Characterization and Identification of Neocosmospora solani and Fusarium oxysporum Causing Root Necrosis and Wilting of Orange Trees in Chile
Abstract
1. Introduction
2. Results
2.1. Characterization of Disease Symptoms in Orchards
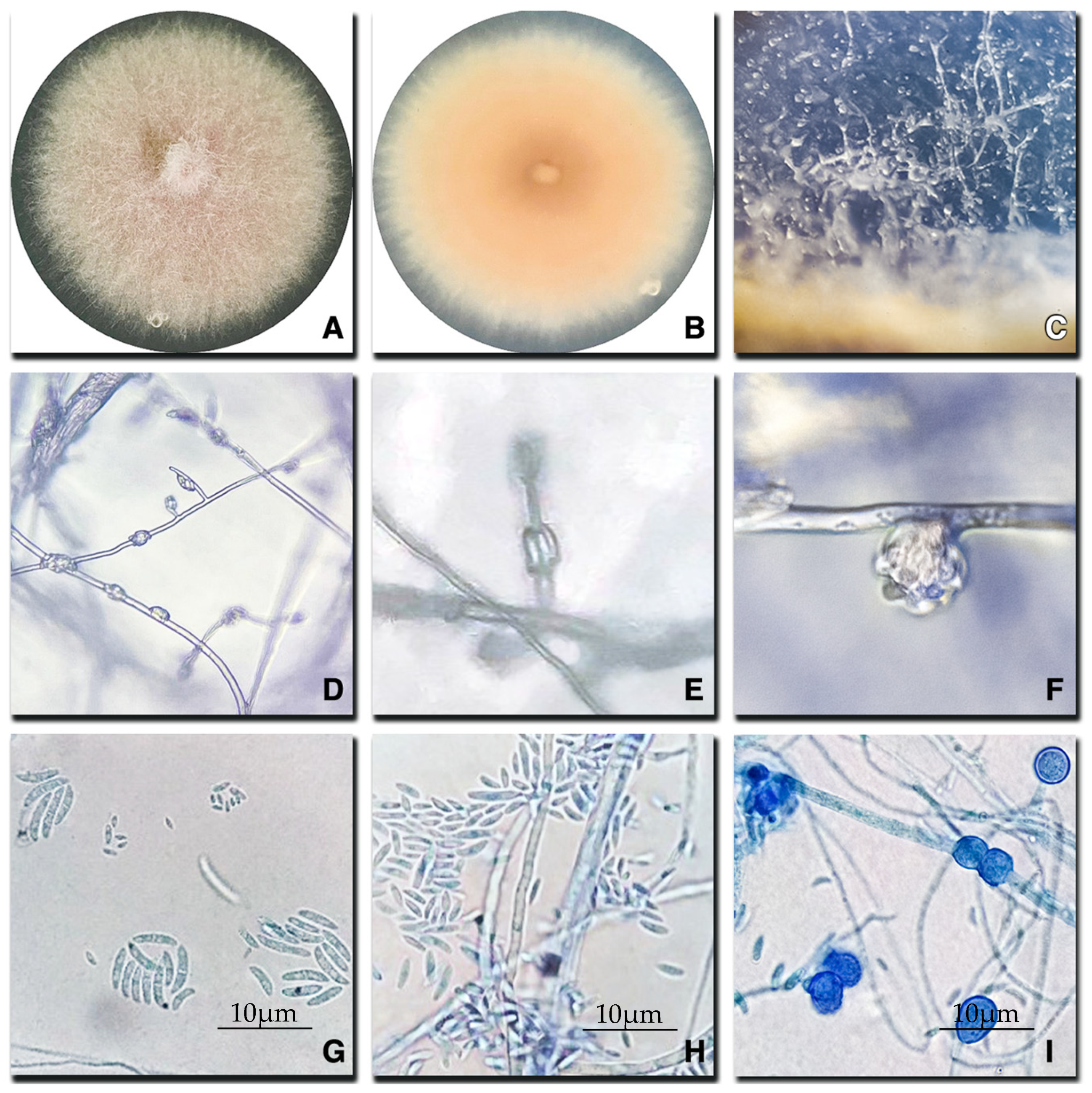
2.2. Morphological Characterization of Fusarium Species
2.3. Phylogenetic Identification
2.4. Pathogenicity
3. Discussion
3.1. Fusarioid Species Affecting Orange Trees
3.2. Potential Factors Contributing to Root Rot and Vascular Wilt
4. Materials and Methods
4.1. Field Sampling and Pathogen Isolation
4.2. Morphological Characterization and Identification
4.3. DNA Extraction and Molecular Identification
4.4. Pathogenicity Tests
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ODEPA Superficie Plantada Nacional, Regional, Número de Huertos e Infraestructura Frutícola (Catastro Frutícola). Available online: https://www.odepa.gob.cl/estadisticas-del-sector/estadisticas-productivas (accessed on 12 November 2023).
- Ezrari, S.; Radouane, N.; Tahiri, A.; El Housni, Z.; Mokrini, F.; Özer, G.; Lazraq, A.; Belabess, Z.; Amiri, S.; Lahlali, R. Dry Root Rot Disease, an Emerging Threat to Citrus Industry Worldwide under Climate Change: A Review. Physiol. Mol. Plant Pathol. 2022, 117, 101753. [Google Scholar] [CrossRef]
- Ezrari, S.; Lahlali, R.; Radouane, N.; Tahiri, A.; Asfers, A.; Boughalleb-M’Hamdi, N.; Amiri, S.; Lazraq, A. Characterization of Fusarium Species Causing Dry Root Rot Disease of Citrus Trees in Morocco. J. Plant Dis. Prot. 2020, 128, 431–447. [Google Scholar] [CrossRef]
- Barrette, J. First Annual Report of the California Citrus Institute, 1919–1920; California Citrus Institute: Glendora, CA, USA, 1920. [Google Scholar]
- Spina, S.; Coco, V.; Gentile, A.; Catara, A.; Cirvilleri, G. Association of Fusarium solani With RolABC and Wild Type Troyer Citrange. J. Plant Pathol. 2008, 90, 479–486. Available online: https://api.semanticscholar.org/CorpusID:86087866 (accessed on 13 November 2023).
- El-Mohamedy, R.S.R. Studies on Wilt and Root Rot Disease of Some Citrus Plants in Egypt; Ain Shams University: Cairo, Egypt, 1998. [Google Scholar]
- Liu, H.; Zhou, J.; Liao, J.; Yi, J.; Ma, D.; Deng, J. Grafted twig rot on Citrus sinensis caused by a member of the Fusarium solani species complex. Can. J. Plant Pathol. 2019, 42, 133–139. [Google Scholar] [CrossRef]
- Bozkurt, D.; Rojas, M.; Boisier, J.P.; Valdivieso, J. Climate Change Impacts on Hydroclimatic Regimes and Extremes over Andean Basins in Central Chile. Hydrol. Earth Syst. Sci. Discuss. 2017, 2017, 1–29. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A Climate Dynamics Perspective. Int. J. Climatol. 2019, 40, 421–439. [Google Scholar] [CrossRef]
- Marín, P.; de Ory, A.; Cruz, A.; Magan, N.; González-Jaén, M.T. Potential Effects of Environmental Conditions on the Efficiency of the Antifungal Tebuconazole Controlling Fusarium verticillioides and Fusarium proliferatum Growth Rate and Fumonisin Biosynthesis. Int. J. Food Microbiol. 2013, 165, 251–258. [Google Scholar] [CrossRef]
- Raza, M.M.; Bebber, D.P. Climate Change and Plant Pathogens. Curr. Opin. Microbiol. 2022, 70, 102233. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The Proportion of Soil-Borne Pathogens Increases with Warming at the Global Scale. Nat. Clim. Chang. 2020, 10, 550–554. [Google Scholar] [CrossRef]
- CANNA Research Fusarium—Plagas y Enfermedades Gestión y Control. Available online: http://www.canna.es/fusarium-plagas-enfermedades (accessed on 6 July 2023).
- Sandoval-Denis, M.; Guarnaccia, V.; Polizzi, G.; Crous, P.W. Symptomatic Citrus Trees Reveal a New Pathogenic Lineage in Fusarium and Two New Neocosmospora Species. Persoonia Mol. Phylo. Evol. Fungi 2018, 40, 25. [Google Scholar] [CrossRef]
- Adesemoye, A.; Eskalen, A.; Faber, B.; Bender, G.; Connell, N.O.; Kallsen, C.; Shea, T. Current Knowledge on Fusarium Dry Rot of Citrus. 2011. Available online: https://www.researchgate.net/publication/255704900 (accessed on 13 December 2022).
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed]
- Summerell, B.A. Resolving Fusarium: Current Status of the Genus. Annu. Rev. Phytopathol. 2019, 57, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Kamali-Sarvestani, S.; Mostowfizadeh-Ghalamfarsa, R.; Salmaninezhad, F.; Cacciola, S.O. Fusarium and Neocosmospora Species Associated with Rot of Cactaceae and Other Succulent Plants. J. Fungi 2022, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic Concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a Node or a Foot-Shaped Basal Cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef]
- Van Janse Rensburg, J.C.; Labuschagne, N.; Nemec, S. Occurrence of Fusarium-Produced Naphthazarins in Citrus Trees and Sensitivity of Rootstocks to Isomarticin in Relation to Citrus Blight. Plant Pathol. 2001, 50, 258–265. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of Key Phytopathogenic Fusarium Species: Current Status and Future Challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- Leslie, J.; Summerell, B. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 148, ISBN 9780813819198. [Google Scholar]
- Labuschagne, N.; Kotze, J.M.; Putierill, J.F. Incidence of Fusarium solani and F. oxysporum in Citrus Roots and Infection By F. solani. Phytophy 1987, 19, 315–318. [Google Scholar]
- Graham, J.; Feichtenberger, E. Citrus Phytophthora Diseases: Management Challenges and Successes. J. Citrus Pathol. 2015, 2. [Google Scholar] [CrossRef]
- Krueger, R.; Bender, G. Screening a core collection of Citrus genetic resources for resistance to Fusarium solani. Acta Hortic. 2015, 1065, 155–164. [Google Scholar] [CrossRef]
- Balanagouda, P.; Ganesh, C.T.; Kotari, P.; Rathinavelu, R. Wilt and Gummosis Disease of Subabul Caused by Fusarium equiseti A First Record from India. New Dis. Rep. 2023, 48, e12227. [Google Scholar] [CrossRef]
- Smith, G.; Nemec, S.; Gould, A.; Sonoda, R. Effect of Deep tillage and Soil Amendments on Growth of Rough Lemon Citrus and Root and Soil Microflora Population Densities. Proc. Soil Crop Sci. Soc. Fla. 1989, 48, 165–172. [Google Scholar]
- Guarnaccia, V.; Van Niekerk, J.; Crous, P.W.; Sandoval-Denis, M. Neocosmospora spp. Associated with Dry Root Rot of Citrus in South Africa. Phytopathol. Mediterr. 2021, 60, 79–100. [Google Scholar] [CrossRef]
- Dandurand, L.M.; Menge, J.A. Influence of Fusarium solani on Citrus Root Growth and Population Dynamincs of Phytophthora parasitica and Phytophthora citrophora. Ecol. Epidemiol. 1993, 83, 767–771. [Google Scholar] [CrossRef]
- Strauss, J.; Labuschagne, N. Pathogenicity of Fusarium solani Isolates on Citrus Roots and Evaluation of Different Inoculum Types. Appl. Plant Sci. 1995, 9, 48–52. [Google Scholar]
- Summerell, B.A.; Laurence, M.H.; Liew, E.C.Y.; Leslie, J.F. Biogeography and Phylogeography of Fusarium: A Review. Fungal Divers. 2010, 44, 3–13. [Google Scholar] [CrossRef]
- Nemec, S. Symptomatology and Histopathology of Fibrous Roots of Rough Remon (Citrus limon) Infected With Fusarium solani. Mycopathol. 1978, 63, 35–40. [Google Scholar] [CrossRef]
- Olsen, A. Diseases of Citrus in Arizona; The University of Arizona: Tucson, AZ, USA, 2000; pp. 1–15. [Google Scholar]
- Villarroel, C.; Vásquez, R.; Aravena, C.; Gotelli, C.; Vilches, C. Reporte Anual de la Evolución del Clima en Chile 2017. Available online: https://www.researchgate.net/publication/328198104_REPORTE_ANUAL_DE_LA_EVOLUCION_DEL_CLIMA_EN_CHILE_2017 (accessed on 15 May 2024).
- Villarroel, C.; Vásquez Yañez, R.; Aravena, C.; Gotelli, C.; Vilches, C. Reporte Anual de la Evolución del Clima en Chile 2019. Available online: https://www.researchgate.net/publication/342716781 (accessed on 15 May 2024).
- Villarroel, C.; Vásquez Yañez, R.; Aravena, C.; Gotelli, C.; Vilches, C. Reporte Anual de la Evolución del Clima en Chile 2022. Available online: https://www.researchgate.net/publication/371142452_REPORTE_ANUAL_DE_LA_EVOLUCION_DEL_CLIMA_EN_CHILE_2022 (accessed on 8 July 2024).
- Ezrari, S.; Radouane, N.; Tahiri, A.; Amiri, S.; Lazraq, A.; Lahlali, R. Environmental Effects of Temperature and Water Potential on Mycelial Growth of Neocosmospora solani and Fusarium spp. Causing Dry Root Rot of Citrus. Curr. Microbiol. 2021, 78, 3092–3103. [Google Scholar] [CrossRef]
- Baker, R.A. Toxin Production by Fusarium solani From Fibrous Roots of Blight-Diseased Citrus. Phytopatho 1981, 71, 951. [Google Scholar] [CrossRef]
- Cruzat, G.R.; Ionannidis, N.D. Resultados y Lecciones En Biocontrol de Enfermedades Fungosas Con Trichoderma; Enei, G.G., Ed.; Fundación para la Innovación Agraria: Santiago de Chile, Chile, 2008; Volume 62, ISBN 978-956-328-077-7. [Google Scholar]
- Burger, M.E. Tolerance of Citrus Rootstocks to Root Pathogens; University of Pretoria: Pretoria, South Afirca, 2001. [Google Scholar]
- El-Mohamedy, R.S.R.; Hasabo, S.A. Response of Some Citrus Rootstocks to Infection with Fusarium solani and Citrus Nematode Tylenchulus semipenetrans under Greenhouse Conditions. Egypt. J. Phytopathol. 2005, 33, 11–25. Available online: https://www.researchgate.net/publication/237393875 (accessed on 22 June 2022).
- Sáenz, C.; Osorio, E.; Estrada, B.; Poot, W.; Delgado, R.; Rodríguez, R. Principales Enfermedades En Cítricos Resumen. Rev. Mex. Cienc. Agric. 2019, 10, 1653–1665. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Arapid DND Isolation Procedure For Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics; Academic Press: San Diego, CA, USA, 1990. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular Systematics and Phylogeography of the Gibberella fujikuroi Species Complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic Relationships among Ascomycetes: Evidence from an RNA Polymerse II Subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Rashed, A.E.E.D.; Amer, H.M.; El-Seddek, M.; Moustafa, H.E.D. Sequence Alignment Using Machine Learning-Based Needleman-Wunsch Algorithm. IEEE Access 2021, 9, 109522–109535. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular-Evolution-and-Phylogenetics; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Kumar, S.; Thakur, M.; Rani, A. Trichoderma: Mass Production, Formulation, Quality Control, Delivery and Its Scope in Commercialization in India for the Management of Plant Diseases. Afr. J. Agric. Res. 2014, 9, 3838–3852. [Google Scholar]



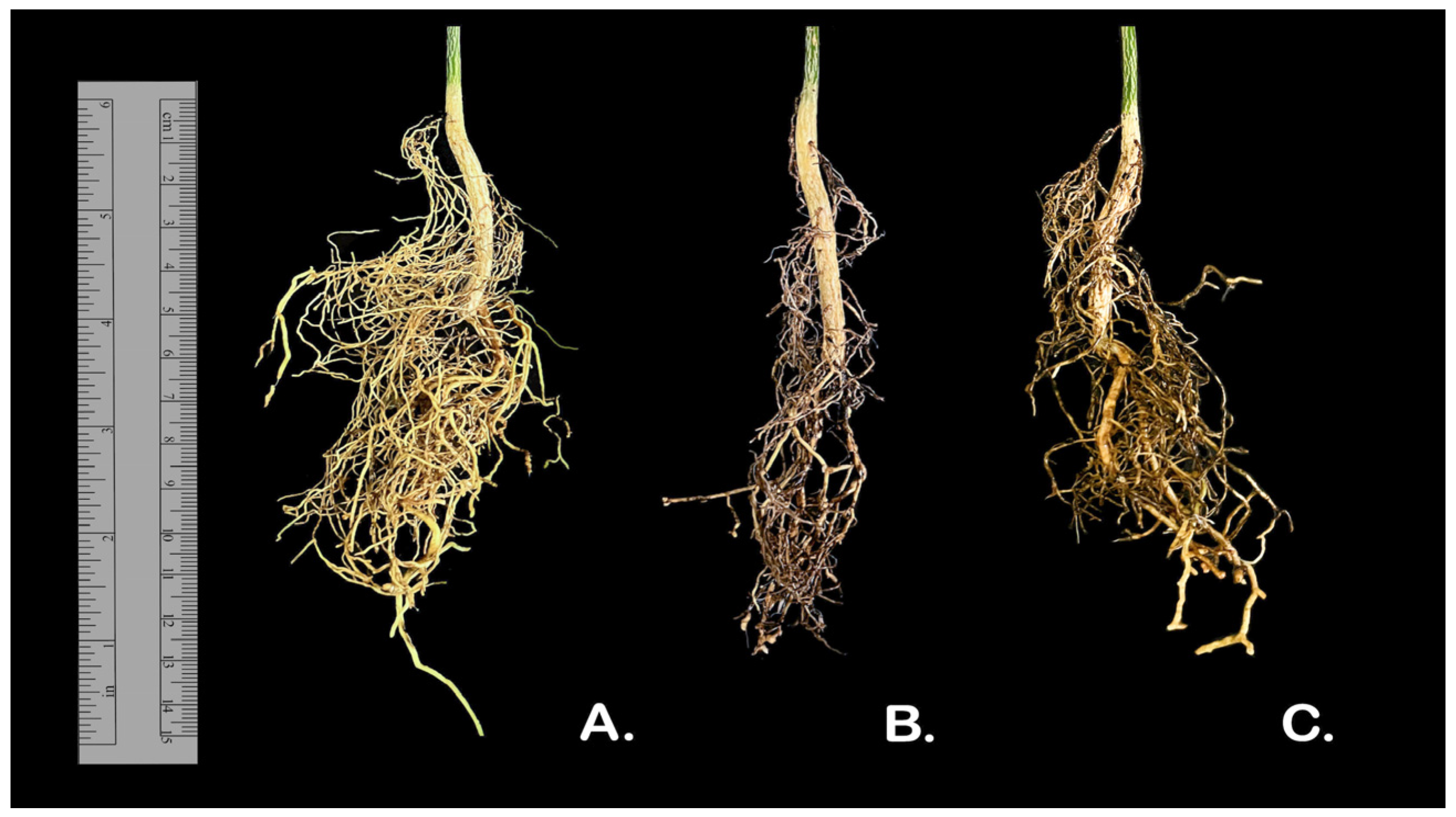
| Isolates | Root Lenght (cm) | Necrotic Root Length (cm) | Percent Necrotic Length/Root Lenght NL/RL (%) |
|---|---|---|---|
| Uninoculated Control | 15.6 ± 0.94 a | 2.3 ± 0.04 a | 15.1 ± 1.04 a |
| N. solani (syn. F. solani) | 13.3 ± 0.24 a | 6.9 ± 0.04 b | 51.5 ± 3.00 b |
| F. oxysporum | 13.3 ± 0.41 a | 5.7 ± 0.61 b | 42.9 ± 3.65 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzón-Nivia, M.A.; Mártiz Mártiz, J.; Moya-Elizondo, E.A.; Ruiz, B.; Cornejo, J.C.; Valdés-Gómez, H.A. Characterization and Identification of Neocosmospora solani and Fusarium oxysporum Causing Root Necrosis and Wilting of Orange Trees in Chile. Plants 2025, 14, 376. https://doi.org/10.3390/plants14030376
Garzón-Nivia MA, Mártiz Mártiz J, Moya-Elizondo EA, Ruiz B, Cornejo JC, Valdés-Gómez HA. Characterization and Identification of Neocosmospora solani and Fusarium oxysporum Causing Root Necrosis and Wilting of Orange Trees in Chile. Plants. 2025; 14(3):376. https://doi.org/10.3390/plants14030376
Chicago/Turabian StyleGarzón-Nivia, María A., Johanna Mártiz Mártiz, Ernesto A. Moya-Elizondo, Braulio Ruiz, Julio C. Cornejo, and Héctor A. Valdés-Gómez. 2025. "Characterization and Identification of Neocosmospora solani and Fusarium oxysporum Causing Root Necrosis and Wilting of Orange Trees in Chile" Plants 14, no. 3: 376. https://doi.org/10.3390/plants14030376
APA StyleGarzón-Nivia, M. A., Mártiz Mártiz, J., Moya-Elizondo, E. A., Ruiz, B., Cornejo, J. C., & Valdés-Gómez, H. A. (2025). Characterization and Identification of Neocosmospora solani and Fusarium oxysporum Causing Root Necrosis and Wilting of Orange Trees in Chile. Plants, 14(3), 376. https://doi.org/10.3390/plants14030376







