Impacts of Human Activity and Climate Change on the Suitable Habitats for Xanthium spinosum in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Processing for the Distribution of Xanthium spinosum L.
2.2. Preprocessing of Environmental Data
2.3. Model Prediction and Evaluation for the Suitable Habitats for Xanthium spinosum L.
2.4. Distribution and Area of the Suitable Habitats for Xanthium spinosum L.
2.5. Changes in the Spatial Patterns of the Suitable Habitats for Xanthium spinosum L.
3. Results
3.1. Evaluation of Model Accuracy
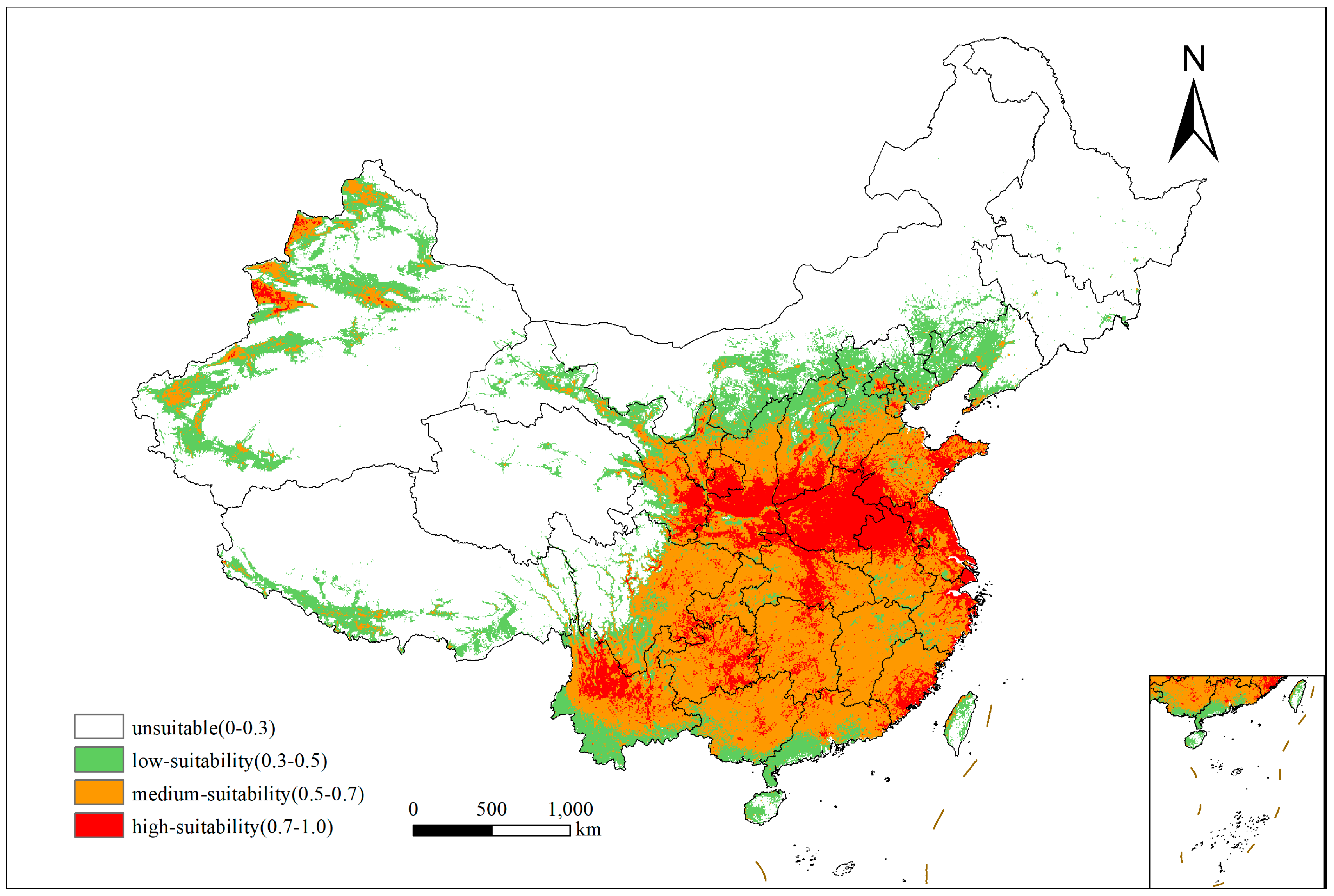
3.2. Current Distribution of the Suitable Habitats for Xanthium spinosum L. in China
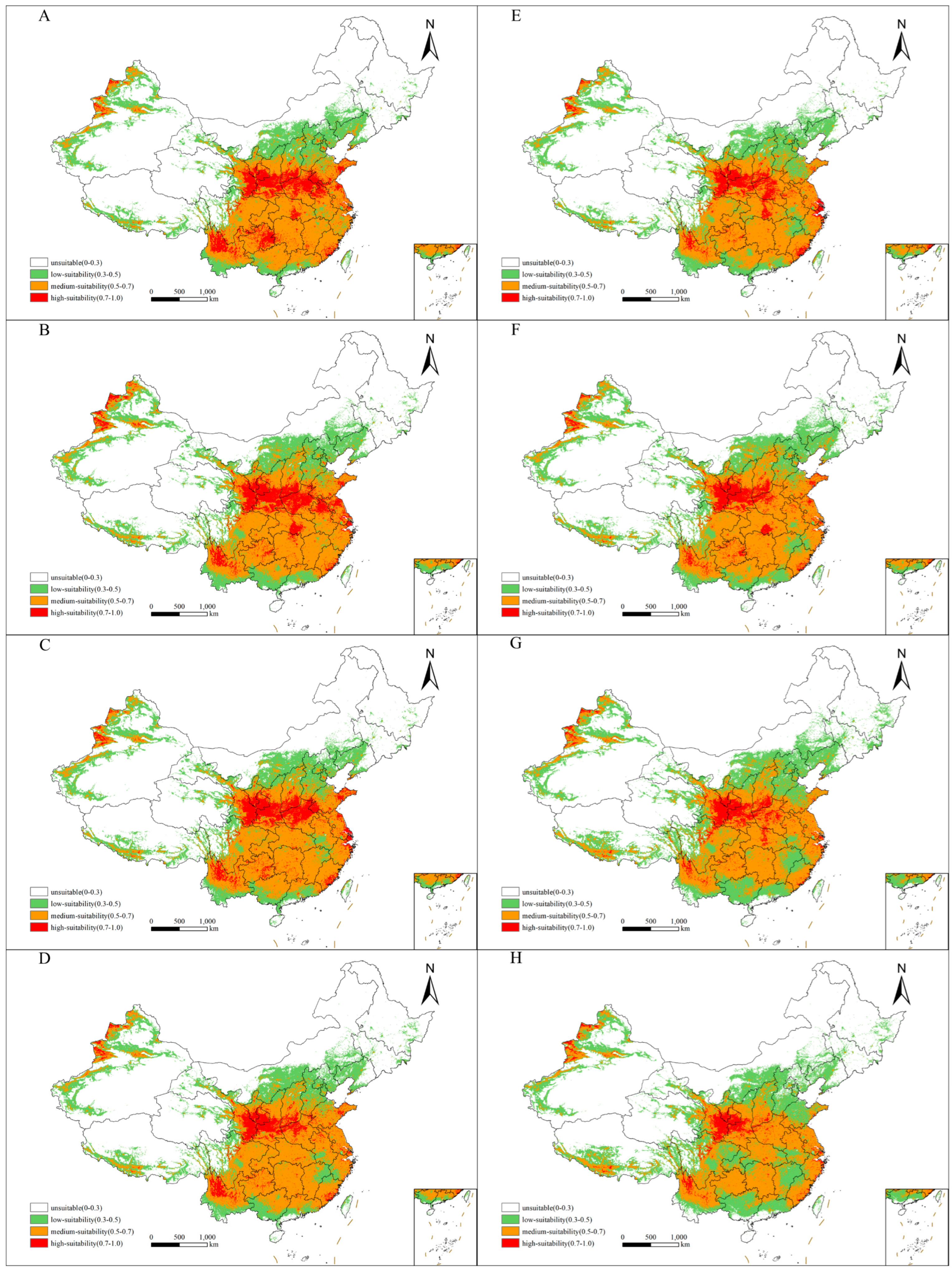
3.3. Future Distribution of Suitable Habitats for Xanthium spinosum L. in China
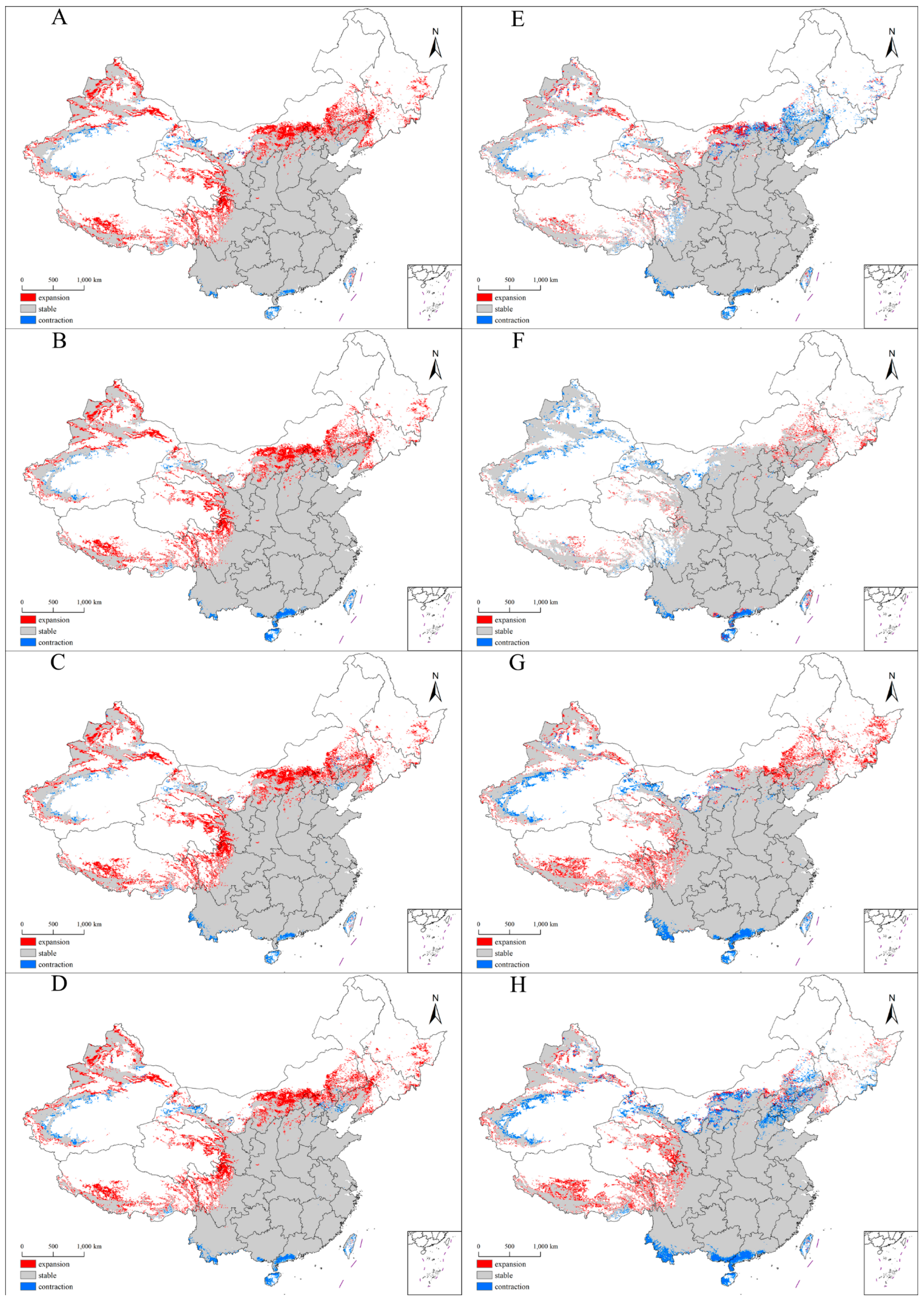
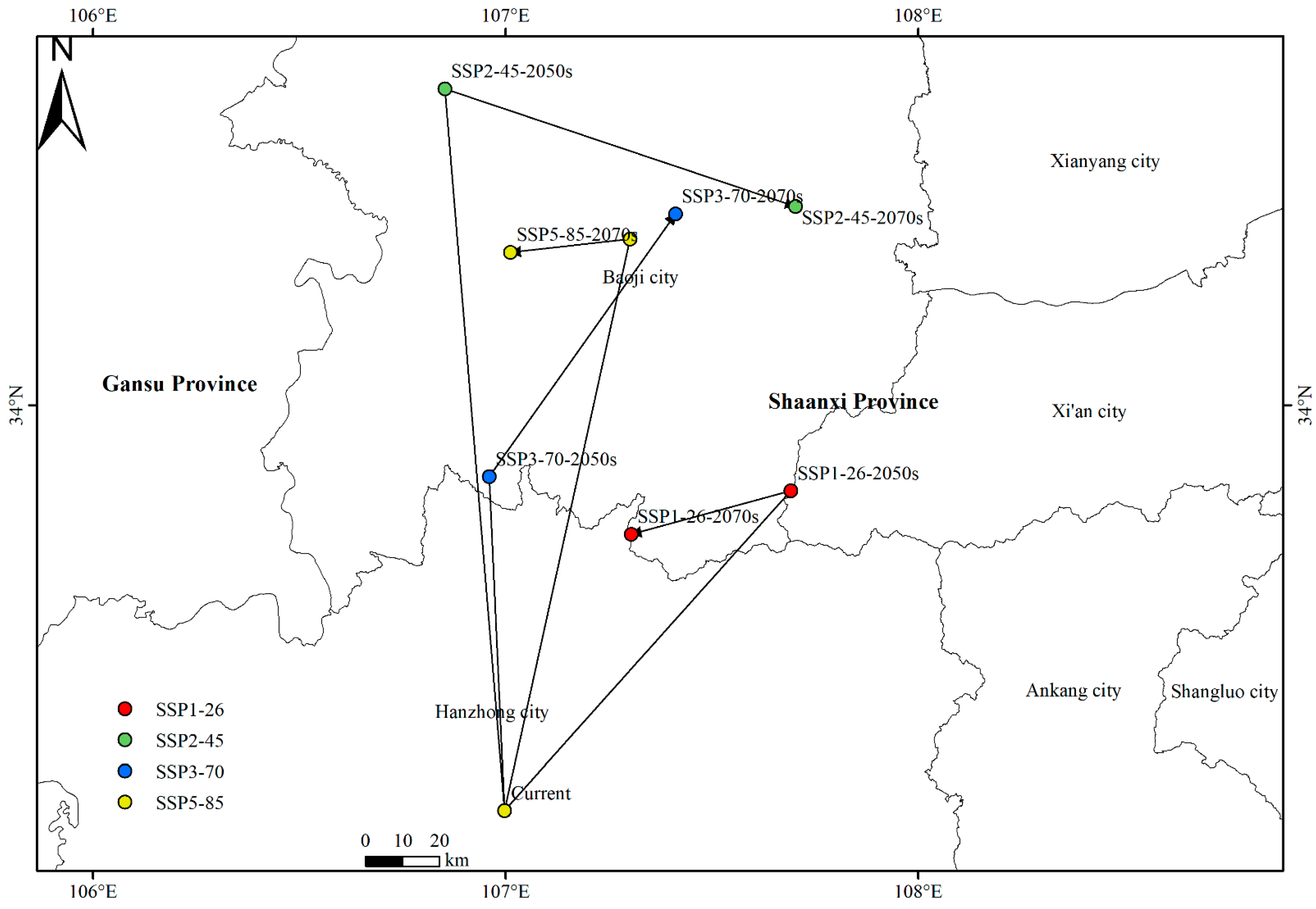
3.4. Future Distribution Patterns of the Suitable Habitats for Xanthium spinosum L. in China
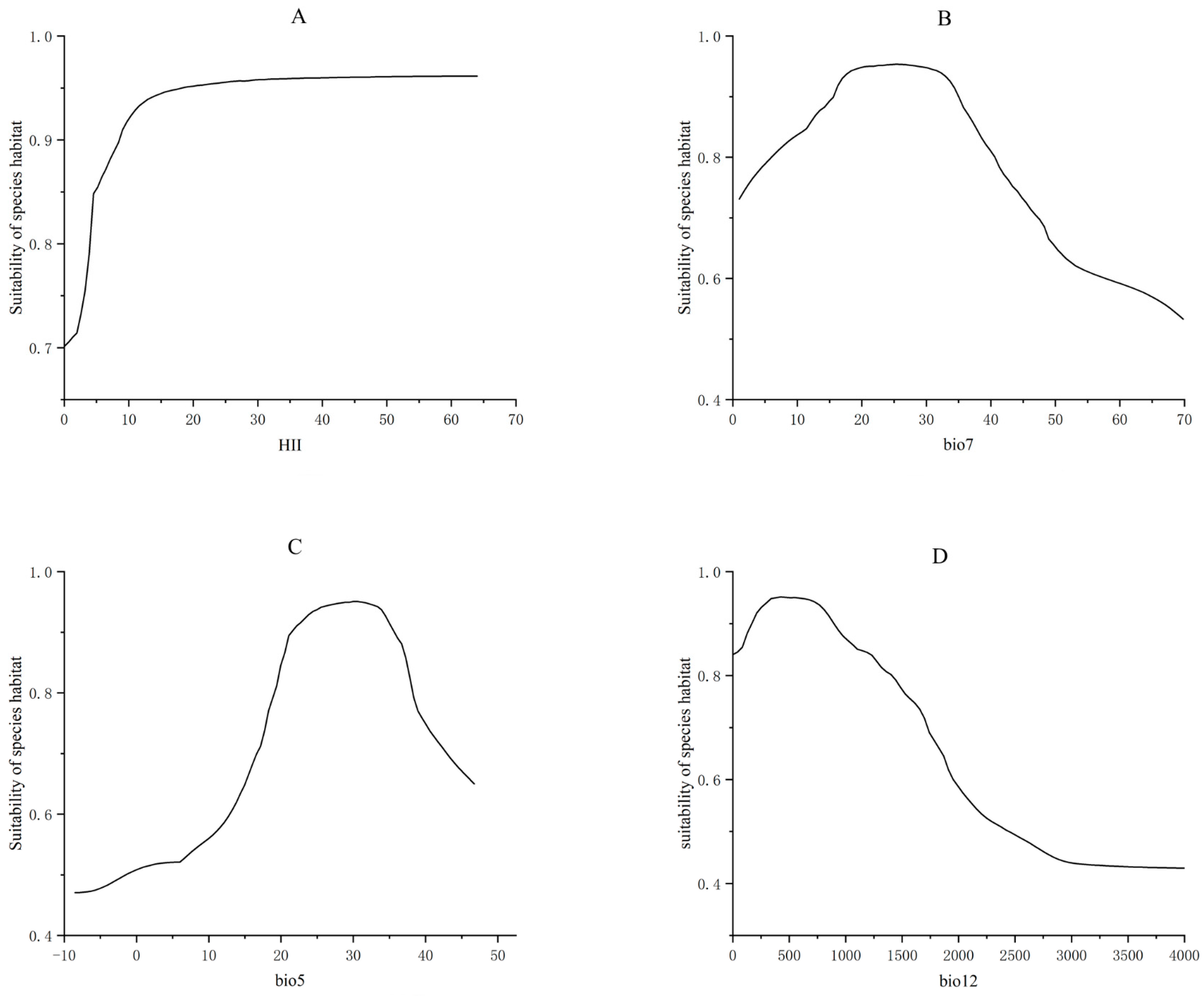
3.5. Analysis of the Environmental Factors Influencing the Distribution of Xanthium spinosum L.
4. Discussion
4.1. Assessment of Biomod2 Model Prediction Performance
4.2. Change Pattern and Shift Direction of the Suitable Habitats for Xanthium spinosum L. Under Future Climate Change
4.3. Human Activities and Climatic Factors Influencing the Distribution of Xanthium spinosum L.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.Y.; Xu, R.M. Overview of exotic animal invasions in China. Bull. Biol. 2007, 1–4, 64. [Google Scholar] [CrossRef]
- Jones, B.A.; McDermott, S.M. Health Impacts of Invasive Species through an Altered Natural Environment: Assessing Air Pollution Sinks as a Causal Pathway. Environ. Resour. Econ. 2018, 71, 23–43. [Google Scholar] [CrossRef]
- Alley, R.B.; Berntsen, T.; Bindoff, N.L.; Chidthaisong, A.; Friedlingstein, P.; Gregory, J.M.; Hegerl, G.C.; Heimann, M.; Hewitson, B.; Hoskins, B.J.; et al. Summary for Policymakers. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Canavan, S.; Richardson, D.M.; Visser, V.; Le Roux, J.J.; Vorontsova, M.S.; Wilson, J.R. The Global Distribution of Bamboos: Assessing Correlates of Introduction and Invasion. AoB Plants 2017, 9, plw078. [Google Scholar] [CrossRef] [PubMed]
- Clements, D.R.; Jones, V.L. Rapid Evolution of Invasive Weeds Under Climate Change: Present Evidence and Future Research Needs. Front. Agron. 2021, 3, 664034. [Google Scholar] [CrossRef]
- Jarvis, C. Order Out of Chaos: Linnaean Plant Names and Their Types; Linnean Society: London, UK, 2007. [Google Scholar]
- Löve, D.; Dansereau, P. Biosystematic Studies on Xanthium: Taxonomic Appraisal and Ecological Status. Can. J. Bot. 1959, 37, 173–208. [Google Scholar] [CrossRef]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds. Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977. [Google Scholar]
- Chen, Y.L.; Chen, S.R. A new synonym of Xanthium sibiricum Patrin ex Widder. J. Syst. Evol. 2004, 42, 191. [Google Scholar]
- Zhao, L.Q.; Zang, C.X.; Yang, J. Distribution of a Invasive Species-Xanthium spinosum L. in Inner Mongolia and Ning Xia. J. Inn. Mong. Univ. 2006, 37, 308–310. [Google Scholar]
- Du, Z.Z.; Xu, W.B.; Yan, P.; Wang, S.S.; Guo, Y. Three Newly Recorded Alien Invasive Plants of Xanthium in Xinjiang. Xinjiang Agric. Sci. 2012, 49, 879–886. [Google Scholar] [CrossRef]
- Song, X.Y.; He, J.Y. Status and Prospect of Research on Alien Invasive Plant Xanthium spinosum L. J. Weed Sci. 2023, 41, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Ma, M. Seed overwinter performance of two invasive plants: Xanthium italicum Moretti and X. spinosum Linnaeus in Xinjiang. Acta Ecol. Sin. 2017, 37, 7181–7186. [Google Scholar] [CrossRef][Green Version]
- Gu, W.; Ma, M. Study on reproductive biology characteristics of invasive plant Xanthium spinosum L. J. Shihezi Univ. 2019, 37, 332–338. [Google Scholar] [CrossRef]
- Liang, Q.L.; Liu, Z.Q.; Lu, P.; Chen, W.M. Distribution and Growth Characteristics of Xanthium spinosum in Yili River Valley of Xinjiang. J. Weed Sci. 2017, 35, 25–29. [Google Scholar] [CrossRef]
- Dong, F.H.; Leng, J.M.; Liu, Y.; Yu, W.L.; Zhao, Y. Allelopathic Effects of Aqueous Extracts from Xanthium Spinosum on Seed Germination and Seedling Growth of Lactuca Sativa Var. Longifolia. Acta Prataculturae Sin. 2017, 26, 146–160. [Google Scholar] [CrossRef]
- Yuan, Z.G.; Liu, Y.; Shao, H.; Zhao, Y.; Hu, Y.X. Allelopathy of Each Part of Invasive Plant Xanthium spinosum L. J. Henan Agric. Sci. 2017, 46, 73–77. [Google Scholar] [CrossRef]
- Nick, P. Xanthium Spinosum (Bathurst Burr). CABI Compend. 2022. [Google Scholar] [CrossRef]
- Lang, Q. Population Distribution and Ecological Characteristics of Xanthium spinosum L. in Yili Region. Rural. Pract. Sci. Technol. 2020, 8, 78–79. [Google Scholar]
- Tao, Y.Y.; Zhao, Y.; Hu, Y.X.; Shang, T.C.; Zhang, Z.Y.; Lang, Q.; Liu, Y. Effects of simulated nitrogen deposition on the growth of Xanthium spinosum. Chin. J. Ecol. 2020, 39, 3971–3978. [Google Scholar] [CrossRef]
- Xiao, Y.K.; He, J.X.; Aishan, T.; Sui, X.Q.; Zhou, Y.F.; Yimingniyazi, A. Effects of Different Degrees of Xanthium Spinosum Invasion on the Invasibility of Plant Communities in the Yili Grassland of Northwest China. Biology 2023, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Beery, S.; Cole, E.; Parker, J.; Perona, P.; Winner, K. Species Distribution Modeling for Machine Learning Practitioners: A Review. In Proceedings of the 4th ACM SIGCAS Conference on Computing and Sustainable Societies, Virtual, 28 June–2 July 2021; Association for Computing Machinery: New York, NY, USA, 2021; pp. 329–348. [Google Scholar] [CrossRef]
- Brambilla, M.; Ficetola, G.F. Species Distribution Models as a Tool to Estimate Reproductive Parameters: A Case Study with a Passerine Bird Species. J. Anim. Ecol. 2012, 81, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Moraitis, M.L.; Valavanis, V.D.; Karakassis, I. Modelling the Effects of Climate Change on the Distribution of Benthic Indicator Species in the Eastern Mediterranean Sea. Sci. Total Environ. 2019, 667, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiao, N.; Shen, M.; Li, J. Comparison between Optimized MaxEnt and Random Forest Modeling in Predicting Potential Distribution: A Case Study with Quasipaa Boulengeri in China. Sci. Total Environ. 2022, 842, 156867. [Google Scholar] [CrossRef] [PubMed]
- Nelder, J.A.; Wedderburn, R.W. Generalized Linear Models. J. R. Stat. Soc. Ser. A Stat. Soc. 1972, 135, 370–384. [Google Scholar] [CrossRef]
- Ho, T.K. Random Decision Forests. In Proceedings of the 3rd International Conference on Document Analysis and Recognition, Montreal, QC, Canada, 14–16 August 1995; Volume 1, pp. 278–282. [Google Scholar] [CrossRef]
- Bajkova, A.T. The Generalization of Maximum Entropy Method for Reconstruction of Complex Functions. Astron. Astrophys. Trans. 1992, 1, 313–320. [Google Scholar] [CrossRef]
- Segurado, P.; Araujo, M.B. An Evaluation of Methods for Modelling Species Distributions. J. Biogeogr. 2004, 31, 1555–1568. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble Forecasting of Species Distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Levinson, D. The Ensemble Approach to Forecasting: A Review and Synthesis. Transp. Res. Part C Emerg. Technol. 2021, 132, 103357. [Google Scholar] [CrossRef]
- Thuiller, W. BIOMOD–Optimizing Predictions of Species Distributions and Projecting Potential Future Shifts under Global Change. Glob. Change Biol. 2003, 9, 1353–1362. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD–a Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Guan, B.; Guo, H.; Chen, S.; Li, D.; Liu, X.; Gong, X.I.; Ge, G. Shifting Ranges of Eleven Invasive Alien Plants in China in the Face of Climate Change. Ecol. Inform. 2020, 55, 101024. [Google Scholar] [CrossRef]
- Jia, T.; Qi, Y.H.; Zhao, H.X.; Xian, X.Q.; Li, J.Y.; Huang, H.K.; Yu, W.T.; Liu, W.X. Estimation of Climate-Induced Increased Risk of Centaurea Solstitialis L. Invasion in China: An Integrated Study Based on Biomod2. Front. Ecol. Evol. 2023, 11, 1113474. [Google Scholar] [CrossRef]
- Xu, L.; Fan, Y.; Zheng, J.H.; Guan, J.Y.; Lin, J.; Wu, J.G.; Liu, L.; Wu, R.; Liu, Y.J. Impacts of Climate Change and Human Activity on the Potential Distribution of Aconitum Leucostomum in China. Sci. Total Environ. 2024, 912, 168829. [Google Scholar] [CrossRef] [PubMed]
- Feijó, A.; Ge, D.Y.; Wen, Z.X.; Cheng, J.L.; Xia, L.; Yang, Q.S. Exploring GBIF Database and Extracting Climate Data from Georeferenced Localities with R Software. Bio-101 2021, e1010609. [Google Scholar] [CrossRef]
- Qin, Y.J.; Wang, C.; Zhao, Z.H.; Pan, X.B.; Li, Z.H. Climate Change Impacts on the Global Potential Geographical Distribution of the Agricultural Invasive Pest, Bactrocera Dorsalis (Hendel)(Diptera: Tephritidae). Clim. Change 2019, 155, 145–156. [Google Scholar] [CrossRef]
- Jiang, Y.B.; Wang, T.J.; Wu, Y.P.; Hu, R.G.; Huang, K.; Shao, X.M. Past Distribution of Epiphyllous Liverworts in China: The Usability of Historical Data. Ecol. Evol. 2018, 8, 7436–7450. [Google Scholar] [CrossRef]
- Zhao, G.H.; Cui, X.Y.; Sun, J.J.; Li, T.T.; Wang, Q.I.; Ye, X.Z.; Fan, B.G. Analysis of the Distribution Pattern of Chinese Ziziphus Jujuba under Climate Change Based on Optimized Biomod2 and MaxEnt Models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Sharma, R.; Khan, S.; Kaul, V. Predicting the Potential Habitat Suitability and Distribution of “Weed-Onion”(Asphodelus Tenuifolius Cavan.) in India under Predicted Climate Change Scenarios. J. Agric. Food Res. 2023, 14, 100697. [Google Scholar] [CrossRef]
- Qin, Z.; Yang, J.H.; Gan, T.; Zhang, J.E.; Liu, Y.M.; Liu, J.M.; Yao, F.C.; Zhao, B.L. Prediction of the Breeding and Wintering Ranges of Pomacea Canaliculata in China Using Ensemble Models. J. Environ. Inform. 2024, 44, 73–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Na, X.D.; Li, W.L. Impacts of Climate Changes on the Potential Habitat Suitability of Grus Japonensis on Migration Routes. Ecol. Indic. 2024, 166, 112462. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Xian, X.Q.; Zhao, H.X.; Wang, R.; Huang, H.K.; Chen, B.X.; Zhang, G.F.; Liu, W.X.; Wan, F.H. Climate Change Has Increased the Global Threats Posed by Three Ragweeds (Ambrosia L.) in the Anthropocene. Sci. Total Environ. 2023, 859, 160252. [Google Scholar] [CrossRef] [PubMed]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Qiao, H.J.; Hu, J.H.; Huang, J.H. Theoretical Basis, Future Directions, and Challenges for Ecological Niche Models. Sci. Sin. 2013, 43, 915–927. [Google Scholar] [CrossRef]
- Tanaka, K.R.; Torre, M.P.; Saba, V.S.; Stock, C.A.; Chen, Y. An Ensemble High-resolution Projection of Changes in the Future Habitat of American Lobster and Sea Scallop in the Northeast US Continental Shelf. Divers. Distrib. 2020, 26, 987–1001. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Wei, H.Y.; Guo, Y.L.; Gu, W. Potential distribution of Panax ginseng and its predicted responses to climate change. Chin. J. Appl. Ecol. 2016, 27, 3607–3615. [Google Scholar] [CrossRef]
- Zhu, N. Modelling the Suitable Habitat Distribution of Magnolia Officinalis Using Ensemble Model. J. Sichuan Agric. Univ. 2019, 37, 481–489. [Google Scholar] [CrossRef]
- McGeoch, M.A.; Butchart, S.H.; Spear, D.; Marais, E.; Kleynhans, E.J.; Symes, A.; Chanson, J.; Hoffmann, M. Global Indicators of Biological Invasion: Species Numbers, Biodiversity Impact and Policy Responses. Divers. Distrib. 2010, 16, 95–108. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of Climate Change on the Future of Biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.M.; Settele, J.; Brondízio, E.; Ngo, H.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.; Butchart, S.; et al. The Global Assessment Report on Biodiversity and Ecosystem Services: Summary for Policy Makers; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2019. [Google Scholar]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will Climate Change Promote Future Invasions? Glob. Change Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Sayit, H.; Nurbay, A.; Xu, Z.L.; Arman, J.; Shao, H.; Vinira, Y. Simulation of potential distribution patterns of the invasive plant species Xanthium spinosum L. (Bathurst burr) in Xinjiang under climate change. Acta Ecologica Sinica 2019, 39, 1551–1559. [Google Scholar] [CrossRef]
- BenDor, T.K.; Metcalf, S.S. The Spatial Dynamics of Invasive Species Spread. Syst. Dyn. Rev. J. Syst. Dyn. Soc. 2006, 22, 27–50. [Google Scholar] [CrossRef]
- BenDor, T.K.; Metcalf, S.S.; Fontenot, L.E.; Sangunett, B.; Hannon, B. Modeling the Spread of the Emerald Ash Borer. Ecol. Model. 2006, 197, 221–236. [Google Scholar] [CrossRef]
- McDermott, S.M.; Finnoff, D.C. Impact of Repeated Human Introductions and the Allee Effect on Invasive Species Spread. Ecol. Model. 2016, 329, 100–111. [Google Scholar] [CrossRef]
- Mairal, M.; Chown, S.L.; Shaw, J.; Chala, D.; Chau, J.H.; Hui, C.; Kalwij, J.M.; Münzbergová, Z.; Jansen van Vuuren, B.; Le Roux, J.J. Human Activity Strongly Influences Genetic Dynamics of the Most Widespread Invasive Plant in the sub-Antarctic. Mol. Ecol. 2022, 31, 1649–1665. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.P. A Framework for Using Niche Models to Estimate Impacts of Climate Change on Species Distributions. Ann. New York Acad. Sci. 2013, 1297, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Marzloff, M.P.; Oliver, E.C.; Barrett, N.S.; Holbrook, N.J.; James, L.; Wotherspoon, S.J.; Johnson, C.R. Differential Vulnerability to Climate Change Yields Novel Deep-Reef Communities. Nat. Clim. Change 2018, 8, 873–878. [Google Scholar] [CrossRef]
- Lu, X.M.; Siemann, E.; Shao, X.; Wei, H.; Ding, J.Q. Climate Warming Affects Biological Invasions by Shifting Interactions of Plants and Herbivores. Glob. Change Biol. 2013, 19, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Guo, S.L.; Ma, C.L.; Yang, J.X.; Kang, X.L.; Li, R. Impact of Climate Change on the Potential Geographical Distribution Patterns of Luculia Pinceana Hook. f. since the Last Glacial Maximum. Forests 2024, 15, 253. [Google Scholar] [CrossRef]
- Gu, R.; Wei, S.P.; Li, J.R.; Zheng, S.H.; Li, Z.T.; Liu, G.L.; Fan, S.H. Predicting the Impacts of Climate Change on the Geographic Distribution of Moso Bamboo in China Based on Biomod2 Model. Eur. J. Forest Res. 2024, 143, 1499–1512. [Google Scholar] [CrossRef]
- Larson, C.D.; Pollnac, F.W.; Schmitz, K.; Rew, L.J. Climate Change and Micro-Topography Are Facilitating the Mountain Invasion by a Non-Native Perennial Plant Species. NeoBiota 2021, 65, 23–45. [Google Scholar] [CrossRef]
- Wiens, J.J.; Graham, C.H. Niche Conservatism: Integrating Evolution, Ecology, and Conservation Biology. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef]
- Li, K.X.; Liu, Z.; Yan, B.H.; Guo, J.L. A multi-factor comprehensive analysis of the distribution and development potentiality of geothermal resource in Baoji region. J. Baoji Univ. Arts Sci. 2022, 42, 86–92. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B. Biodiversity Redistribution under Climate Change: Impacts on Ecosystems and Human Well-Being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Lenoir, J.; Nuñez, M.A.; Pauchard, A.; Geron, C.; Bussé, G.; Milbau, A.; Nijs, I. Microclimate Variability in Alpine Ecosystems as Stepping Stones for Non-native Plant Establishment above Their Current Elevational Limit. Ecography 2018, 41, 900–909. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.P.; Fan, Y.W.; Liu, C.; Wu, P.; Li, Q.Y.; Yin, W.L. Effects of Biophysical Constraints, Climate and Phylogeny on Forest Shrub Allometries along an Altitudinal Gradient in Northeast China. Sci. Rep. 2017, 7, 43769. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.B.; Li, S.P. Modern Coexistence Theory as a Framework for Invasion Ecology. Biodivers. Sci. 2020, 28, 1362. [Google Scholar] [CrossRef]
- Essl, F.; Biró, K.; Brandes, D.; Broennimann, O.; Bullock, J.M.; Chapman, D.S.; Chauvel, B.; Dullinger, S.; Fumanal, B.; Guisan, A. Biological Flora of the British Isles: Ambrosia Artemisiifolia. J. Ecol. 2015, 103, 1069–1098. [Google Scholar] [CrossRef]
- Kueffer, C. Plant Invasions in the Anthropocene. Science 2017, 358, 724–725. [Google Scholar] [CrossRef]
- Yang, H.J.; Li, Y.; Wu, M.Y.; Zhang, Z.; Li, L.H.; Wan, S.Q. Plant Community Responses to Nitrogen Addition and Increased Precipitation: The Importance of Water Availability and Species Traits. Glob. Change Biol. 2011, 17, 2936–2944. [Google Scholar] [CrossRef]
- Lu, Z.J.; Yang, H.B.; Du, W.; Li, Z.Z.; Sun, F.G.; Yang, J.L.; Chen, C.F.; Gao, Y.; Wang, S.M.; Wu, P. Preliminary Study on Control Effects of Five Herbicides on Invasive Species of Xanthium sibiricum in Ningxia. Chin. Agric. Sci. Bull. 2024, 40, 129–132. [Google Scholar] [CrossRef]
| Models | TSS | AUC | ||||
|---|---|---|---|---|---|---|
| Mean | SD | CV | Mean | SD | CV | |
| ANN | 0.7798 | 0.0132 | 0.0170 | 0.9471 | 0.0057 | 0.0060 |
| CTA | 0.8455 | 0.0301 | 0.0356 | 0.9528 | 0.0105 | 0.0110 |
| FDA | 0.7812 | 0.0079 | 0.0102 | 0.9516 | 0.0035 | 0.0037 |
| GAM | 0.6821 | 0.0092 | 0.0135 | 0.9200 | 0.0039 | 0.0043 |
| GLM | 0.7858 | 0.0075 | 0.0096 | 0.9571 | 0.0016 | 0.0017 |
| MARS | 0.7930 | 0.0070 | 0.0089 | 0.9574 | 0.0023 | 0.0024 |
| RF | 0.9861 | 0.0044 | 0.0044 | 0.9999 | 0.0003 | 0.0003 |
| SRE | 0.5680 | 0.0052 | 0.0092 | 0.7841 | 0.0028 | 0.0035 |
| Climate Scenario | Period | High-Suitability Habitat | Medium-Suitability Habitat | Low-Suitability Habitat | Total Suitable Habitat |
|---|---|---|---|---|---|
| Current | 1970–2000 | 77.8056 | 208.6410 | 128.2930 | 414.7396 |
| SSP-1-26 | 2050s | 60.3160 | 227.5660 | 161.5590 | 449.4410 |
| 2070s | 41.0694 | 220.1440 | 174.2380 | 435.4514 | |
| SSP-2-45 | 2050s | 61.2865 | 230.8140 | 174.4440 | 466.5445 |
| 2070s | 42.5226 | 243.8400 | 185.0760 | 471.4386 | |
| SSP-3-70 | 2050s | 53.9792 | 231.4700 | 171.8660 | 457.3152 |
| 2070s | 37.2483 | 226.3350 | 219.1390 | 482.7223 | |
| SSP-5-85 | 2050s | 40.1059 | 238.7590 | 179.2450 | 458.1099 |
| 2070s | 26.5990 | 207.4980 | 224.1910 | 458.2880 |
| Climate Scenario | Area (×104 km2) | Change (%) | |||||
|---|---|---|---|---|---|---|---|
| Expansion | Unchanged | Contraction | Expansion | Unchanged | Contraction | ||
| Current vs. 2050s | SSP1-26 | 41.24 | 408.20 | 6.54 | 9.94 | 98.42 | 1.58 |
| SSP2-45 | 59.93 | 406.62 | 8.12 | 14.45 | 98.04 | 1.96 | |
| SSP3-70 | 50.12 | 407.20 | 7.54 | 12.08 | 98.18 | 1.82 | |
| SSP5-85 | 54.12 | 403.99 | 10.75 | 13.05 | 97.41 | 2.59 | |
| 2050s vs. 2070s | SSP1-26 | 2.43 | 433.02 | 16.42 | 0.54 | 96.35 | 3.65 |
| SSP2-45 | 13.05 | 458.39 | 8.15 | 2.80 | 98.25 | 1.75 | |
| SSP3-70 | 39.81 | 442.91 | 14.41 | 8.71 | 96.85 | 3.15 | |
| SSP5-85 | 29.19 | 429.10 | 29.01 | 6.37 | 93.67 | 6.33 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, Y.; Wang, R.; Guo, L.; Ji, Y.; Chen, Y.; Hao, L.; Lin, K. Impacts of Human Activity and Climate Change on the Suitable Habitats for Xanthium spinosum in China. Plants 2025, 14, 306. https://doi.org/10.3390/plants14030306
Liu Y, Li Y, Wang R, Guo L, Ji Y, Chen Y, Hao L, Lin K. Impacts of Human Activity and Climate Change on the Suitable Habitats for Xanthium spinosum in China. Plants. 2025; 14(3):306. https://doi.org/10.3390/plants14030306
Chicago/Turabian StyleLiu, Yabin, Yuyu Li, Rui Wang, Lizhu Guo, Yu Ji, Yihao Chen, Lifen Hao, and Kejian Lin. 2025. "Impacts of Human Activity and Climate Change on the Suitable Habitats for Xanthium spinosum in China" Plants 14, no. 3: 306. https://doi.org/10.3390/plants14030306
APA StyleLiu, Y., Li, Y., Wang, R., Guo, L., Ji, Y., Chen, Y., Hao, L., & Lin, K. (2025). Impacts of Human Activity and Climate Change on the Suitable Habitats for Xanthium spinosum in China. Plants, 14(3), 306. https://doi.org/10.3390/plants14030306






