tridentata (Scop.) R.M.Bateman, Pridgeon & M.W.Chase subsp. tridentata | O-TUB |
| Neotinea ustulata (L.) R.M.Bateman, Pridgeon & M.W.Chase | O-TUB |
| Neottia cordata (L.) Rich. | R |
| Neottia nidus-avis (L.) Rich. | R |
| Neottia ovata (L.) Bluff & Fingerh. | R |
| Nigritella rhellicani Teppner & E.Klein | P-TUB |
| Ophrys apifera Huds. | O-TUB |
| Ophrys insectifera L. subsp. insectifera | O-TUB |
| Ophrys scolopax subsp. cornuta (Steven) E.G.Camus | O-TUB |
| Ophrys sphegodes Mill. subsp. sphegodes | O-TUB |
| Orchis mascula subsp. speciosa (Mutel) Hegi | O-TUB |
| Orchis militaris L. subsp. militaris | O-TUB |
| Orchis pallens L. | O-TUB |
| Orchis purpurea Huds. subsp. purpurea | O-TUB |
| Orchis simia Lam. subsp. simia | O-TUB |
| Platanthera bifolia (L.) Rich. | P-TUB |
| Platanthera chlorantha (Custer) Rchb | P-TUB |
| Pseudorchis albida (L.) Á.Löve & D.Löve | P-TUB |
| Spiranthes spiralis (L.) Chevall. | O-TUB |
| Traunsteinera globosa (L.) Rchb. | O-TUB |
| Environmental Factors | The Importance of Environmental Factors in the Random Forest Model | ||||
|---|---|---|---|---|---|
| Total Number of Orchids | Rhizomatous Orchids | Palmate Tuberous Orchids | Ovoid Tuberous Orchids | ||
| Altitudinal factors | Altitudinal range | 3 | 2 | Not important | Not important |
| Maximum altitude | Not important | Not important | 1 | Not important | |
| Climatic factors | Minimum values of mean temperature of driest quarter (BIO9) | Not important | Not important | 4 | Not important |
| Habitat types expressed through associative names of vegetation units | Abieti-Fagenion | 1 | 3 | 2 | Not important |
| Ostryo-Carpinion orientalis | 2 | 1 | Not important | 1 | |
| Pinion nigrae | 4 | 4 | Not important | 3 | |
| Vaccinio-Piceetea | Not important | Not important | 3 | Not important | |
| Asplenietea trichomanis | Not important | Not important | Not important | 2 | |
| Molinio-Arrhenatheretea | Not important | Not important | Not important | 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djordjević, V.; Lakušić, D.; Novković, I.; Stevanović, V.; Tsiftsis, S. Factors Influencing Orchid Species Richness in the Central Balkans: The Importance of Belowground Organ Types. Plants 2025, 14, 443. https://doi.org/10.3390/plants14030443
Djordjević V, Lakušić D, Novković I, Stevanović V, Tsiftsis S. Factors Influencing Orchid Species Richness in the Central Balkans: The Importance of Belowground Organ Types. Plants. 2025; 14(3):443. https://doi.org/10.3390/plants14030443
Chicago/Turabian StyleDjordjević, Vladan, Dmitar Lakušić, Ivan Novković, Vladimir Stevanović, and Spyros Tsiftsis. 2025. "Factors Influencing Orchid Species Richness in the Central Balkans: The Importance of Belowground Organ Types" Plants 14, no. 3: 443. https://doi.org/10.3390/plants14030443
APA StyleDjordjević, V., Lakušić, D., Novković, I., Stevanović, V., & Tsiftsis, S. (2025). Factors Influencing Orchid Species Richness in the Central Balkans: The Importance of Belowground Organ Types. Plants, 14(3), 443. https://doi.org/10.3390/plants14030443







