Abscisic Acid Can Play a Dual Role in the Triticum aestivum–Stagonospora nodorum Pathosystem
Abstract
1. Introduction
2. Results
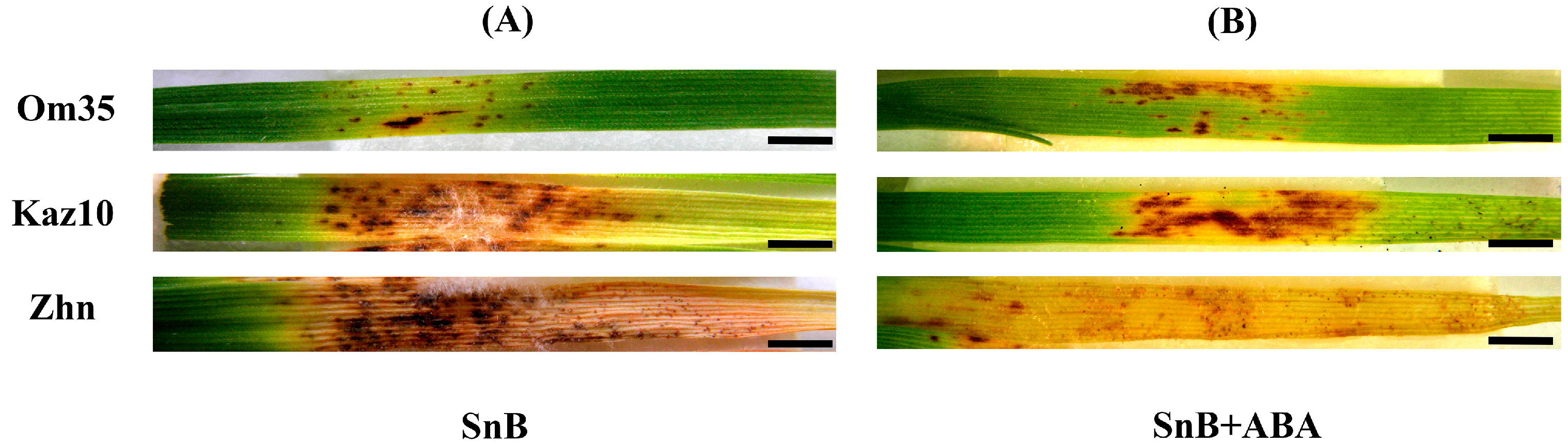
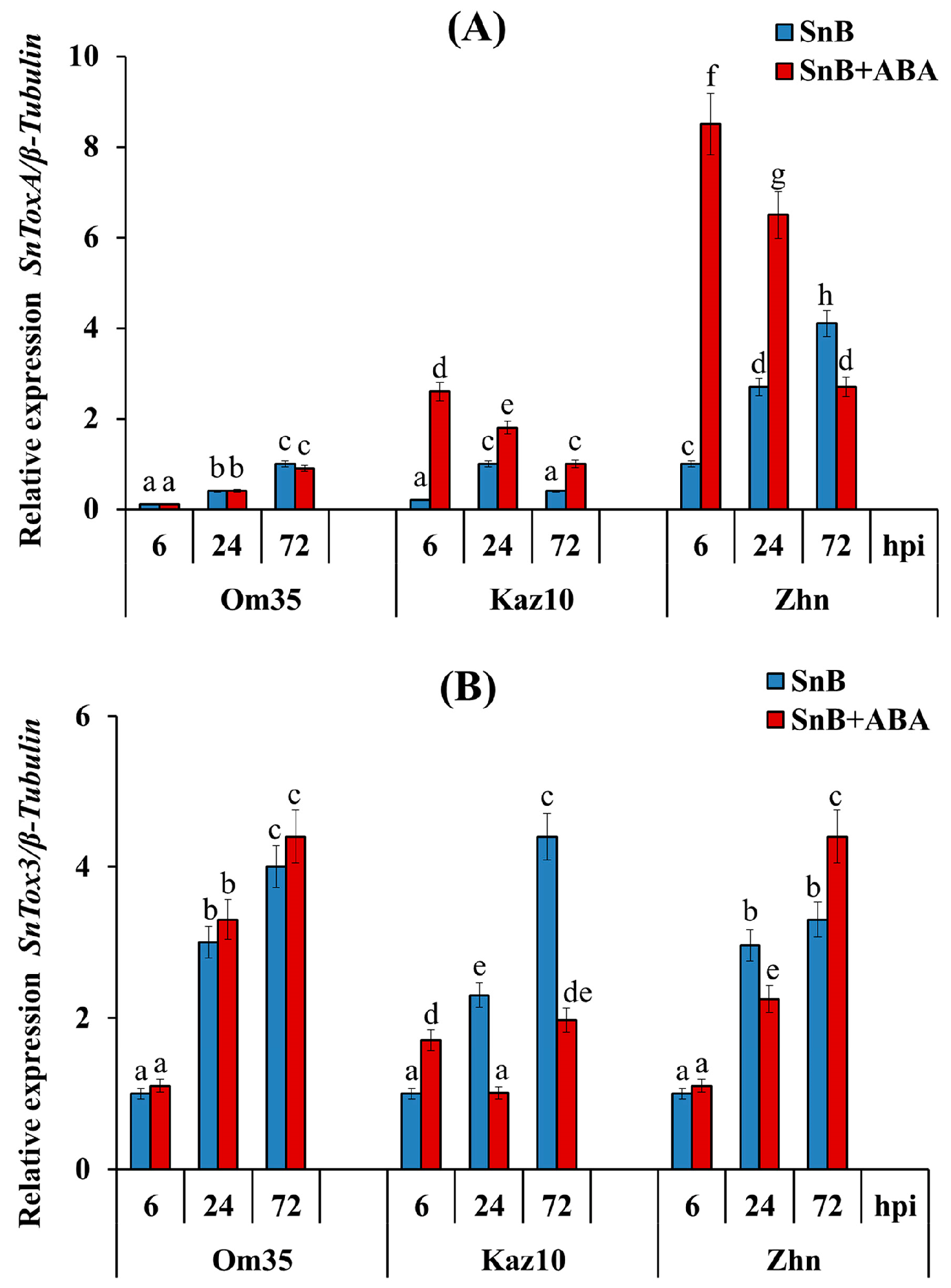
2.1. The Role of SnTox3, SnToxA, and Phytohormone ABA in the Development of Defense Reactions in the Three Wheat Genotypes
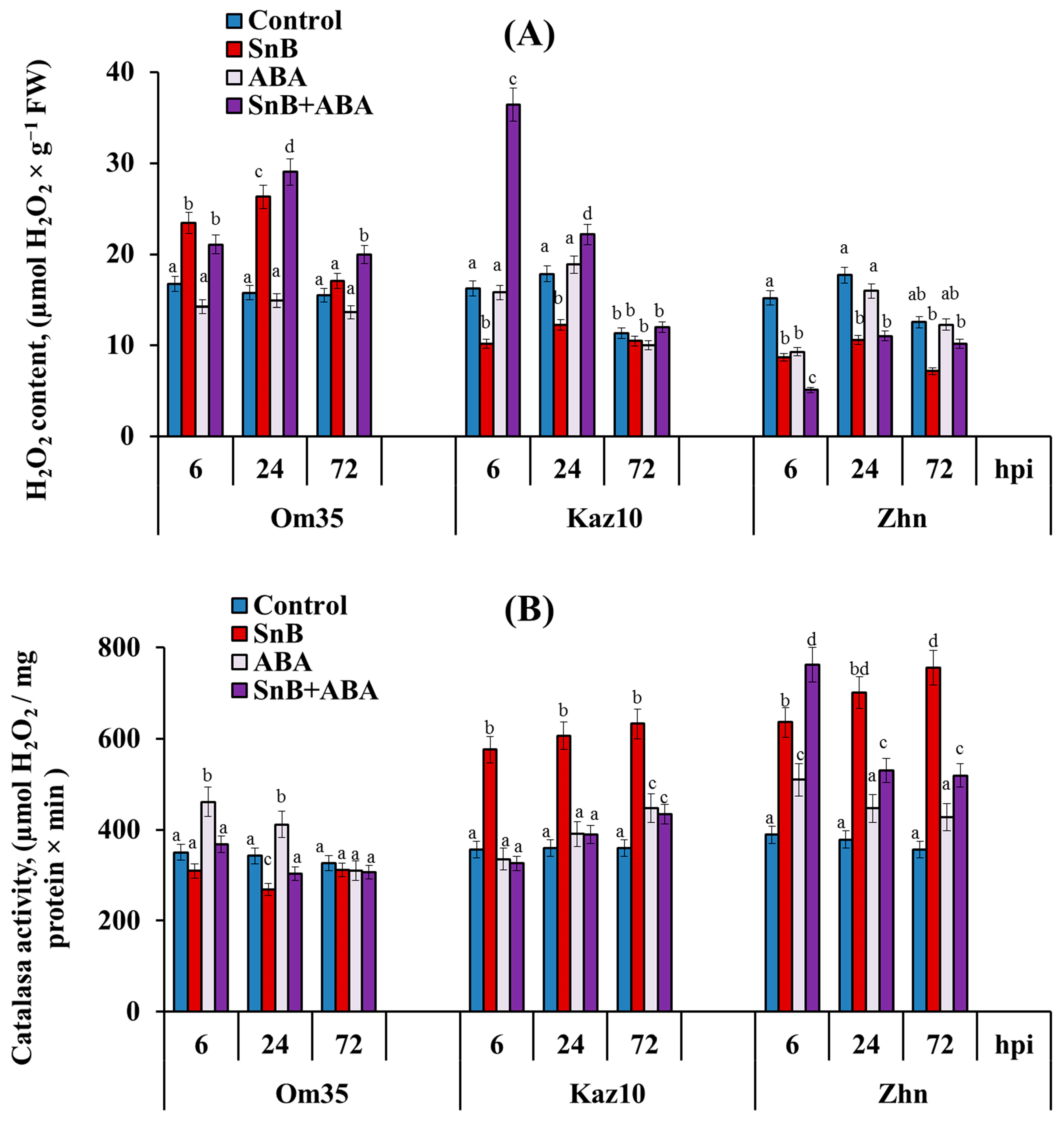
2.2. The Role of SnTox3, SnToxA, and Phytohormone ABA in the Regulation of the Redox Status of the Three Wheat Genotypes
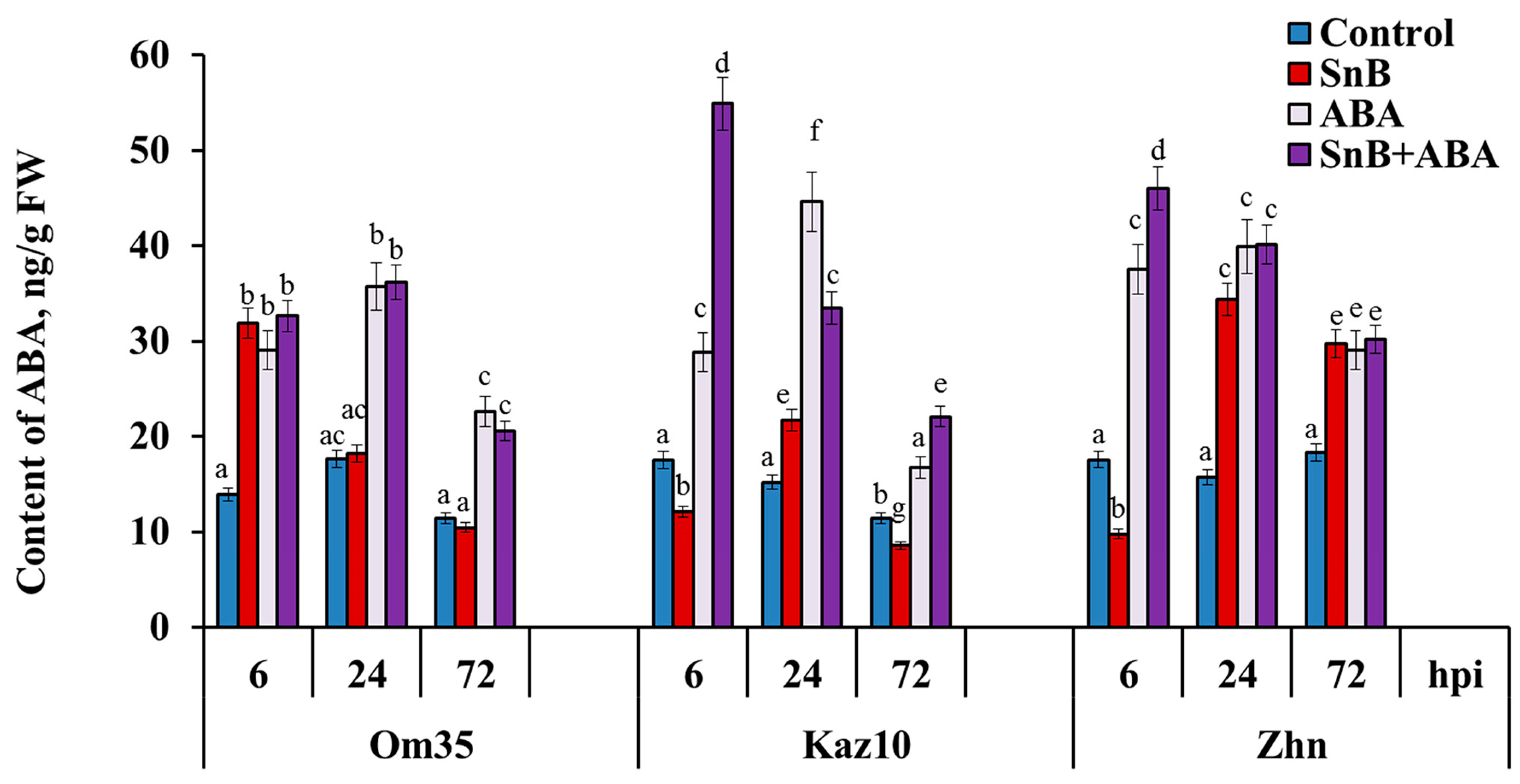
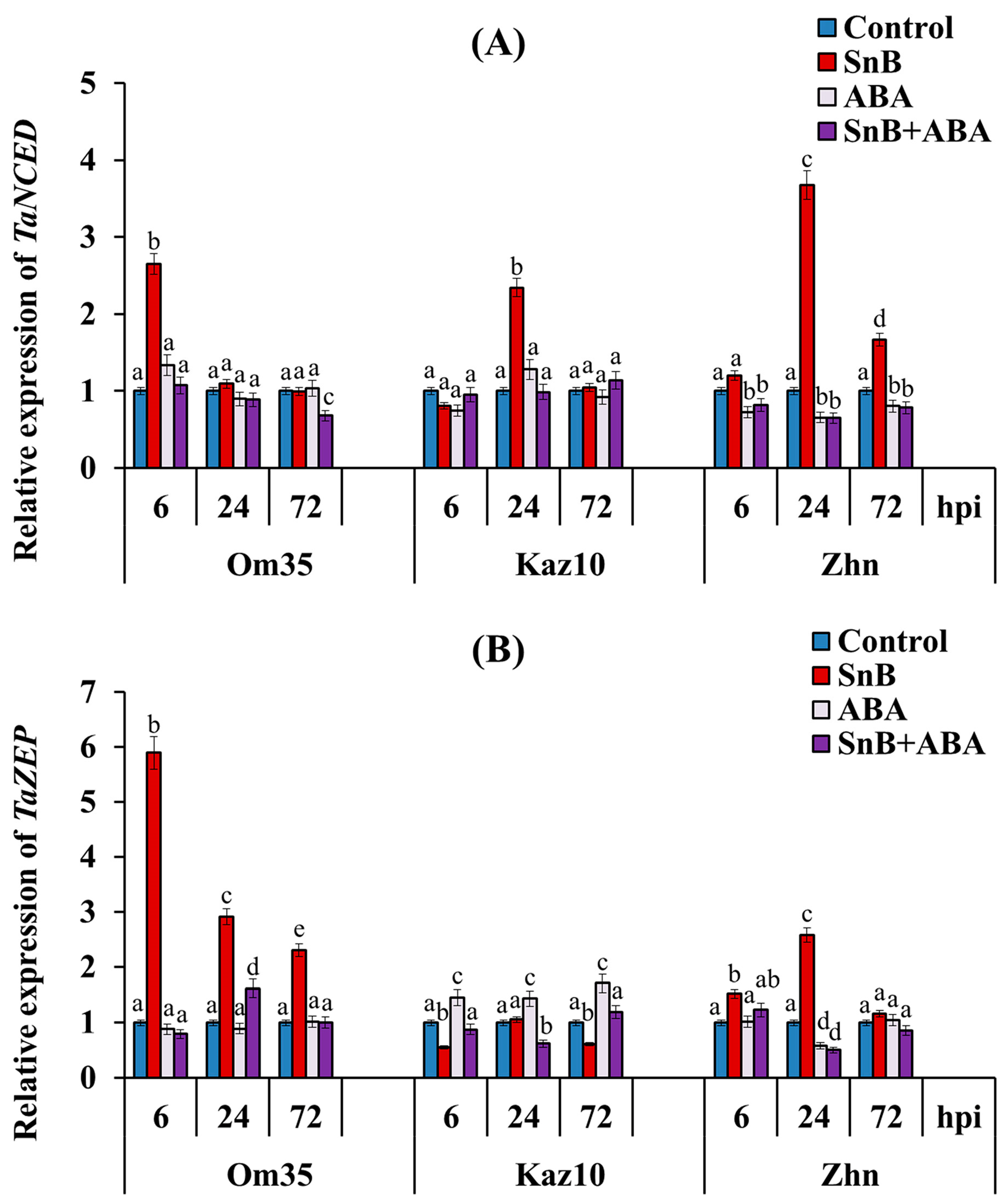
2.3. The Role of SnTox3 and SnToxA in the Regulation of the Endogenous ABA Content in the Three Wheat Genotypes
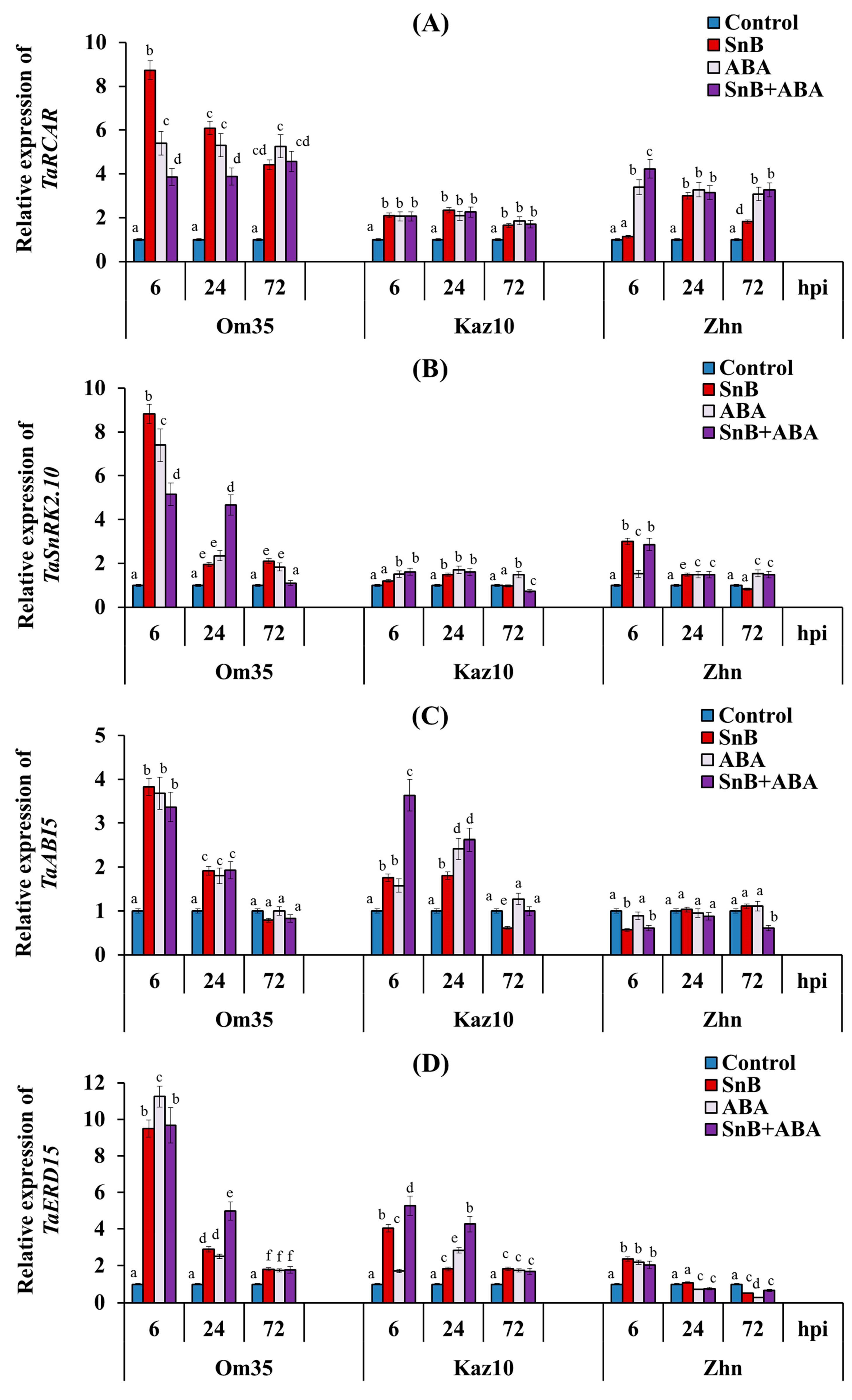
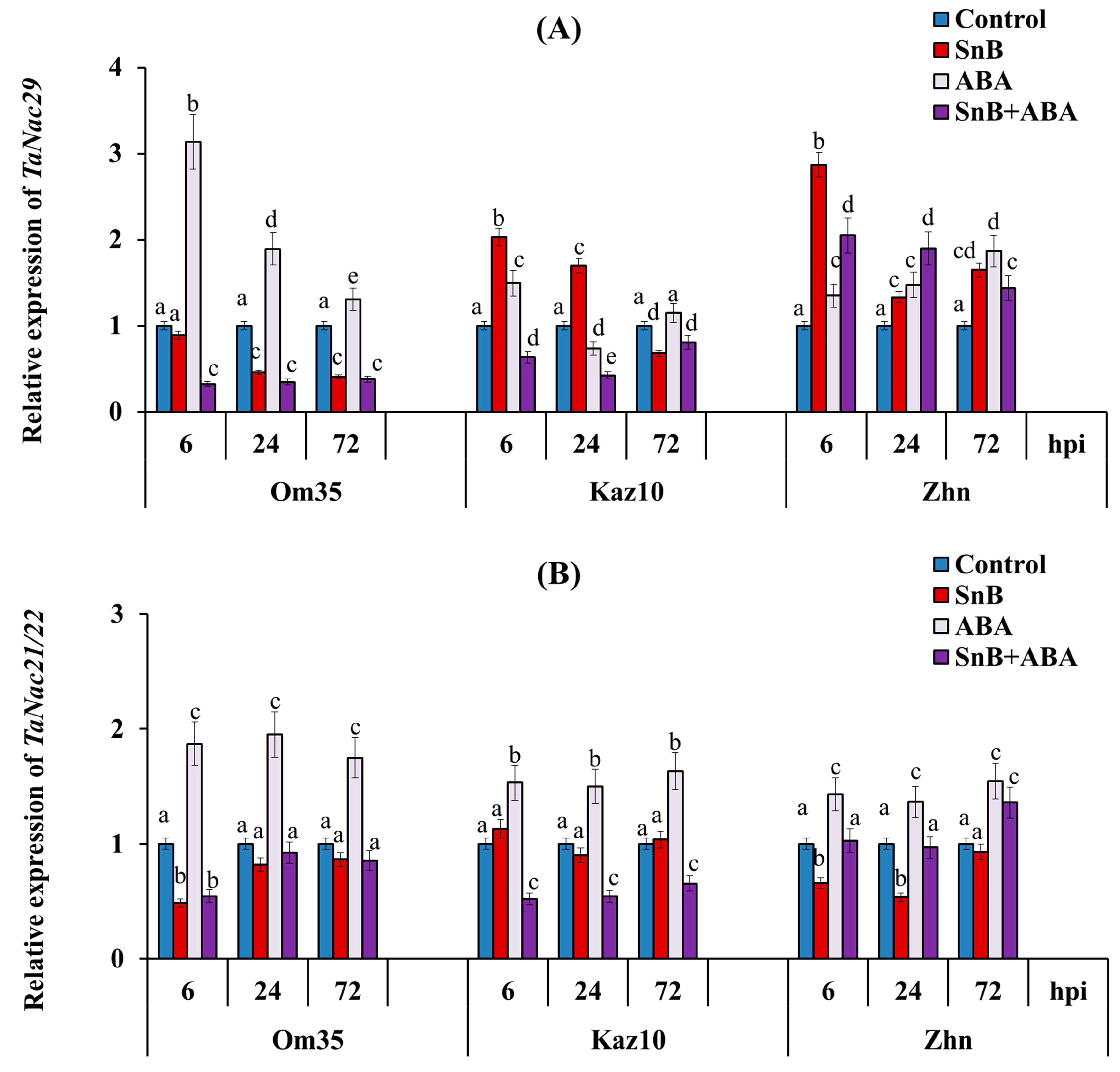
2.4. The Role of SnTox3 and SnToxA in Manipulating the ABA Signaling Pathway and the Role of the Hormone ABA in the Development of the Resistance/Susceptibility of Wheat to the S. nodorum SnB Isolate
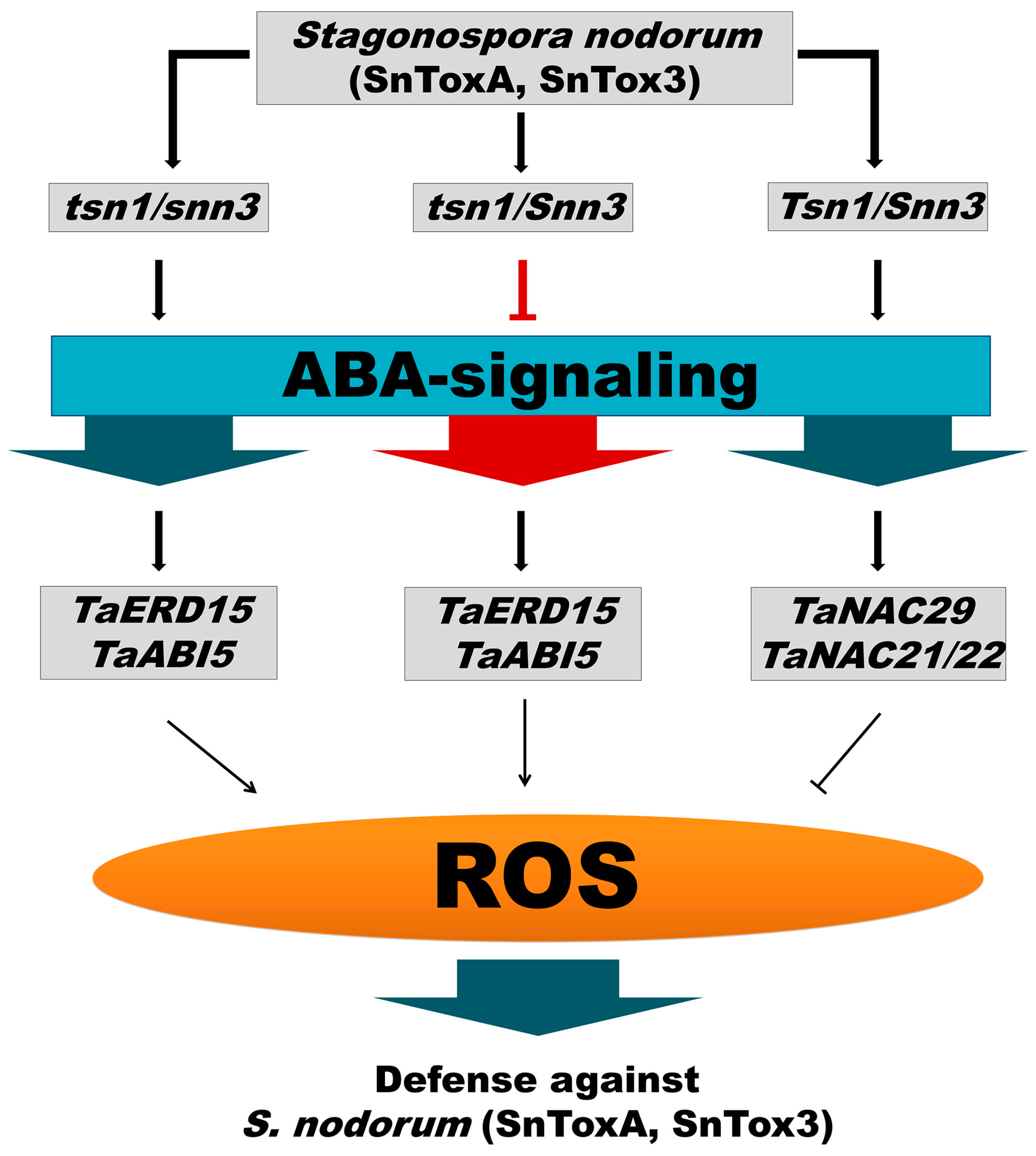
3. Discussion
4. Materials and Methods
4.1. Plant and Fungi Materials
4.2. Experimental Design
4.3. Analysis of Hydrogen Peroxide Content and Catalase Activity
4.4. Enzyme-Linked Immunosorbent Assay (ELISA) of ABA Content in Wheat Plants
4.5. Gene Expression Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.D.G.; Dang, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.C.; Oliver, R.P.; Solomon, P.S.; Moffat, C.S. Proteinaceous necrotrophic effectors in fungal virulence. Funct. Plant Biol. 2010, 37, 907–912. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Si, J.; Han, Z.; Chen, D. Action Mechanisms of Effectors in Plant-Pathogen Interaction. Int. J. Mol. Sci. 2022, 23, 6758. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Panigrahi, G.K.; Jung, G.Y.; Lee, Y.J.; Shin, K.H.; Sahoo, A.; Choi, E.S.; Lee, E.; Kim, K.M.; Yang, S.H.; et al. Pathogen-associated molecular pattern-triggered immunity involves proteolytic degradation of core nonsense-mediated mRNA decay factors during the early defense response. Plant Cell 2020, 32, 1081–1101. [Google Scholar] [CrossRef]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Kim, C.-Y.; Song, H.; Lee, Y.-H. Ambivalent response in pathogen defense: A double-edged sword? Plant Comm. 2022, 3, 100415. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Faris, J.D.; Oliver, R.P.; Syme, R.; McDonald, M.C.; McDonald, B.A.; Solomon, P.S.; Lu, S.; Shelver, W.L.; et al. The cysteine rich necrotrophic effector SnTox1 produced by Stagonospora nodorum triggers susceptibility of wheat lines harboring Snn1. PLoS Pathog. 2012, 8, e1002467. [Google Scholar] [CrossRef]
- Winterberg, B.; Du Fall, A.L.; Song, X.; Pascovici, D.; Care, N.; Molloy, M.; Ohms, S.; Solomon, P.S. The necrotrophic effector protein SnTox3 re-programs metabolism and elicits a strong defence response in susceptible wheat leaves. BMC Plant Biol. 2014, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zhang, Z.; Friesen, T.L.; Raats, D.; Fahima, T.; Brueggeman, R.S.; Lu, S.; Trick, H.N.; Liu, Z.; Chao, W.; et al. The hijacking of a receptor kinase–driven pathway by a wheat fungal pathogen leads to disease. Sci. Adv. 2016, 2, e1600822. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Mir, Z.A. Plant Immunity: At the Crossroads of Pathogen Perception and Defense Response. Plants 2024, 13, 1434. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grantm, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Dong, X. Salicylic acid in plant immunity and beyond. Plant Cell 2024, 36, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.-J.; Bressan, R.A.; Song, C.-P.; Zhu, J.-K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Gietler, M.; Fidler, J.; Labudda, M.; Nykiel, M. Abscisic Acid—Enemy or Savior in the Response of Cereals to Abiotic and Biotic Stresses? Int. J. Mol. Sci. 2020, 21, 4607. [Google Scholar] [CrossRef]
- Hewage, K.A.H.; Yang, J.-F.; Wang, D.; Hao, G.-F.; Yang, G.-F.; Zhu, J.-K. Chemical Manipulation of Abscisic Acid Signaling: A New Approach to Abiotic and Biotic Stress Management in Agriculture. Adv. Sci. 2020, 7, 2001265. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.V. Abscisic Acid in the Plants–Pathogen Interaction. Russ. J. Plant Physiol. 2009, 56, 742–752. [Google Scholar] [CrossRef]
- Ton, J.; Mauch-Mani, B. Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J. 2004, 38, 119–130. [Google Scholar] [CrossRef] [PubMed]
- De Vleesschauwer, D.; Yang, Y.; Cruz, C.V.; Hofte, M. Abscisic acid-induced resistance against the brown spot pathogen Cochliobolus miyabeanus in rice involves MAPK-mediated repression of ethylene signaling. Physiol. Plant. 2010, 152, 2036–2052. [Google Scholar] [CrossRef] [PubMed]
- Spence, C.A.; Lakshmanan, V.; Donofrio, N.; Bais, H.P. Crucial Roles of Abscisic Acid Biogenesis in Virulence of Rice Blast Fungus Magnaporthe oryzae. Front. Plant Sci. 2015, 1, 1082. [Google Scholar] [CrossRef]
- Han, X.; Kahmann, R. Manipulation of phytohormone pathways by effectors of filamentous plant pathogens. Front. Plant Sci. 2019, 10, 822. [Google Scholar] [CrossRef]
- Boba, A.; Kostyn, K.; Kochneva, Y.; Wojtasik, W.; Mierziak, J.; Prescha, A.; Augustyniak, B.; Grajzer, M.; Szopa, J.; Kulma, A. Abscisic Acid—Defensive Player in Flax Response to Fusarium culmorum Infection. Molecules 2022, 27, 2833. [Google Scholar] [CrossRef] [PubMed]
- Adie, B.; Chico, J.M.; Rubio-Somoza, I.; Solano, R. Modulation of Plant Defenses by Ethylene. J. Plant Growth Regul. 2007, 26, 60–177. [Google Scholar] [CrossRef]
- Haugrud, A.R.P.; Zhang, Z.; Friesen, T.L.; Faris, J.D. Genetics of resistance to septoria nodorum blotch in wheat. Theor. Appl. Genet. 2022, 135, 3685–3707. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.C.; Solomon, P.S. Just the surface: Advances in the discovery and characterization of necrotrophic wheat effectors. Curr. Opin. Microbiol. 2018, 46, 14–18. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Rybak, K.; Furuki, E.; Breen, S.; Solomon, P.S.; Oliver, R.P.; Tan, K.C. Differential effector gene expression underpins epistasis in a plant fungal disease. Plant J. 2016, 87, 343–354. [Google Scholar] [CrossRef]
- Breen, S.; Williams, S.J.; Winterberg, B.; Kobe, B.; Solomon, P.S. Wheat PR-1 proteins are targeted by necrotrophic pathogen effector proteins. Plant J. 2016, 88, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Veselova, S.V.; Nuzhnaya, T.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Khusnutdinova, E.K.; Maksimov, I.V. Ethylene-cytokinin interaction determines early defense response of wheat against Stagonospora nodorum Berk. Biomolecules 2021, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Veselova, S.; Nuzhnaya, T.; Maksimov, I. The Role of Salicylic, Jasmonic Acid and Ethylene in the Development of the Resistance/Susceptibility of Wheat to the SnTox1-Producing Isolate of the Pathogenic Fungus Stagonospora nodorum (Berk.). Plants 2024, 13, 2546. [Google Scholar] [CrossRef]
- Virdi, S.K.; Liu, Z.; Overlander, M.E.; Zhang, Z.; Xu, S.S.; Friesen, T.L.; Faris, J.D. New Insights into the Roles of Host Gene-Necrotrophic Effector Interactions in Governing Susceptibility of Durum Wheat to Tan Spot and Septoria Nodorum Blotch. G3 (Bethesda) 2016, 6, 4139–4150. [Google Scholar] [CrossRef] [PubMed]
- Faris, J.D.; Zhang, Z.; Lu, H.; Lu, S.; Reddy, L.; Cloutier, S.; Fellers, J.P.; Meinhardt, S.W.; Rasmussen, J.B.; Xu, S.S.; et al. A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens. Proc. Natl. Acad. Sci. USA 2010, 107, 13544–13549. [Google Scholar] [CrossRef] [PubMed]
- Veselova, S.; Nuzhnaya, T.; Burkhanova, G.; Rumyantsev, S.; Maksimov, I. Reactive oxygen species in host plant are required for an early defense response against attack of Stagonospora nodorum Berk. necrotrophic effectors SnTox. Plants 2021, 10, 1586. [Google Scholar] [CrossRef] [PubMed]
- Nuzhnaya, T.; Veselova, S.; Burkhanova, G.; Rumyantsev, S.; Shoeva, O.; Shein, M.; Maksimov, I. Novel sources of resistance to Stagonospora nodorum and role of effector-susceptibility gene interactions in wheat of russian breeding. Int. J. Plant Biol. 2023, 14, 377–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Li, Y.; Zhang, Z.; Yang, L.; Wang, M.; Zhang, Y.; Zhang, J.; Li, C.; Li, L.; et al. Wheat TaSnRK2.10 phosphorylates TaERD15 and TaENO1 and confers drought tolerance when overexpressed in rice. Plant Physiol. 2023, 191, 1344–1364. [Google Scholar] [CrossRef]
- Kariola, T.; Brader, G.; Helenius, E.; Li, J.; Heino, P.; Palva, E.T. Early responsive to dehydration 15, a negative regulator of abscisic acid responses in Arabidopsis. Plant Physiol. 2006, 142, 1559–1573. [Google Scholar] [CrossRef] [PubMed]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The Role and Regulation of ABI5 (ABA-Insensitive 5) in Plant Development, Abiotic Stress Responses and Phytohormone Crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef]
- Feng, H.; Duan, X.; Zhang, Q.; Li, X.; Wang, B.; Huang, L.; Wang, X.; Kang, Z. The target gene of tae-miR164, a novel NAC transcription factor from the NAM subfamily, negatively regulates resistance of wheat to stripe rust. Mol. Plant Pathol. 2014, 15, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Guo, H.; Zhang, M.; Wang, Q.; Zhang, H.; Ji, W. Large-Scale Cloning and Comparative Analysis of TaNAC Genes in Response to Stripe Rust and Powdery Mildew in Wheat (Triticum aestivum L.). Genes 2020, 11, 1073. [Google Scholar] [CrossRef]
- Dong, B.; Liu, Y.; Huang, G.; Song, A.; Chen, S.; Jiang, J.; Chen, F.; Fang, W. Plant NAC transcription factors in the battle against pathogens. BMC Plant Biol. 2024, 24, 958. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.Y.; Yoshioka, K.; Desveaux, D. The roles of ABA in plant–pathogen interactions. J. Plant Res. 2011, 124, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.V.; Ganiev, R.M.; Khairullin, R.M. Changes in the levels of IAA, ABA, and cytokinins in wheat seedlings infected with Tilletia caries. Russ. J. Plant Physiol. 2002, 49, 221–224. [Google Scholar] [CrossRef]
- Sivakumaran, A.; Akinyemi, A.; Mandon, J.; Cristescu, S.M.; Hall, M.A.; Harren, F.J.M.; Mur, L.A.J. ABA Suppresses Botrytis cinerea Elicited NO Production in Tomato to Influence H2O2 Generation and Increase Host Susceptibility. Front. Plant Sci. 2016, 7, 709. [Google Scholar] [CrossRef]
- Wang, W.-R.; Liang, J.-H.; Wang, G.-F.; Sun, M.-X.; Peng, F.-T.; Xiao, Y.-S. Overexpression of PpSnRK1α in tomato enhanced salt tolerance by regulating ABA signaling pathway and reactive oxygen metabolism. BMC Plant Biol. 2020, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, Y.; Li, B.; Chang, J.; Chen, M.; Li, K.; Yang, G.; He, G. TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biol. 2015, 15, 268. [Google Scholar] [CrossRef]
- Faris, J.D.; Zhang, Z.; Rasmussen, J.B.; Friesen, T.L. Variable expression of the Stagonospora nodorum effector SnToxA among isolates is correlated with levels of disease in wheat. Mol. Plant-Microbe Interact. 2011, 24, 1419–1426. [Google Scholar] [CrossRef]
- Nuzhnaya, T.V.; Sorokan, A.V.; Burkhanova, G.F.; Maksimov, I.V.; Veselova, S.V. The Role of Cytokinins and Abscisic Acid in the Growth, Development and Virulence of the Pathogenic Fungus Stagonospora nodorum (Berk.). Biomolecules 2024, 14, 517. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. Intervention of Phytohormone Pathways by Pathogen Effectors. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef]
- de Torres, M.; Mansfield, J.W.; Grabov, N.; Brown, I.R.; Ammouneh, H.; Tsiamis, G.; Forsyth, A.; Robatzek, S.; Grant, M.; Boch, J. Pseudomonas syringae effector AvrPtoB suppresses basal defence in Arabidopsis. Plant J. 2006, 47, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.P.; Tan, C.M.; Li, M.Y.; Lin, H.; Deng, W.L.; Yang, J.Y. The AvrB_AvrC domain of AvrXccC of Xanthomonas campestris pv. campestris is required to elicit plant defense responses and manipulate ABA homeostasis. Mol. Plant Microbe Interact. 2013, 26, 419–430. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; He, Q.; Li, S.; Liu, W.; Lin, C.; Miao, W. A Candidate Secreted Effector Protein of Rubber Tree Powdery Mildew Fungus Contributes to Infection by Regulating Plant ABA Biosynthesis. Front. Microbiol. 2020, 11, 591387. [Google Scholar] [CrossRef]
- Gordon, C.S.; Rajagopalan, N.; Risseeuw, E.P.; Surpin, M.; Ball, F.J.; Barber, C.J.; Buhrow, L.M.; Clark, S.M.; Page, J.E.; Todd, C.D.; et al. Characterization of Triticum aestivum abscisic acid receptors and a possible role for these in mediating Fusarium head blight susceptibility in wheat. PLoS ONE 2016, 11, e0164996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Mao, X.; Jing, R.; Jia, H. Differential activation of the wheat SnRK2 family by abiotic stresses. Front. Plant Sci. 2016, 7, 420. [Google Scholar] [CrossRef]
- Lei, L.; Stevens, D.M.; Coaker, G. Phosphorylation of the Pseudomonas effector AvrPtoB by Arabidopsis SnRK2.8 Is Required for Bacterial Virulence. Mol. Plant. 2020, 13, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Jiang, Y.; Shi, M.; Wu, X.; Wu, G. ABI5 acts downstream of miR159 to delay vegetative phase change in Arabidopsis. New Phytol. 2021, 231, 339–350. [Google Scholar] [CrossRef]
- Nuzhnaya, T.; Veselova, S.; Burkhanova, G.; Maksimov, I. Virulence Factors of the Fungal Pathogen Stagonospora nodorum Manipulate Hormonal Signaling Pathways in Triticum aestivum L. by Regulating Host Plant MicroRNA Expressions. Front. Biosci. (Elite Ed) 2023, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Sasani, S.T.; Soltani, B.M.; Mehrabi, R.; Padasht-Dehkaei, H.S.F. Expression alteration of candidate rice miRNAs in response to sheath blight disease. Iran. J. Biotechnol. 2020, 18, e2451. [Google Scholar] [CrossRef]
- Peng, X.; Feng, C.; Wang, Y.-T.; Zhang, X.; Wang, Y.-Y.; Sun, Y.-T.; Xiao, Y.-Q.; Zhai, Z.-F.; Zhou, X.; Du, B.-Y.; et al. miR164g-MsNAC022 acts as a novel module mediating drought response by transcriptional regulation of reactive oxygen species scavenging systems in apple. Hortic. Res. 2022, 9, uhac192. [Google Scholar] [CrossRef] [PubMed]
- Bindschedler, L.V.; Dewdney, J.; Blee, K.A. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 2006, 47, 851–863. [Google Scholar] [CrossRef]
- Rumyantsev, S.D.; Veselova, S.V.; Burkhanova, G.F.; Alekseev, V.Y.; Maksimov, I.V. Bacillus subtilis 26D Triggers Induced Systemic Resistance against Rhopalosiphum padi L. by Regulating the Expression of Genes AGO, DCL and microRNA in Bread Spring Wheat. Microorganisms 2023, 11, 2983. [Google Scholar] [CrossRef]
- Bouizgarne, B. Bacteria for plant growth promotion and disease management. In Bacteria in Agrobiology: Disease Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Chapter 2; pp. 15–46. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Veselov, D.S.; Sharipova, G.V.; Akhiyarova, R.G.; Dodd, I.C.; Veselov, S.Y. Water relations and growth of original barley plants and its ABA-deficient mutants at increased air temperature. Russ. J. Plant Physiol. 2014, 61, 188–193. [Google Scholar] [CrossRef]
- Veselov, S.Y.; Timergalina, L.N.; Akhiyarova, G.R.; Kudoyarova, G.R.; Korobova, A.V.; Ivanov, I.; Arkhipova, T.N.; Prinsen, E. Study of cytokinin transport from shoots to roots of wheat plants is informed by a novel method of differential localization of free cytokinin bases or their ribosylated forms by means of their specific fixation. Protoplasma 2018, 255, 1581–1594. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Korobova, A.V.; Akhiyarova, G.R.; Arkhipova, T.N.; Zaytsev, D.Y.; Prinsen, E.; Egutkin, N.L.; Medvedev, S.S.; Veselov, S.Y. Accumulation of cytokinins in roots and their export to the shoots of durum wheat plants treated with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP). J. Exp. Bot. 2014, 65, 2287–2294. [Google Scholar] [CrossRef]
- Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Cherepanova, E.A.; Alekseev, V.Y.; Sarvarova, E.R.; Kasimova, A.R.; Maksimov, I.V. By modulating the hormonal balance and ribonuclease activity of tomato plants Bacillus subtilis induces defense response against potato virus X and potato Virus Y. Biomolecules 2022, 12, 288. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Iqbal, N.; Czékus, Z.; Poór, P.; Ördög, A. Ethylene-dependent regulation of oxidative stress in the leaves of fusaric acid-treated tomato plants. Plant Physiol. Biochem. 2023, 196, 841–849. [Google Scholar] [CrossRef] [PubMed]
| Variant of Treatment | Cultivars (Genotypes) | ||
|---|---|---|---|
| Omskaya 35 (tsn1/snn3) | Kazakhstanskaya 10 (tsn1/Snn3) | Zhnitsa (Tsn1/Snn3) | |
| S. nodorum (SnToxA/SnTox3) * | 8 ± 0.5 a | 61 ± 4 b | 81 ± 5 d |
| S. nodorum + Abscisic acid | 9 ± 0.4 a | 29 ± 2 c | 97 ± 6 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselova, S.; Nuzhnaya, T.; Burkhanova, G.; Rumyantsev, S.; Maksimov, I. Abscisic Acid Can Play a Dual Role in the Triticum aestivum–Stagonospora nodorum Pathosystem. Plants 2025, 14, 355. https://doi.org/10.3390/plants14030355
Veselova S, Nuzhnaya T, Burkhanova G, Rumyantsev S, Maksimov I. Abscisic Acid Can Play a Dual Role in the Triticum aestivum–Stagonospora nodorum Pathosystem. Plants. 2025; 14(3):355. https://doi.org/10.3390/plants14030355
Chicago/Turabian StyleVeselova, Svetlana, Tatyana Nuzhnaya, Guzel Burkhanova, Sergey Rumyantsev, and Igor Maksimov. 2025. "Abscisic Acid Can Play a Dual Role in the Triticum aestivum–Stagonospora nodorum Pathosystem" Plants 14, no. 3: 355. https://doi.org/10.3390/plants14030355
APA StyleVeselova, S., Nuzhnaya, T., Burkhanova, G., Rumyantsev, S., & Maksimov, I. (2025). Abscisic Acid Can Play a Dual Role in the Triticum aestivum–Stagonospora nodorum Pathosystem. Plants, 14(3), 355. https://doi.org/10.3390/plants14030355








