VsMATE1-Mediated Citrate Efflux Is Involved in Al Resistance in Common Vetch (Vicia sativa L.)
Abstract
1. Introduction
2. Results
2.1. Growth Response to Al in Seven Legume Species
2.2. Comparison of Al Tolerance in Eleven Common Vetch Collections
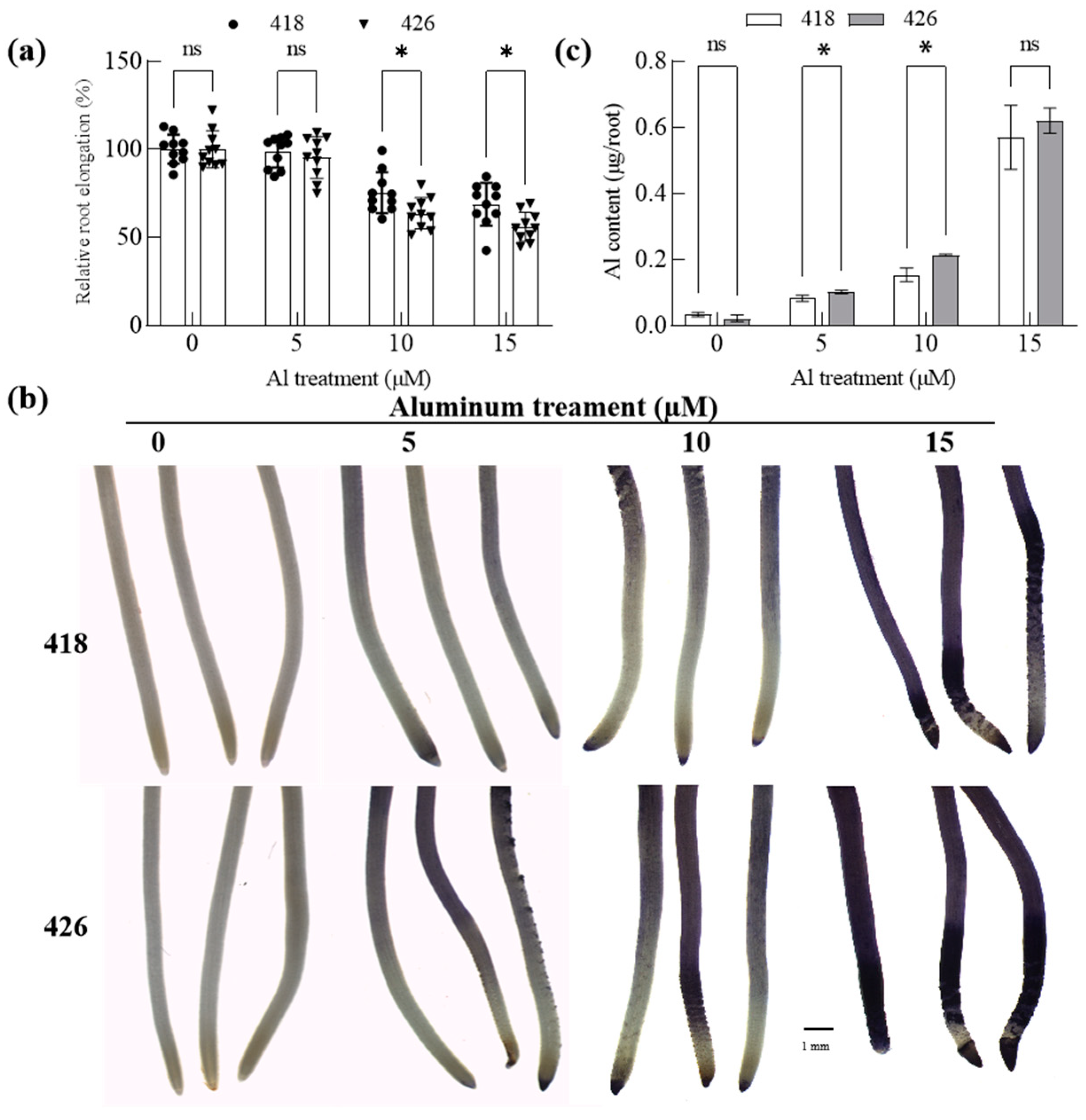
2.3. The Al-Resistant Common Vetch Accumulated Less Al than the Al-Sensitive One
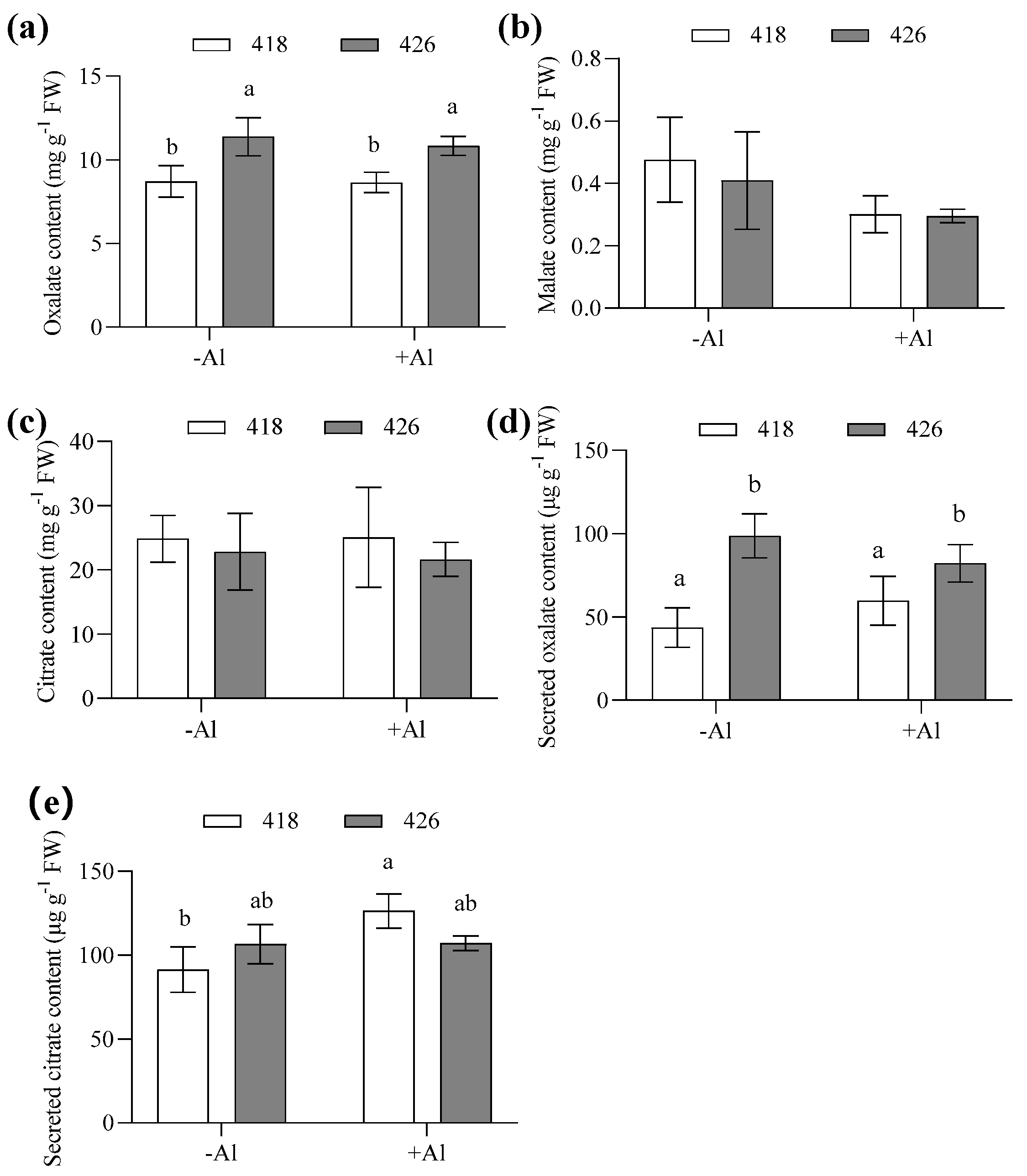
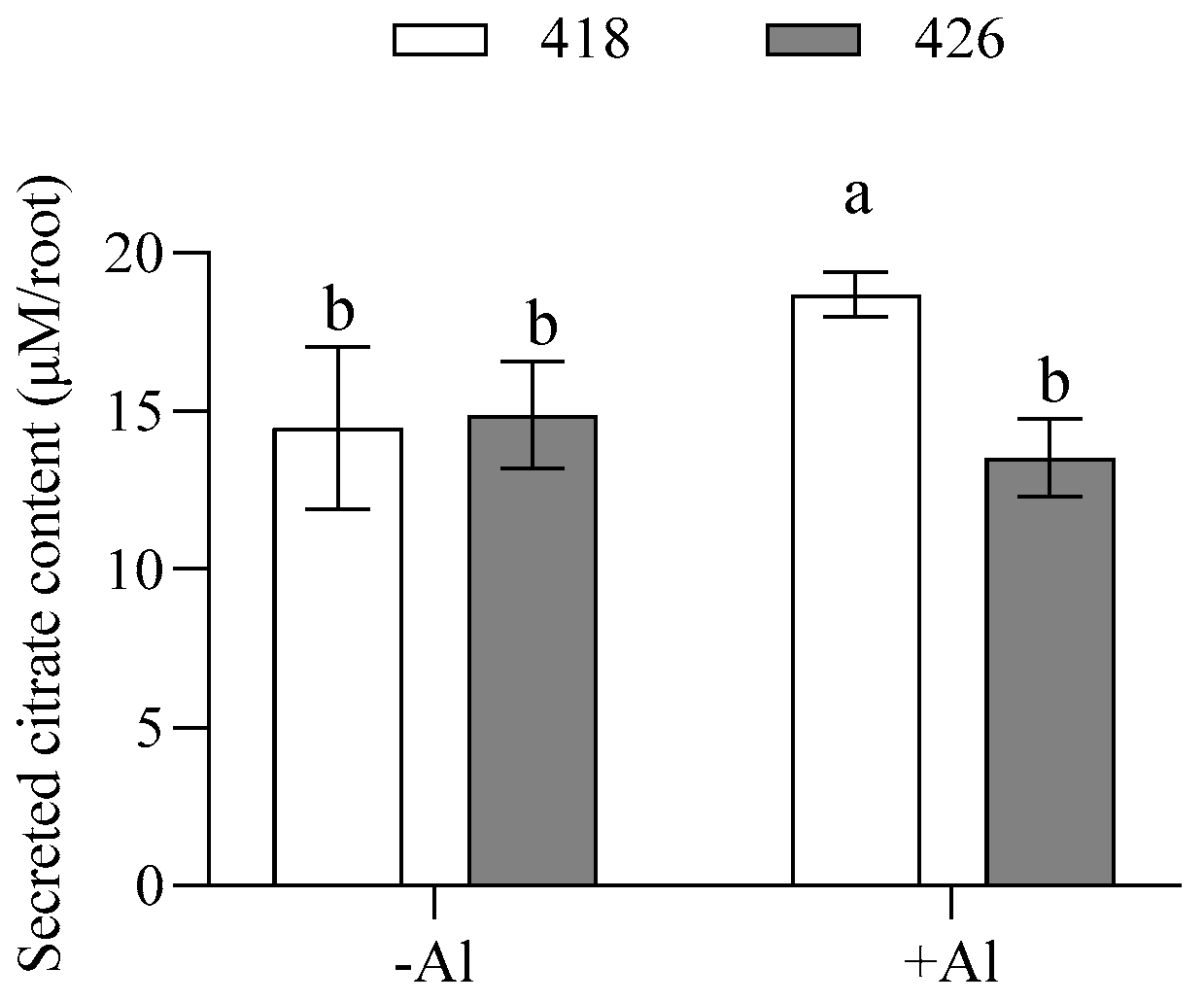
2.4. The Al-Resistant Common Vetch Released More Citrate than the Al-Sensitive One
2.5. VsMATE1 Responds to Al More Dramatically and Faster in the Al-Resistant Common Vetch than the Sensitive One
3. Discussion
3.1. Common Vetch Is an Al-Resistant Legume with Great Potential for Application in Acidic Soils
3.2. VsMATE1-Mediated Citrate Exclusion May Play an Important Role in Al Resistance in Common Vetch
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Treatments
4.2. Determination of Al Content
4.3. Measurements of Internal and External OAs
4.4. Identification and Cloning of VsMATE1
4.5. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Al | Aluminum |
| OA | Organic acid |
| ICP-OES | Inductively coupled plasma mass spectrometry |
| HPLC | High-performance liquid chromatography |
References
- Xu, J.; Si, L.L.; Zhang, X.; Cao, K.; Wang, J.H. Various green manure-fertilizer combinations affect the soil microbial community and function in immature red soil. Front. Microbiol. 2023, 14, 1255056. [Google Scholar] [CrossRef]
- Pypers, P.; Verstraete, S.; Thi, C.P.; Merckx, R. Changes in mineral nitrogen, phosphorus availability and salt-extractable aluminium following the application of green manure residues in two weathered soils of South Vietnam. Soil Biol. Biochem. 2005, 37, 163–172. [Google Scholar] [CrossRef]
- Qin, R.J.; Chen, F.X. Amelioration of aluminum toxicity in red soil through use of barnyard and green manure. Commun. Soil Sci. Plan. 2005, 36, 1875–1889. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. Differential soil acidity tolerance of tropical legume cover crops. Commun. Soil Sci. Plan. 2009, 40, 1148–1160. [Google Scholar] [CrossRef]
- Mackay, A.D.; Caradus, J.R.; Pritchard, M.W. Variation for aluminum tolerance in white clover. Plant Soil 1990, 123, 101–105. [Google Scholar] [CrossRef]
- Scheffer-Basso, S.M.; Prior, B.C. Aluminum toxicity in roots of legume seedlings assessed by topological analysis. Acta Sci.-Agron. 2015, 37, 61–68. [Google Scholar] [CrossRef][Green Version]
- Kidd, D.; Premaratne, M.; Wisdom, J.; Nicol, D.; Ryan, M.H. An agronomic study of legacy effects from annual legume pastures in acid soils. J. Agron. Crop Sci. 2023, 209, 439–458. [Google Scholar] [CrossRef]
- Quinones, M.A.; Lucas, M.M.; Pueyo, J.J. Adaptive mechanisms make lupin a choice crop for acidic soils affected by aluminum toxicity. Front. Plant Sci. 2022, 12, 810692. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Hu, X.; Wang, L.J.; Schultze-Kraft, R.; Wang, W.Q.; Chen, Z.J. Characterization of SgALMT genes reveals the function of SgALMT2 in conferring aluminum tolerance in Stylosanthes guianensis through the mediation of malate exudation. Plant Physiol. Biochem. 2024, 208, 108535. [Google Scholar] [CrossRef] [PubMed]
- Alzueta, C.; Caballero, R.; Rebole, A.; Trevino, J.; Gil, A. Crude protein fractions in common vetch (Vicia sativa L.) fresh forage during pod filling. J. Anim. Sci. 2001, 79, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Parra, E.; De la Rosa, L. Designing novel strategies for improving old legumes: An overview from common vetch. Plants 2023, 12, 1275. [Google Scholar] [CrossRef]
- Basheer-Salimia, R.; Aloweidat, M.Y.; Al-Salimiya, M.A.; Hamdan, Y.A.S.; Sayara, T.A.S. Comparative study of five legume species under drought conditions. Legume Res. 2021, 44, 712–717. [Google Scholar] [CrossRef]
- Xu, L.X.; Tang, G.J.; Wu, D.; Zhang, J.G. Yield and nutrient composition of forage crops and their effects on soil characteristics of winter fallow paddy in South China. Front. Plant Sci. 2024, 14, 1292114. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Li, Y.; Hou, Y.; Zhao, Z.; Meng, L.; Liu, J.; Wang, J.; Xiong, B.; Wang, Z. Cover crops control weed and improve soil qualities in citrus orchard. J. Soil. Sci. Plant Nutr. 2023, 23, 6827–6837. [Google Scholar] [CrossRef]
- Ligaba, A.; Yamaguchi, M.; Shen, H.; Sasaki, T.; Yamamoto, Y.; Matsumoto, H. Phosphorus deficiency enhances plasma membrane H-ATPase activity and citrate exudation in greater purple lupin (Lupinus pilosus). Funct. Plant Biol. 2004, 31, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Shen, J.B.; Zhang, W.H.; Zhang, F.S.; Neumann, G. Citrate exudation from white lupin induced by phosphorus deficiency differs from that induced by aluminum. New Phytol. 2007, 176, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.P.; Neuhäuser, B.; Neumann, G.; Ludewig, U. LaALMT1 mediates malate release from phosphorus-deficient white lupin root tips and metal root to shoot translocation. Plant Cell Environ. 2020, 43, 1691–1706. [Google Scholar] [CrossRef]
- Silva, I.R.; Smyth, T.J.; Raper, C.D.; Carter, T.E.; Rufty, T.W. Differential aluminum tolerance in soybean: An evaluation of the role of organic acids. Physiol. Plant 2001, 112, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.L.; Zhu, H.F.; Wen, S.L.; Liu, L.S.; Gou, L.M.; Guo, Z.F. Citrate synthesis and exudation confer Al resistance in alfalfa (Medicago sativa L.). Plant Soil 2020, 449, 319–329. [Google Scholar] [CrossRef]
- Jia, Y.; Pradeep, K.; Vance, W.H.H.; Zhang, X.; Weir, B.; Wei, H.R.; Deng, Z.W.; Zhang, Y.J.; Xu, X.X.; Zhao, C.X.; et al. Identification of two chickpea multidrug and toxic compound extrusion transporter genes transcriptionally upregulated upon aluminum treatment in root tips. Front. Plant Sci. 2022, 13, 909045. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Ji, F.; Zhang, Y.Y.; Hou, J.J.; Liu, W.W.; Huang, J.J.; Liang, W.H. Interactions between hydrogen sulphide and nitric oxide regulate two soybean citrate transporters during the alleviation of aluminium toxicity. Plant Cell Environ. 2019, 42, 2340–2356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Z.B.; Gong, L.; Chen, A.L.; Liu, N.; Li, S.; Sun, H.R.; Yang, Z.M.; You, J.F. Functional characterization of three MATE genes in relation to aluminum-induced citrate efflux from soybean root. Plant Soil 2019, 443, 121–138. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Cui, W.; Gong, L.; He, Y.; Zhang, Q.; Meng, X.; Yang, Z.; You, J. Characterization of GmMATE13 in its contribution of citrate efflux and aluminum resistance in soybeans. Front. Plant Sci. 2022, 13, 1027560. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; Vinecky, F.; Duarte, K.E.; Santiago, T.R.; das Chagas Noqueli Casari, R.A.; Hell, A.F.; da Cunha, B.A.; Martins, P.K.; da Cruz Centeno, D.; de Oliveira Molinari, P.A.; et al. Enhanced aluminum tolerance in sugarcane: Evaluation of SbMATE overexpression and genome-wide identification of ALMTs in Saccharum spp. BMC Plant Biol. 2021, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.R.; Delhaize, E.; Randall, P.J. Malate efflux from root apices and tolerance to aluminum are highly correlated in wheat. Aust. J. Plant Physiol. 1995, 22, 531–536. [Google Scholar] [CrossRef]
- Jin, D.H.; Chen, J.L.; Kang, Y.M.; Yang, F.; Yu, D.W.; Liu, X.Q.; Yan, C.C.; Guo, Z.F.; Zhang, Y. Genome-wide characterization, transcriptome profiling, and functional analysis of the ALMT gene family in Medicago for aluminum resistance. J. Plant Physiol. 2024, 297, 154262. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Liao, H. Organic acid anions: An effective defensive weapon for plants against aluminum toxicity and phosphorus deficiency in acidic soils. J. Genet. Genom. 2016, 43, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.H.; Lu, P.; Liu, Y.Y.; Hou, Z.G.; Fu, L.R.; Shi, J.; Guo, Z.F.; Zhu, H.F. Comprehensive evaluation of phosphate deficiency tolerance in common vetch germplasms and the adaption mechanism to phosphate deficiency. J. Plant Physiol. 2024, 302, 154317. [Google Scholar] [CrossRef]
- Liao, H.; Wan, H.; Shaff, J.; Wang, X.; Yan, X.; Kochian, L.V. Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system. Plant Physiol. 2006, 141, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Dong, X.Y.; Zheng, L.; Shen, R.F.; Lan, P. Can aluminum tolerant wheat cultivar perform better under phosphate deficient conditions? Int. J. Mol. Sci. 2018, 19, 2964. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, Y.J.; Zhou, Y.; Yang, J.L.; Zheng, S.J. Protecting cell walls from binding aluminum by organic acids contributes to aluminum resistance. J. Integr. Plant Biol. 2009, 51, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.J.; Tian, Z.; Yang, Z.Y.; Yin, X.Y.; Dong, R. Comparative transcriptomic analysis reveals coordinated mechanisms of different genotypes of common vetch root in response to Al stress. Environ. Exp. Bot. 2023, 213, 105450. [Google Scholar] [CrossRef]
- Bürgmann, H.; Meier, S.; Bunge, M.; Widmer, F.; Zeyer, J. Effects of model root exudates on structure and activity of a soil diazotroph community. Environ. Microbiol. 2005, 7, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.; Ma, Q.; Shi, Q.; Cai, Z.; Zhang, Y.; Cheng, Y.; Nian, H. High aluminum stress drives different rhizosphere soil enzyme activities and bacterial community structure between aluminum-tolerant and aluminum-sensitive soybean genotypes. Plant Soil 2019, 440, 409–425. [Google Scholar] [CrossRef]
- Furukawa, J.; Yamaji, N.; Wang, H.; Mitani, N.; Murata, Y.; Sato, K.; Katsuhara, M.; Takeda, K.; Ma, J.F. An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 2007, 48, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Raman, H.; Zhou, M.X.; Ryan, P.R.; Delhaize, E.; Hebb, D.M.; Coombes, N.; Mendham, N. High-resolution mapping of the locus and identification of a candidate gene controlling aluminium tolerance in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2007, 115, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Nguyen, V.; Ward, C.; Liu, Z.; Searle, I.R. Chromosome-level assembly of the common vetch (Vicia sativa) reference genome. GigaByte 2022, 2022, 38. [Google Scholar] [CrossRef]
- Yang, J.L.; Fan, W.; Zheng, S.J. Mechanisms and regulation of aluminum-induced secretion of organic acid anions from plant roots. J. Zhejiang Univ. Sci. B 2019, 20, 513–527. [Google Scholar] [CrossRef]
- Dong, R.; Dong, D.; Luo, D.; Zhou, Q.; Chai, X.; Zhang, J.; Xie, W.; Liu, W.; Dong, Y.; Wang, Y.; et al. Transcriptome analyses reveal candidate pod shattering-associated genes involved in the pod ventral sutures of common vetch (Vicia sativa L.). Front. Plant Sci. 2017, 8, 649. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Shi, J.; He, L.; Hou, Z.; Guo, Z.; Zhu, H. VsMATE1-Mediated Citrate Efflux Is Involved in Al Resistance in Common Vetch (Vicia sativa L.). Plants 2025, 14, 290. https://doi.org/10.3390/plants14020290
Yan W, Shi J, He L, Hou Z, Guo Z, Zhu H. VsMATE1-Mediated Citrate Efflux Is Involved in Al Resistance in Common Vetch (Vicia sativa L.). Plants. 2025; 14(2):290. https://doi.org/10.3390/plants14020290
Chicago/Turabian StyleYan, Wenhui, Jia Shi, Ling He, Zigang Hou, Zhenfei Guo, and Haifeng Zhu. 2025. "VsMATE1-Mediated Citrate Efflux Is Involved in Al Resistance in Common Vetch (Vicia sativa L.)" Plants 14, no. 2: 290. https://doi.org/10.3390/plants14020290
APA StyleYan, W., Shi, J., He, L., Hou, Z., Guo, Z., & Zhu, H. (2025). VsMATE1-Mediated Citrate Efflux Is Involved in Al Resistance in Common Vetch (Vicia sativa L.). Plants, 14(2), 290. https://doi.org/10.3390/plants14020290






