Metabolic and Nutritional Responses of Contrasting Aluminium-Tolerant Banana Genotypes Under Al Stress
Abstract
1. Introduction
2. Results
2.1. Plant Growth After Al Treatment
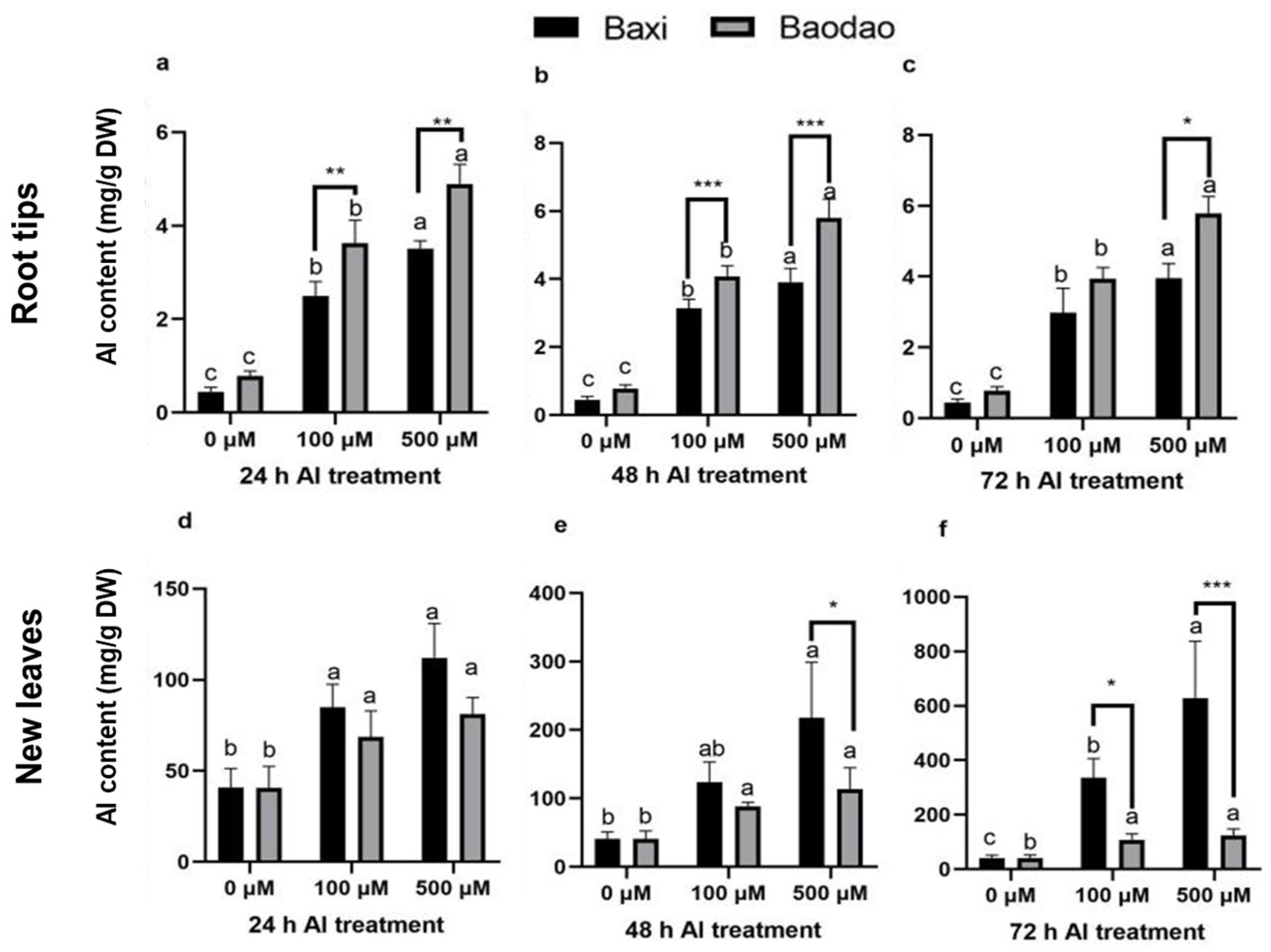
2.2. Al Uptake and Distribution in Banana Tissues
2.3. Enzyme Activities Related to Carbon Metabolism in Root Tips
2.4. Primary Metabolites Alteration
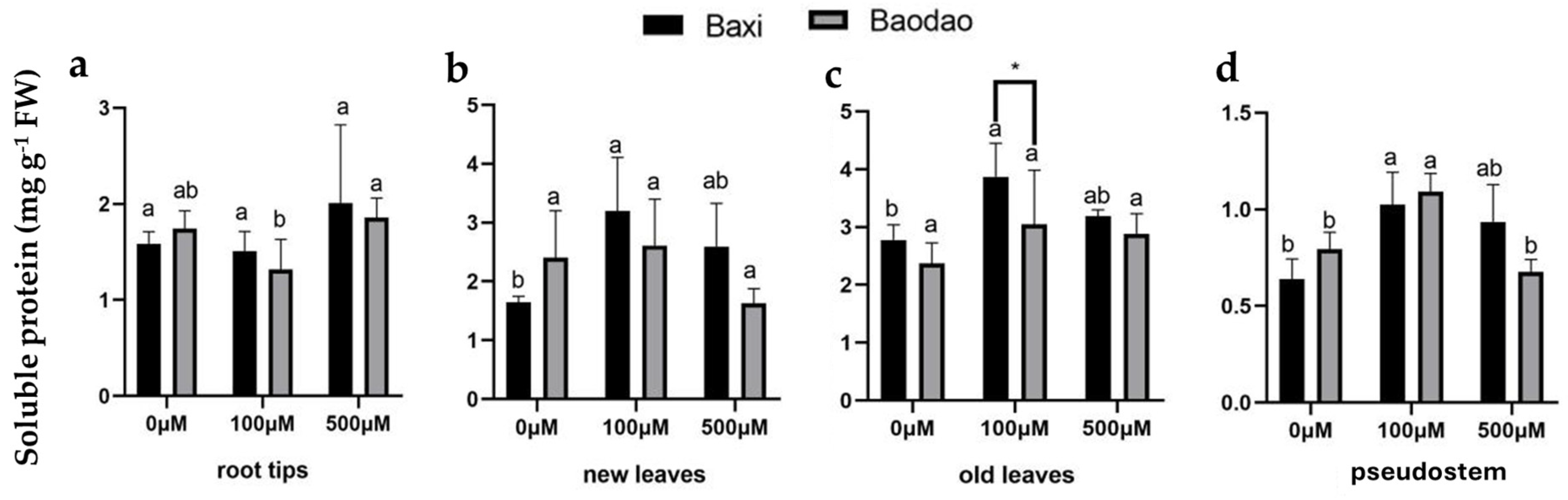
2.4.1. Soluble Protein Content
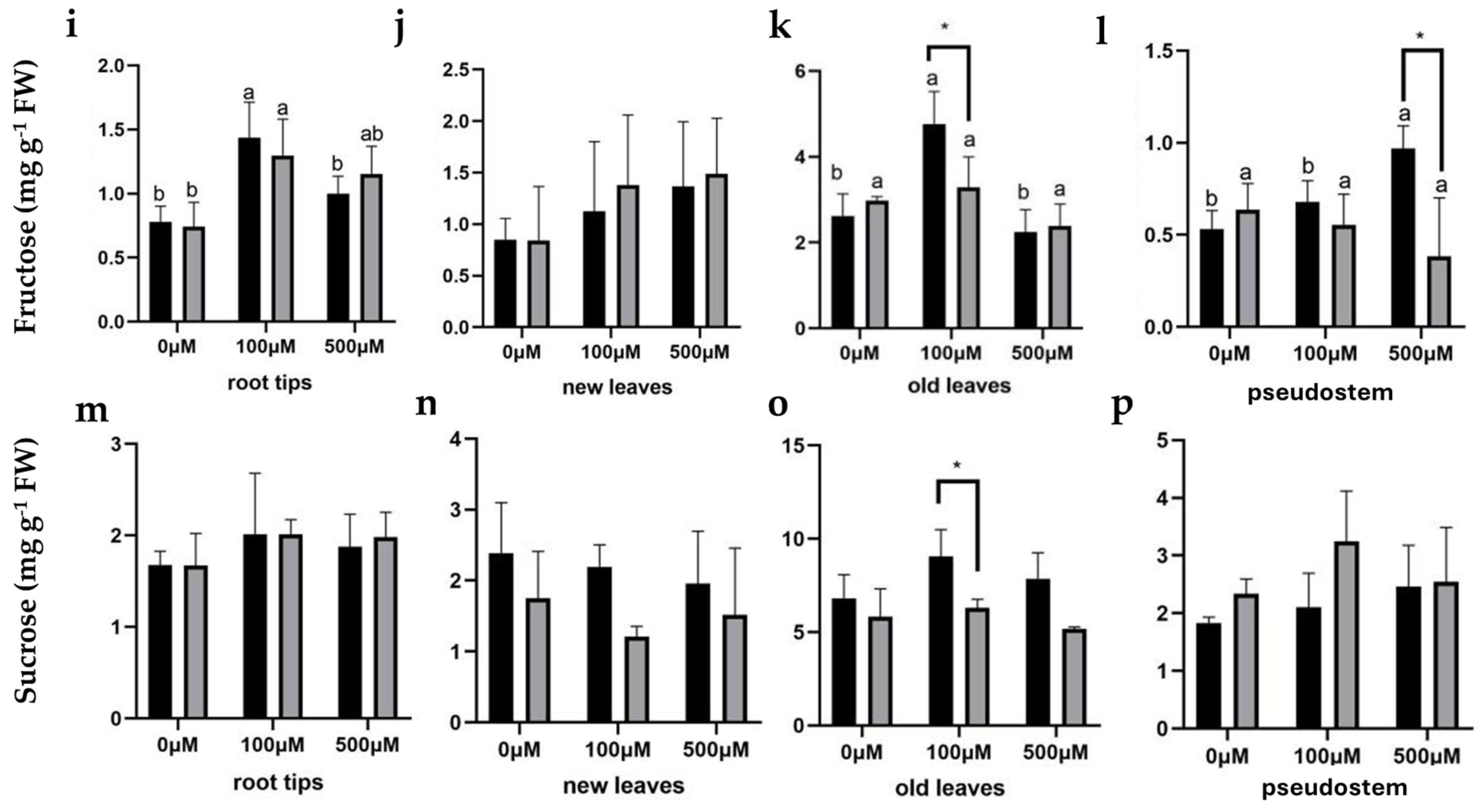
2.4.2. Carbohydrates
2.5. Mineral Nutrient Content Changes in the Tissues of Banana Genotypes with Al Treatment
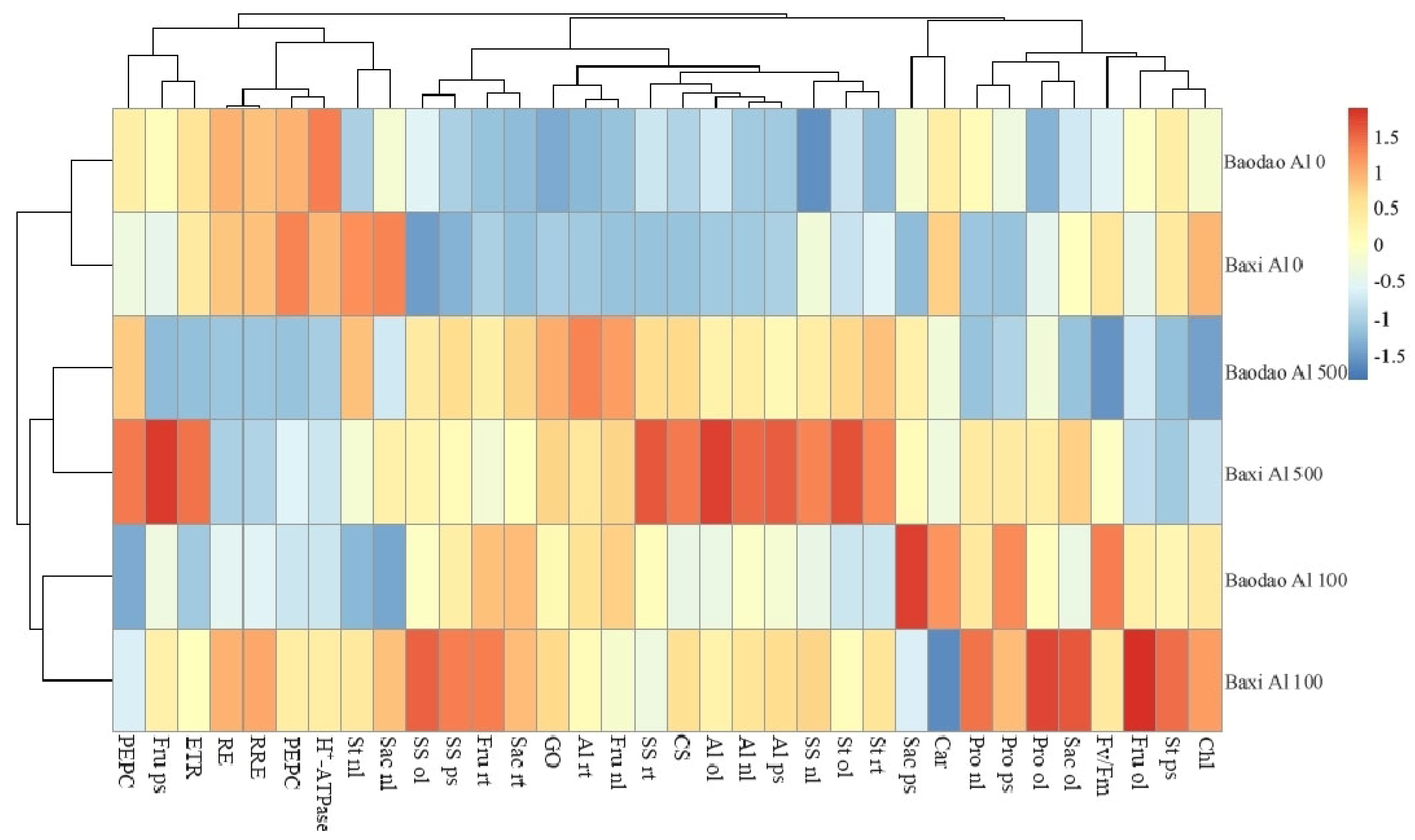
2.6. Heat Map of the Carbohydrate Metabolism Enzymes, Metabolites, and Root Elongation
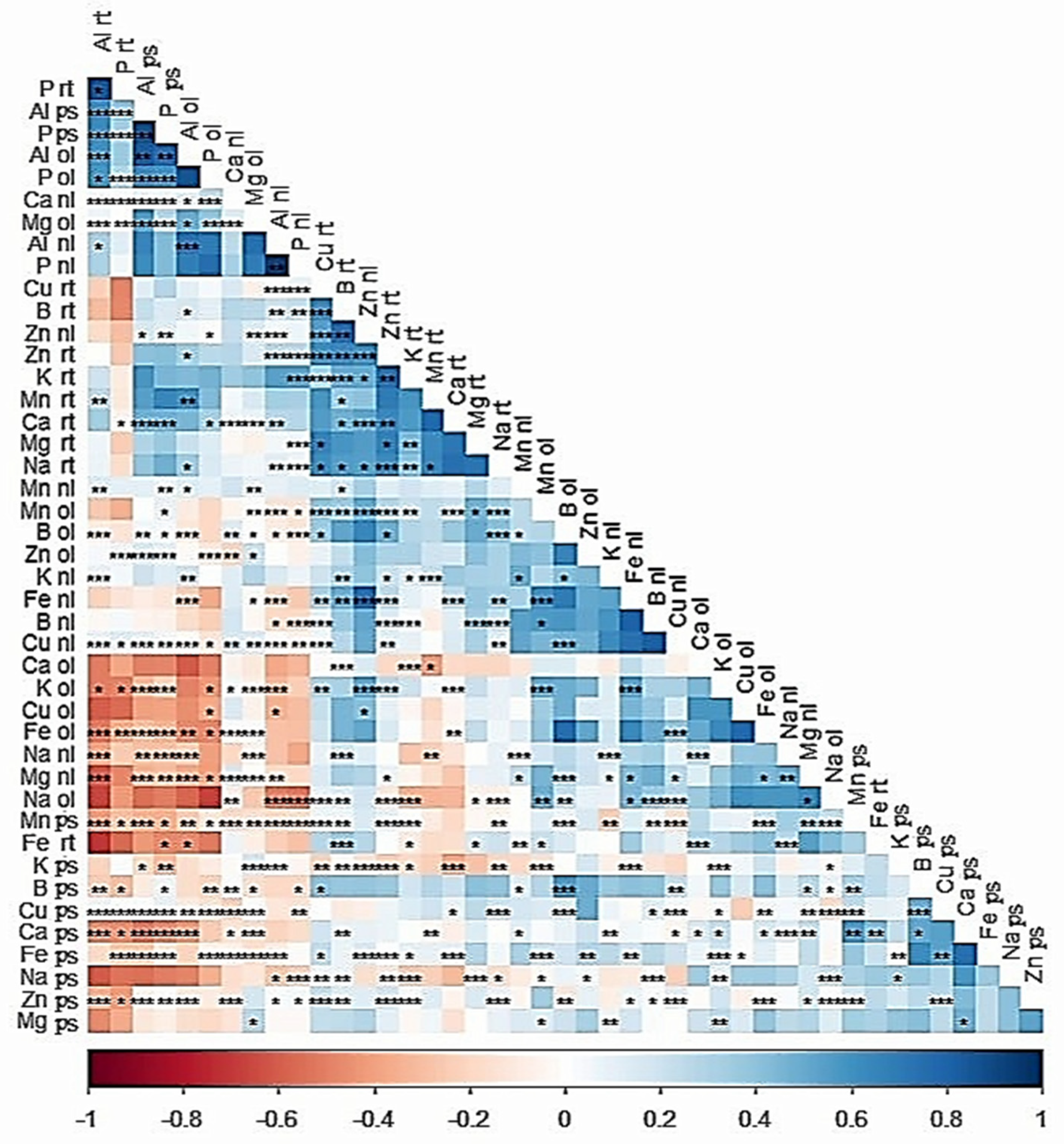
2.7. Correlation of Mineral Nutrient Content in Tissues
3. Discussion
3.1. Differential Responses of Badao and Baxi Genotypes to Al Stress
3.2. The Al-Sensitive Genotype Accumulates More Al in Root Tips with Limited Translocation
3.3. Tolerant Genotype Showed More Efficient and Regulated Metabolic Adjustment
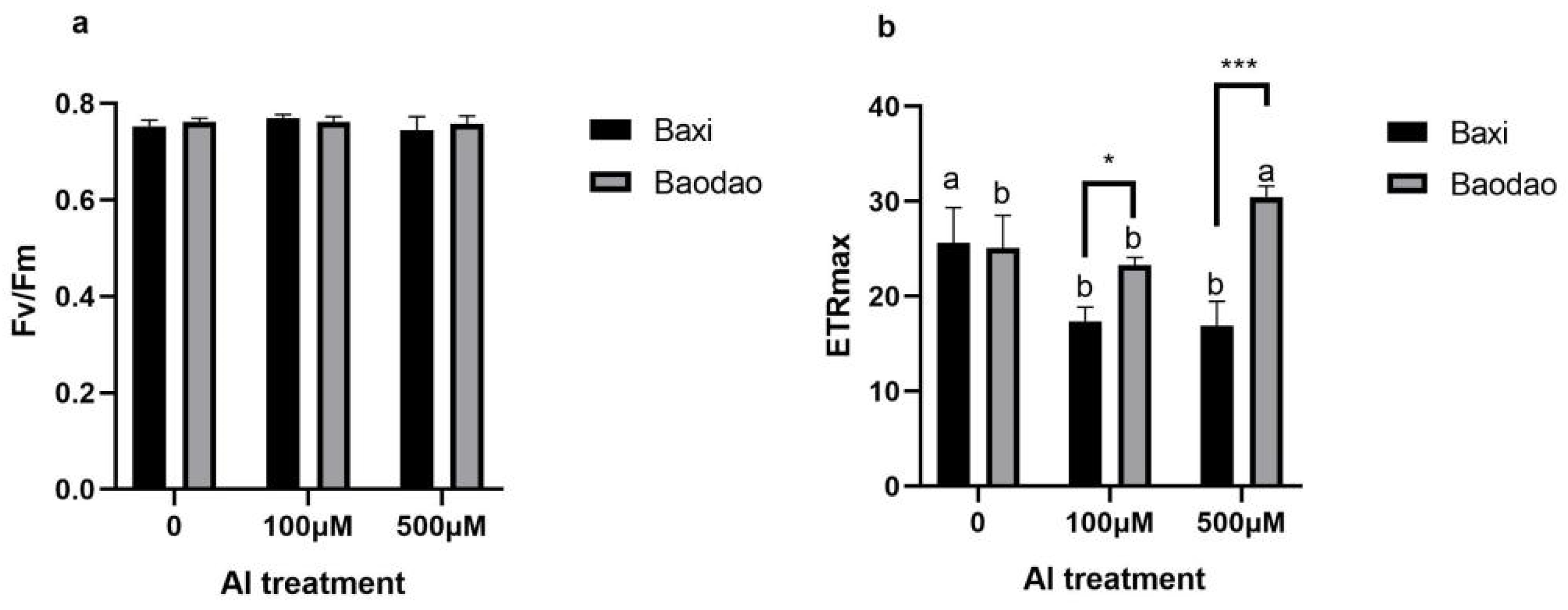
3.4. Tolerant Genotype Modulates ETRmax as a Protective Strategy Without Compromising PSII Efficiency
3.5. Prolonged Al Exposure and Increased Concentration Induce Greater Nutrient Imbalance in the Sensitive Banana Genotype
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Root Length and Elongation Rate Measurement
4.3. Carbon Metabolism Enzyme Activity Assay
4.4. Soluble Protein Content Assay
4.5. Soluble Sugar, Sucrose, Fructose, and Starch Content Measurement
4.5.1. Soluble Sugar
4.5.2. Sucrose
4.5.3. Fructose
4.5.4. Starch
4.6. Chlorophyll Fluorescence Measurement
4.7. Al and Nutrient Content Determination
5. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, C.; Shabala, S.; Chen, Z.H.; Zhou, M.; Zhao, C. Aluminium tolerance and stomata operation: Towards optimising crop performance in acid soil. Plant Physiol. Biochem. 2024, 210, 108626. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Oleg, Y.; Darya, S.; Denis, K.; Anna, S.; Tatiana, A.; Edgar, S.; Vera, S.; Andrey, B. Beneficial aluminium immobilizing microorganisms inhabiting the rhizosphere of pea. Biol. Commun. 2023, 68, 74–85. [Google Scholar]
- Nogueirol, R.C.; Monteiro, F.A.; Gratão, P.L.; Borgo, L.; Azevedo, R.A. Tropical Soils with High Aluminum Concentrations Cause Oxidative Stress in Two Tomato Genotypes. Environ. Monit. Assess. 2015, 187, 73. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Change Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, W.; Li, C.; Chang, W.; Zhang, S.; Zeng, Y.; Zeng, C.; Peng, M. Effect of different rates of nitrogen fertilization on crop yield, soil properties and leaf physiological attributes in banana under subtropical regions of China. Front. Plant Sci. 2020, 11, 613760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bei, S.; Li, B.; Zhang, J.; Christie, P.; Li, X. Organic Fertilizer, but Not Heavy Liming, Enhances Banana Biomass, Increases Soil Organic Carbon and Modifies Soil Microbiota. Appl. Soil. Ecol. 2019, 136, 67–79. [Google Scholar] [CrossRef]
- Rahman, R.; Upadhyaya, H. Aluminium Toxicity and Its Tolerance in Plant: A Review. J. Plant Biol. 2021, 64, 101–121. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic Loss of Inorganic Carbon by Nitrogen-induced Soil Acidification in Chinese Croplands. Glob. Change Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef] [PubMed]
- Ningombam, L.; Hazarika, B.N.; Singh, Y.D.; Singh, R.P.; Yumkhaibam, T. Aluminium stress tolerance by Citrus plants: A consolidated review. Physiol. Mol. Biol. Plants 2024, 30, 705–718. [Google Scholar] [CrossRef]
- Hu, Y.; Khan, S.; Yin, L.; Tang, H.; Huang, J. Investigating aluminum toxicity effects on callose deposition, oxidative stress, and nutrient homeostasis in banana genotypes. Environ. Sci. Pollut. Res. 2024, 31, 31287–31303. [Google Scholar] [CrossRef] [PubMed]
- Rufyikiri, G.; Dufey, J.E.; Nootens, D.; Delvaux, B. Effect of aluminium on bananas (Musa Spp.) cultivated in acid solutions. II. Water and nutrient uptake. Fruits 2001, 56, 5–16. [Google Scholar] [CrossRef]
- Li, D.; Shu, Z.; Ye, X.; Zhu, J.; Pan, J.; Wang, W.; Chang, P.; Cui, C.; Shen, J.; Fang, W.; et al. Cell wall pectin methyl-esterification and organic acids of root tips involve in aluminum tolerance in Camellia sinensis. Plant Physiol. Biochem. 2017, 119, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ullah, S.; Xu, Y.; Bai, T.; Ye, S.; Jiang, W.; Yang, M. Effects of Elevated Aluminum Concentration and Distribution on Root damage, cell wall polysaccharides, and nutrient uptake in different tolerant eucalyptus clones. Int. J. Mol. Sci. 2022, 23, 13438. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Friend, A.L. Aluminum fractions in root tips of slash pine and loblolly pine families differing in Al resistance. Tree Physiol. 2005, 25, 245–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barceló, J.; Poschenrieder, C. Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: A review. Environ. Exp. Bot. 2002, 48, 75–92. [Google Scholar] [CrossRef]
- Yan, L.; Riaz, M.; Liu, J.; Yu, M.; Cuncang, J. The aluminum tolerance and detoxification mechanisms in plants; recent advances and prospects. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1491–1527. [Google Scholar] [CrossRef]
- Varghese, E.M.; Kour, B.; Ramya, S.; Kumar, N.S.; Jisha, M.S.; Ramakrishnan, B. Chapter 25—Rhizosphere microbe-mediated alleviation of aluminum and iron toxicity in acidic soils. In Rhizosphere Engineering; Dubey, R.C., Kumar, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 499–526. ISBN 978-0-323-89973-4. [Google Scholar]
- Silva, S.; Pinto-Carnide, O.; Martins-Lopes, P.; Matos, M.; Guedes-Pinto, H.; Santos, C. Differential aluminium changes on nutrient accumulation and root differentiation in an al sensitive vs. tolerant wheat. Environ. Exp. Bot. 2010, 68, 91–98. [Google Scholar] [CrossRef]
- Mohanty, S.; Das, A.B.; Das, P.; Mohanty, P. Effect of a low dose of aluminum on mitotic and meiotic activity, 4C DNA content, and pollen sterility in rice, Oryza sativa L. Cv. Lalat. Ecotoxicol. Environ. Saf. 2004, 59, 70–75. [Google Scholar] [CrossRef]
- Chakraborty, N.; Das, A.; Pal, S.; Roy, S.; Sil, S.; Adak, M.; Hasanuzzaman, M. Exploring Aluminum tolerance mechanisms in plants with reference to rice and Arabidopsis: A comprehensive review of genetic, metabolic, and physiological adaptations in acidic soils. Plants 2024, 13, 1760. [Google Scholar] [CrossRef]
- Furlan, F.; Borgo, L.; Rabêlo, F.H.S.; Rossi, M.L.; Martinelli, A.P.; Azevedo, R.A.; Lavres, J. Aluminum-induced stress differently modifies Urochloa genotypes responses on growth and regrowth: Root-to-shoot al-translocation and oxidative stress. Theor. Exp. Plant Physiol. 2018, 30, 141–152. [Google Scholar] [CrossRef]
- Hayes, K.L.; Mui, J.; Song, B.; Sani, E.S.; Eisenman, S.W.; Sheffield, J.B.; Kim, B. Effects, uptake, and translocation of aluminum oxide nanoparticles in lettuce: A comparison study to phytotoxic aluminum ions. Sci. Total Environ. 2020, 719, 137393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, W.; Cao, Y.; Cai, Y.; Lyi, S.M.; Wu, W.; Kang, Y.; Liang, C.; Liu, J. An exclusion mechanism is epistatic to an internal detoxification mechanism in aluminum resistance in Arabidopsis. BMC Plant Biol. 2020, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z. Uptake of aluminium by plant cells. New Phytol. 1996, 134, 389–406. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Li, D.; Jia, X.; Zhou, D.; Li, J.; Lyi, S.M.; Hou, S.; Huang, Y.; Kochian, L.V.; et al. NIP1;2 is a plasma membrane-localized transporter mediating aluminum uptake, translocation, and tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 5047–5052. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Kocjan, A.; Kwasniewska, J.; Szurman-Zubrzycka, M. Understanding plant tolerance to aluminum: Exploring mechanisms and perspectives. Plant Soil 2024, 1–25. [Google Scholar] [CrossRef]
- Chen, L.-S.; Tang, N.; Jiang, H.-X.; Yang, L.-T.; Li, Q.; Smith, B.R. Changes in organic acid metabolism differ between roots and leaves of citrus grandis in response to phosphorus and aluminum interactions. J. Plant Physiol. 2009, 166, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
- Miyao, M.; Fukayama, H. Metabolic consequences of overproduction of phosphoenolpyruvate carboxylase in C3 plants. Arch. Biochem. Biophys. 2003, 414, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Doubnerová, V.; Ryšlavá, H. What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci. 2011, 180, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, M.R.; Ward, C.L.; Botha, F.C.; Valentine, A.J. Routes of pyruvate synthesis in phosphorus-deficient lupin roots and nodules. New Phytol. 2006, 169, 399–408. [Google Scholar] [CrossRef]
- Osaki, M.; Nursyamsi, D.; Begum, H.H.; Watanabe, T. Study on aluminium resistance in relation to organic-acid anion exudation from roots of PEPC transgenic rice plants. In Plant Nutrition; Horst, W.J., Schenk, M.K., Bürkert, A., Claassen, N., Flessa, H., Frommer, W.B., Goldbach, H., Olfs, H.-W., Römheld, V., Sattelmacher, B., et al., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2001; pp. 514–515. ISBN 978-0-7923-7105-2. [Google Scholar]
- Wang, Q.; Yi, Q.; Hu, Q.; Zhao, Y.; Nian, H.; Li, K.; Yu, Y.; Izui, K.; Chen, L. Simultaneous overexpression of citrate synthase and phosphoenolpyruvate carboxylase in leaves augments citrate exclusion and al resistance in transgenic tobacco. Plant Mol. Biol. Rep. 2012, 30, 992–1005. [Google Scholar] [CrossRef]
- Rangel, A.F.; Rao, I.M.; Braun, H.-P.; Horst, W.J. Aluminum resistance in common bean (Phaseolus vulgaris) involves induction and maintenance of citrate exudation from root apices. Physiol. Plant. 2010, 138, 176–190. [Google Scholar] [CrossRef]
- Ermolayev, V. Comparison of Al-induced gene expression in sensitive and tolerant soybean cultivars. J. Exp. Bot. 2003, 54, 2745–2756. [Google Scholar] [CrossRef]
- Li, W.; Finnegan, P.M.; Dai, Q.; Guo, D.; Yang, M. Metabolic Acclimation Supports Higher Aluminium-induced secretion of citrate and malate in an aluminium-tolerant hybrid clone of eucalyptus. BMC Plant Biol. 2021, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Efraín Ramírez-Benítez, J.; Chee-González, L.; Teresa Hernandez-Sotomayor, S.M. Aluminium induces changes in organic acids metabolism in Coffea arabica suspension cells with differential Al-tolerance. J. Inorg. Biochem. 2008, 102, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.R.; Novais, R.F.; Jham, G.N.; Barros, N.F.; Gebrim, F.O.; Nunes, F.N.; Neves, J.C.L.; Leite, F.P. Responses of eucalypt species to aluminum: The possible involvement of low molecular weight organic acids in the al tolerance mechanism. Tree Physiol. 2004, 24, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Moneim, D.; Contreras, R.; Silva-Navas, J.; Gallego, F.J.; Figueiras, A.M.; Benito, C. On the consequences of aluminium stress in rye: Repression of two mitochondrial malate dehydrogenase mRNAs. Plant Biol. J. 2015, 17, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhu, H.; Wen, S.; Liu, L.; Gou, L.; Guo, Z. Citrate synthesis and exudation confer al resistance in alfalfa (Medicago sativa L.). Plant Soil. 2020, 449, 319–329. [Google Scholar] [CrossRef]
- Yang, Z.M.; Nian, H.; Sivaguru, M.; Tanakamaru, S.; Matsumoto, H. Characterization of aluminium-induced citrate secretion in aluminium-tolerant soybean (Glycine max) plants. Physiol. Plant. 2001, 113, 64–71. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.; Li, D.; Kong, X.; Rengel, Z.; Chen, L.; Yang, Y.; Cui, X.; Chen, Q. The role of the plasma membrane H+-ATPase in plant responses to aluminum toxicity. Front. Plant Sci. 2017, 8, 1757. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Q.L.; Geng, M.J.; Guo, Z.H.; Zhao, Z. Rhizosphere pH difference regulated by plasma membrane H+-ATPase is related to differential Al tolerance of two wheat cultivars. Plant Soil. Environ. 2011, 57, 201–206. [Google Scholar] [CrossRef]
- Zelitch, I.; Schultes, N.P.; Peterson, R.B.; Brown, P.; Brutnell, T.P. High Glycolate oxidase activity is required for survival of maize in normal air. Plant Physiol. 2009, 149, 195–204. [Google Scholar] [CrossRef]
- Zhang, J.; He, Z.; Tian, H.; Zhu, G.; Peng, X. Identification of aluminium-responsive genes in rice cultivars with different aluminium sensitivities. J. Exp. Bot. 2007, 58, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Jin, Y.L.; Jung, W.J.; Avice, J.C.; Morvan-Bertrand, A.; Ourry, A.; Park, C.W.; Kim, T.H. Water-Deficit Accumulates Sugars by Starch Degradation—Not by De Novo Synthesis—In White Clover Leaves (Trifolium repens). Physiol. Plant 2008, 134, 403–411. [Google Scholar] [CrossRef]
- Giannakoula, A.; Moustakas, M.; Mylona, P.; Papadakis, I.; Yupsanis, T. Aluminum tolerance in maize is correlated with increased levels of mineral nutrients, carbohydrates and proline, and decreased levels of lipid peroxidation and Al accumulation. J. Plant Physiol. 2008, 165, 385–396. [Google Scholar] [CrossRef]
- Mishra, P.; Dubey, R.S. Effect of aluminium on metabolism of starch and sugars in growing rice seedlings. Acta Physiol. Plant 2008, 30, 265–275. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Rehman, R.U.; Hakeem, K.R.; Alharby, H.F. Aluminium stress modulates the osmolytes and enzyme defense system in Fagopyrum species. Plant Physiol. Biochem. 2019, 144, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Alvim, M.N.; Ramos, F.T.; Oliveira, D.C.; Isaias, R.; França, M.G.C. Aluminium localization and toxicity symptoms related to root growth inhibition in rice (Oryza sativa L.) seedlings. J. Biosci. 2012, 37, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Rabêlo, F.H.S.; Fernie, A.R.; Navazas, A.; Borgo, L.; Keunen, E.; Silva, B.K.D.A.D.; Cuypers, A.; Lavres, J. A glimpse into the effect of sulfur supply on metabolite profiling, glutathione and phytochelatins in Panicum maximum Cv. Massai exposed to cadmium. Environ. Exp. Bot. 2018, 151, 76–88. [Google Scholar] [CrossRef]
- Cunha, A.R.D.; Ambrósio, A.D.S.; Wolowski, M.; Westin, T.B.; Govêa, K.P.; Carvalho, M.; Barbosa, S. Negative effects on photosynthesis and chloroplast pigments exposed to lead and aluminum: A meta-analysis. Cerne 2020, 26, 232–237. [Google Scholar] [CrossRef]
- Júnior, E.M.F.; Cambraia, J.; Ribeiro, C.; Oliva, M.A.; Oliveira, J.A.; DaMATTA, F.M. The effects of aluminium on the photosynthetic apparatus of two rice cultivars. Exp. Agric. 2014, 50, 343–352. [Google Scholar] [CrossRef]
- Sharma, V.; Yadav, M.; Kumari, N. Aluminium fluoride induced changes in chlorophyll a fluorescence, antioxidants and Psb A gene expression of Brassica juncea cultivars. J. Plant Interact. 2018, 13, 472–482. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Llugany, M.; Barceló, J. Short-term Effects of pH and aluminium on mineral nutrition in maize varieties differing in proton and aluminium tolerance. J. Plant Nutr. 1995, 18, 1495–1507. [Google Scholar] [CrossRef]
- Kichigina, N.E.; Puhalsky, J.V.; Shaposhnikov, A.I.; Azarova, T.S.; Makarova, N.M.; Loskutov, S.I.; Safronova, V.I.; Tikhonovich, I.A.; Vishnyakova, M.A.; Semenova, E.V.; et al. Aluminum exclusion from root zone and maintenance of nutrient uptake are principal mechanisms of Al tolerance in Pisum sativum L. Physiol. Mol. Biol. Plants 2017, 23, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Brito, D.S.; da Silva, A.A.; do Rosário Rosa, V.; Santos, M.F.S.; de Souza, G.A.; Azevedo, A.A.; Dal-Bianco, M.; Oliveira, J.A.; Ribeiro, C. Differential accumulation of aluminum in root tips of soybean seedlings. Braz. J. Bot. 2020, 43, 99–107. [Google Scholar] [CrossRef]
- Lidon, F.C.; Azinheira, H.G.; Barreiro, M.G. Aluminum toxicity in maize: Biomass production and nutrient uptake and translocation. J. Plant Nutr. 2000, 23, 151–160. [Google Scholar] [CrossRef]
- Gaume, A.; Mächler, F.; Frossard, E. Aluminum resistance in two cultivars of Zea mays L.: Root exudation of organic acids and influence of phosphorus nutrition. Plant Soil 2001, 234, 73–81. [Google Scholar] [CrossRef]
- Maron, L.G.; Kirst, M.; Mao, C.; Milner, M.J.; Menossi, M.; Kochian, L.V. Transcriptional profiling of aluminum toxicity and tolerance responses in maize roots. New Phytol. 2008, 179, 116–128. [Google Scholar] [CrossRef]
- Valle, S.R.; Pinochet, D.; Calderini, D.F. Uptake and Use Efficiency of N, P, K, Ca and Al by Al-sensitive and al-tolerant cultivars of wheat under a wide range of soil al concentrations. Field Crops Res. 2011, 121, 392–400. [Google Scholar] [CrossRef]
- Mariano, E.D.; Pinheiro, A.S.; Garcia, E.E.; Keltjens, W.G.; Jorge, R.A.; Menossi, M. Differential aluminium-impaired nutrient uptake along the root axis of two maize genotypes contrasting in resistance to aluminium. Plant Soil 2015, 388, 323–335. [Google Scholar] [CrossRef]
- Påhlsson, A.B. Influence of aluminium on biomass, nutrients, soluble carbohydrates and phenols in beech (Fagus Sylvatica). Physiol. Plant 1990, 78, 79–84. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yaphe, W.; Arsenault, G.P. Improved resorcinol reagent for the determination of fructose, and of 3,6-anhydrogalactose in polysaccharides. Anal. Biochem. 1965, 13, 143–148. [Google Scholar] [CrossRef]
- Han, H.; He, H.; Wu, Z.; Cong, Y.; Zong, S.; He, J.; Fu, Y.; Liu, K.; Sun, H.; Li, Y.; et al. Non-structural carbohydrate storage strategy explains the spatial distribution of treeline species. Plants 2020, 9, 384. [Google Scholar] [CrossRef]





| Genotype | Al (µM) | Time (h) | Root Elongation (cm) | Relative Elongation of Root (%) |
|---|---|---|---|---|
| Baodao | 0 | 24 | 0.38 ± 0.02 c | 100 ± 0.05 a |
| 48 | 0.67 ± 0.01 b | 100 ± 0.08 a | ||
| 72 | 0.95 ± 0.01 a | 100 ± 0.01 a | ||
| 100 | 24 | 0.19 ± 0.01 e | 41.62 ± 0.021 b | |
| 48 | 0.28 ± 0.01 d | 40.94 ± 0.010 b | ||
| 72 | 0.38± 0.01 c | 40.12 ± 0.008 b | ||
| 500 | 24 | 0.07± 0.02 g | 17.82 ± 0.02 c | |
| 48 | 0.18± 0.02 f | 17.31 ± 0.02 c | ||
| 72 | 0.17± 0.01 e | 17.68 ± 0.01 c | ||
| Baxi | 0 | 24 | 0.36 ± 0.01 c | 100 ± 0.01 b |
| 48 | 0.62 ± 0.01 b | 100 ± 0.02 b | ||
| 72 | 0.90 ± 0.01 a | 100 ± 0.02 b | ||
| 100 | 24 | 0.38 ± 0.03 c | 107.50 ± 0.06 a | |
| 48 | 0.61 ± 0.01 b | 99.62 ± 0.01 b | ||
| 72 | 0.87 ± 0.01 a | 97.52 ± 0.01 b | ||
| 500 | 24 | 0.08 ± 0.04 f | 23.54 ± 0.05 c | |
| 48 | 0.13 ± 0.02 e | 21.81 ± 0.02 c | ||
| 72 | 0.18 ± 0.01 d | 20.42 ± 0.01 c | ||
| ANOVA (F-value) | Genotypes | 534.33 *** | 1025.38 *** | |
| Al | 4212.16 *** | 4827.71 *** | ||
| Time | 1744.14 *** | 4.72 * | ||
| Interaction | 34.71 *** | 1.41 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Khan, S.; Qi, Y.; Zhang, C.; Anwar, S.; Yin, L.; Huang, J. Metabolic and Nutritional Responses of Contrasting Aluminium-Tolerant Banana Genotypes Under Al Stress. Plants 2025, 14, 385. https://doi.org/10.3390/plants14030385
Wu X, Khan S, Qi Y, Zhang C, Anwar S, Yin L, Huang J. Metabolic and Nutritional Responses of Contrasting Aluminium-Tolerant Banana Genotypes Under Al Stress. Plants. 2025; 14(3):385. https://doi.org/10.3390/plants14030385
Chicago/Turabian StyleWu, Xinran, Shahbaz Khan, Yucheng Qi, Chuanling Zhang, Sumera Anwar, Liyan Yin, and Jiaquan Huang. 2025. "Metabolic and Nutritional Responses of Contrasting Aluminium-Tolerant Banana Genotypes Under Al Stress" Plants 14, no. 3: 385. https://doi.org/10.3390/plants14030385
APA StyleWu, X., Khan, S., Qi, Y., Zhang, C., Anwar, S., Yin, L., & Huang, J. (2025). Metabolic and Nutritional Responses of Contrasting Aluminium-Tolerant Banana Genotypes Under Al Stress. Plants, 14(3), 385. https://doi.org/10.3390/plants14030385






