Advancing the Taxonomy of the Diatom Pseudo-nitzschia Through an Integrative Study Conducted in the Central and Southeastern Adriatic Sea
Abstract
1. Introduction
2. Results
2.1. Morphological Characterisation
2.2. Phylogenetic Analyses Based on ITS, LSU, and rbcL
3. Discussion
4. Materials and Methods
4.1. Study Area and Sampling
4.2. Cell Isolation and Cultivation
4.3. Morphological Analyses
4.4. DNA Extraction, PCR Amplification, and Sequencing
4.5. Sequence Analysis and Phylogeny
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lelong, A.L.; Hegaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Bates, S.S.; Garrison, D.L.; Horner, R.A. Bloom Dynamics and Physiology of Domoic Acid Producing Pseudo-nitzschia Species. In Physiological Ecology of Harmful Algal Blooms; Springer: Heidelberg, Germany, 1998; pp. 267–292. [Google Scholar]
- Dong, H.C.; Lundholm, N.; Teng, S.T.; Li, A.; Wang, C.; Hu, Y.; Li, Y. Occurrence of Pseudo-nitzschia species and associated domoic acid production along the Guangdong coast, South China Sea. Harmful Algae 2020, 98, 101899. [Google Scholar] [CrossRef]
- Lundholm, N. Bacillariophyta, in IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. Available online: https://www.marinespecies.org/hab (accessed on 19 September 2024).
- Lundholm, N.; Christensen, A.L.; Olesen, A.K.J.; Beszteri, B.; Eggers, S.L.; Krock, B.; Altenburger, A. Diversity, toxicity, and distribution of potentially toxic diatoms in Antarctic waters––With description of Pseudo-nitzschia meridionalis sp. nov. and P. glacialis sp. nov. Harmful Algae 2024, 139, 102724. [Google Scholar] [CrossRef]
- von Dassow, P.; Mikhno, M.; Percopo, I.; Orellana, V.R.; Aguilera, V.; Álvarez, G.; Araya, M.; Cornejo-Guzmán, S.; Llona, T.; Mardones, J.I.; et al. Diversity and toxicity of the planktonic diatom genus Pseudo-nitzschia from coastal and offshore waters of the Southeast Pacific, including Pseudo-nitzschia dampieri sp. nov. Harmful Algae 2023, 130, 102520. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, University of Galway. Available online: https://www.algaebase.org (accessed on 15 September 2024).
- Niu, B.; Pang, J.; Lundholm, N.; Liang, C.; Teng, S.T.; Zheng, Q.; Guo, X.; Li, Y. A Pseudo-nitzschia metabarcoding approach with a calibrated ITS1 reference sequence database applied in the Taiwan Strait. Harmful Algae 2024, 133, 102602. [Google Scholar] [CrossRef]
- Amato, A.; Kooistra, W.H.C.F.; Levialdi Ghiron, J.H.; Mann, D.G.; Pröschold, T.; Montresor, M. Reproductive Isolation among Sympatric Cryptic Species in Marine Diatoms. Protist 2007, 158, 193–207. [Google Scholar] [CrossRef]
- Amato, A.; Kooistra, W.H.C.F.; Montresor, M. Cryptic Diversity: A Long-lasting Issue for Diatomologists. Protist 2019, 170, 1–7. [Google Scholar] [CrossRef]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef]
- Burić, Z.; Viličić, D.; Mihalić, K.C.; Carić, M.; Kralj, K.; Ljubešić, N. Pseudo-nitzschia blooms in the Zrmanja river estuary (Eastern Adriatic sea). Diatom Res. 2008, 23, 51–63. [Google Scholar] [CrossRef]
- Ljubešić, Z.; Bosak, S.; Viličić, D.; Borojević, K.K.; Marić, D.; Godrijan, J.; Ujević, I.; Peharec, P.; Dakovac, T. Ecology and taxonomy of potentially toxic Pseudo-nitzschia species in Lim Bay (north-eastern Adriatic Sea). Harmful Algae 2011, 10, 713–722. [Google Scholar] [CrossRef]
- Arapov, J.; Ujević, I.; Pfannkuchen, D.M.; Godrijan, J.; Bakrač, A.; Ninčević Gladan, Ž.; Marasović, I. Domoic acid in phytoplankton net samples and shellfish from the Krka River estuary in the central Adriatic Sea. Mediterr. Mar. Sci. 2016, 17, 340–350. [Google Scholar] [CrossRef]
- Arapov, J.; Skejić, S.; Bužančić, M.; Bakrač, A.; Vidjak, O.; Bojanić, N.; Ujević, I.; Ninčević Gladan, Ž. Taxonomical diversity of Pseudo-nitzschia from the Central Adriatic Sea. Phycol. Res. 2017, 65, 280–290. [Google Scholar] [CrossRef]
- Arapov, J.; Bužančić, M.; Skejić, S.; Mandić, J.; Bakrač, A.; Straka, M.; Ninčević Gladan, Ž. Phytoplankton dynamics in the middle Adriatic estuary, with a focus on the potentially toxic genus Pseudo-nitzschia. J. Mar. Sci. Eng. 2020, 8, 608. [Google Scholar] [CrossRef]
- Arapov, J.; Tomašević, T.; Bonačić, T.; Pejković, M.; Bužančić, M.; Bušelić, I.; Lepen Pleić, I.; Casabianca, S.; Penna, A.; Skejić, S.; et al. A New Insight into the Taxonomy of Pseudo-nitzschia Genus from the Adriatic Sea: Description of P. brasiliana, P. galaxiae, P. hasleana, and P. Linea. J. Mar. Sci. Eng. 2023, 11, 1370. [Google Scholar] [CrossRef]
- Grbin, D.; Pfannkuchen, M.; Babić, I.; Mejdandžić, M.; Mihanović, H.; Marić Pfannkuchen, D.; Godrijan, J.; Peharec Štefanić, P.; Olujić, G.; Ljubešić, Z. Multigene phylogeny and morphology of newly isolated strain of Pseudo-nitzschia mannii Amato & Montresor (Adriatic Sea). Diatom Res. 2017, 32, 127–131. [Google Scholar] [CrossRef]
- Arapov, J.; Bužančić, M.; Penna, A.; Casabianca, S.; Capellacci, S.; Andreoni, F.; Skejić, S.; Bakrač, A.; Straka, M.; Mandić, J.; et al. High proliferation of Pseudo-nitzschia cf. arenysensis in the Adriatic Sea: Ecological and morphological characterisation. Mediterr. Mar. Sci. 2020, 21, 759–774. [Google Scholar] [CrossRef]
- Percopo, I.; Ruggiero, M.V.; Sarno, D.; Longobardi, L.; Rossi, R.; Piredda, R.; Zingone, A. Phenological segregation suggests speciation by time in the planktonic diatom Pseudo-nitzschia allochrona sp. nov. Ecol. Evol. 2022, 12, e9155. [Google Scholar] [CrossRef]
- Marić Pfannkuchen, D. Potentially Toxic Diatom Genus Pseudo-nitzschia in the Northern Adriatic Sea: Ecological, taxonomic and Molecular featurs. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2013. [Google Scholar]
- Turk Dermastia, T.; Cerino, F.; Stanković, D.; Francé, J.; Ramšak, A.; Žnidarič Tušek, M.; Beran, A.; Natali, V.; Cabrini, M.; Mozetič, P. Ecological time series and integrative taxonomy unveil seasonality and diversity of the toxic diatom Pseudo-nitzschia H. Peragallo in the northern Adriatic Sea. Harmful Algae 2020, 93, 101773. [Google Scholar] [CrossRef]
- Giulietti, S.; Romagnoli, T.; Siracusa, M.; Bacchiocchi, S.; Totti, C.; Accoroni, S. Integrative taxonomy of the Pseudo-nitzschia (Bacillariophyceae) populations in the NW Adriatic Sea, with a focus on a novel cryptic species in the P. delicatissima species complex. Phycologia 2021, 60, 247–264. [Google Scholar] [CrossRef]
- Ujević, I.; Ninčević Gladan, Ž.; Roje, R.; Skejić, S.; Arapov, J.; Marasović, I. Domoic acid-A new toxin in the Croatian adriatic shellfish toxin profile. Molecules 2010, 15, 6835–6849. [Google Scholar] [CrossRef] [PubMed]
- Lundholm, N.; Moestrup, Ø.; Hasle, G.R.; Hoef-Emden, K. A study of the Pseudo-nitzschia pseudodelicatissima/cuspidata complex (Bacillariophyceae): What is P. pseudodelicatissima? J. Phycol. 2003, 39, 797–813. [Google Scholar] [CrossRef]
- Penna, A.; Casabianca, S.; Perini, F.; Bastianini, M.; Riccardi, E.; Pigozzi, S.; Scardi, M. Toxic Pseudo-nitzschia spp. in the northwestern Adriatic Sea: Characterization of species composition by genetic and molecular quantitative analyses. J. Plankton Res. 2013, 35, 352–366. [Google Scholar] [CrossRef]
- Lundholm, N.; Moestrup, Ø.; Kotaki, Y.; Hoef-Emden, K.; Scholin, C.; Miller, P. Inter- and intraspecific variation of the Pseudo-nitzschia delicatissima complex (Bacillariophyceae) illustrated by rRNA probes, morphological data and phylogenetic analyses. J. Phycol. 2006, 42, 464–481. [Google Scholar] [CrossRef]
- Smodlaka Tanković, M.; Baričević, A.; Gerić, M.; Domijan, A.M.; Pfannkuchen, D.M.; Kužat, N.; Ujević, I.; Kuralić, M.; Rožman, M.; Matković, K.; et al. Characterisation and toxicological activity of three different Pseudo-nitzschia species from the northern Adriatic Sea (Croatia). Environ. Res. 2022, 214, 114108. [Google Scholar] [CrossRef]
- Lim, H.C.; Tan, S.N.; Teng, S.T.; Lundholm, N.; Orive, E.; David, H.; Quijano-Scheggia, S.; Leong, S.C.Y.; Wolf, M.; Bates, S.S.; et al. Phylogeny and species delineation in the marine diatom Pseudo-nitzschia (Bacillariophyta) using cox1, LSU, and ITS2 rRNA genes: A perspective in character evolution. J. Phycol. 2018, 54, 234–248. [Google Scholar] [CrossRef]
- Amato, A.; Orsini, L.; D’Alelio, D.; Montresor, M. Life cycle, size reduction patterns, and ultrastructure of the pennate planktonic diatom Pseudo-nitzschia delicatissima (Bacillariophyceae). J. Phycol. 2005, 41, 542–556. [Google Scholar] [CrossRef]
- Artegiani, A.; Bregant, D.; Paschini, E.; Pinardi, N.; Raicich, F.; Russo, A. The Adriatic Sea General Circulation. Part I: Air-Sea Interactions and Water Mass Structure. J. Phys. Oceanogr. 1996, 27, 1492–1513. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Academic Press, Phycological Society of America: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Hasle, G.R.; Fryxell, G.A. Diatoms: Cleaning and Mounting for Light and Electron Microscopy. Trans. Am. Microsc. Soc. 1970, 89, 469–474. [Google Scholar] [CrossRef]
- Noyer, C.; Abot, A.; Trouilh, L.; Le Berre, V.; Dreanno Phytochip, C.; Anton Leberre, V.; Dreanno, C. Phytochip: Development of a DNA-microarray for rapid and accurate identification of Pseudo-nitzschia spp and other harmful algal species. J. Microbiol. Methods 2015, 112, 55–66. [Google Scholar] [CrossRef]
- Adachi, M.; Sako, Y.; Ishida, Y. Restriction fragment length polymorphism of ribosomal DNA internal transcribed spacer and 5.8s regions in Japanese Alexandrium species (Dinophyceae). J. Phycol. 1994, 30, 857–863. [Google Scholar] [CrossRef]
- Scholin, C.A.; Villac, M.C.; Buck, K.R.; Krupp, J.M.; Powers, D.A.; Fryxell, G.A.; Chavez, F.P. Ribosomal DNA Sequences Discriminate Among Toxic and Non-Toxic Pseudonitzschia Species. Nat. Toxins 1994, 2, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Nunn, G.B.; Theisen, B.F.; Christensen, B.; Arctander, P. Simplicity-Correlated Size Growth of the Nuclear 28S Ribosomal RNA D3 Expansion Segment in the Crustacean Order Isopoda. J. Mol. Evol. 1996, 42, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.M.; Simpson, G.E.; Stickle, A.J.; Mann, D.G. Life history and systematics of Petroneis (Bacillariophyta), with special reference to British waters. Eur. J. Phycol. 2005, 40, 61–87. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Masatoshi, N.; Sudhir, K. Molecular Evolution and Phylogenetics; Oxford, University Press: Oxford, UK, 2000. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Yang, Z. Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: Approximate methods. J. Mol. Evol. 1994, 39, 306–314. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Gelfond, J.; Goros, M.; Hernandez, B.; Bokov, A. A System for an Accountable Data Analysis Process in R. R J. 2018, 10, 6–21. [Google Scholar] [CrossRef]
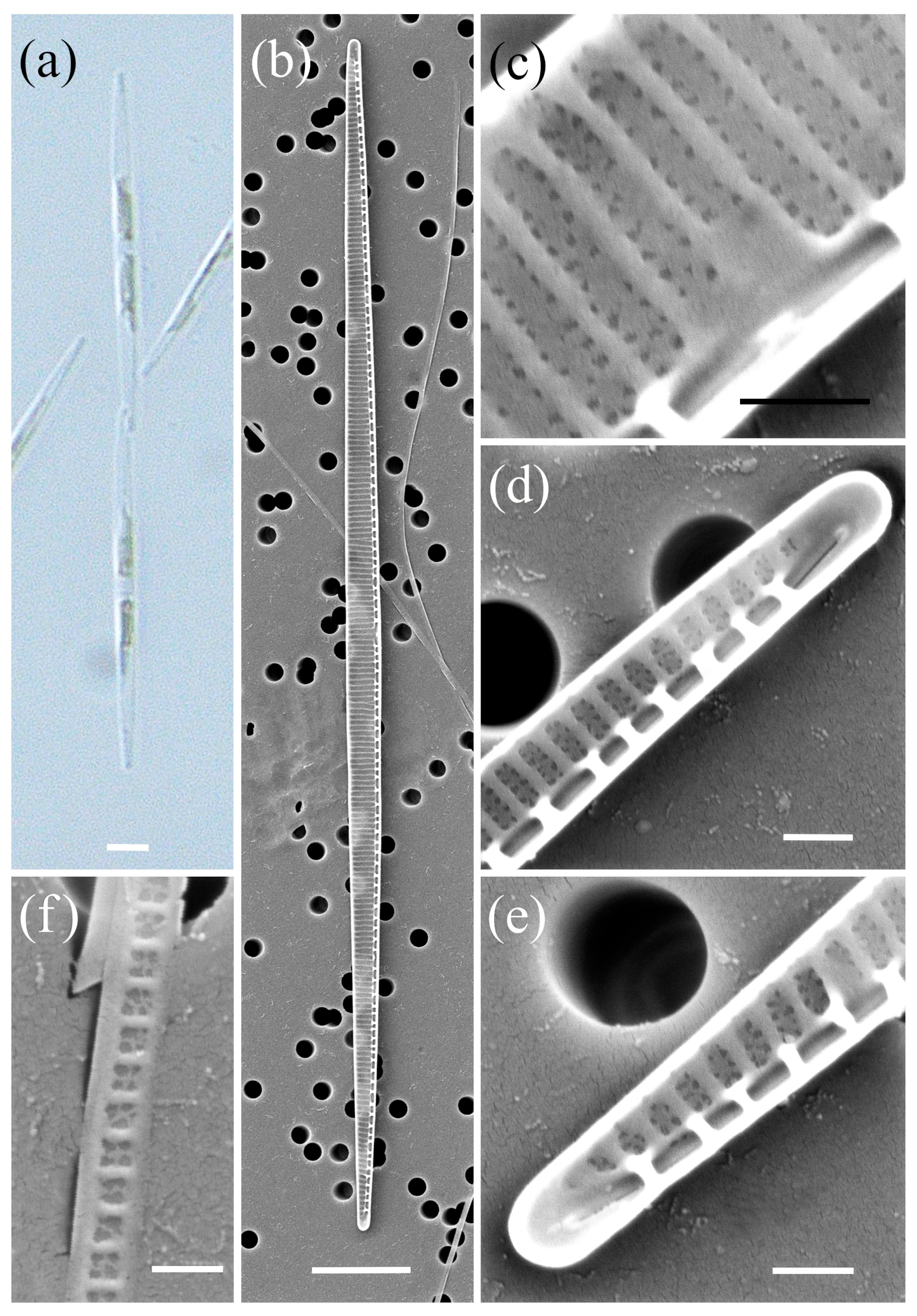


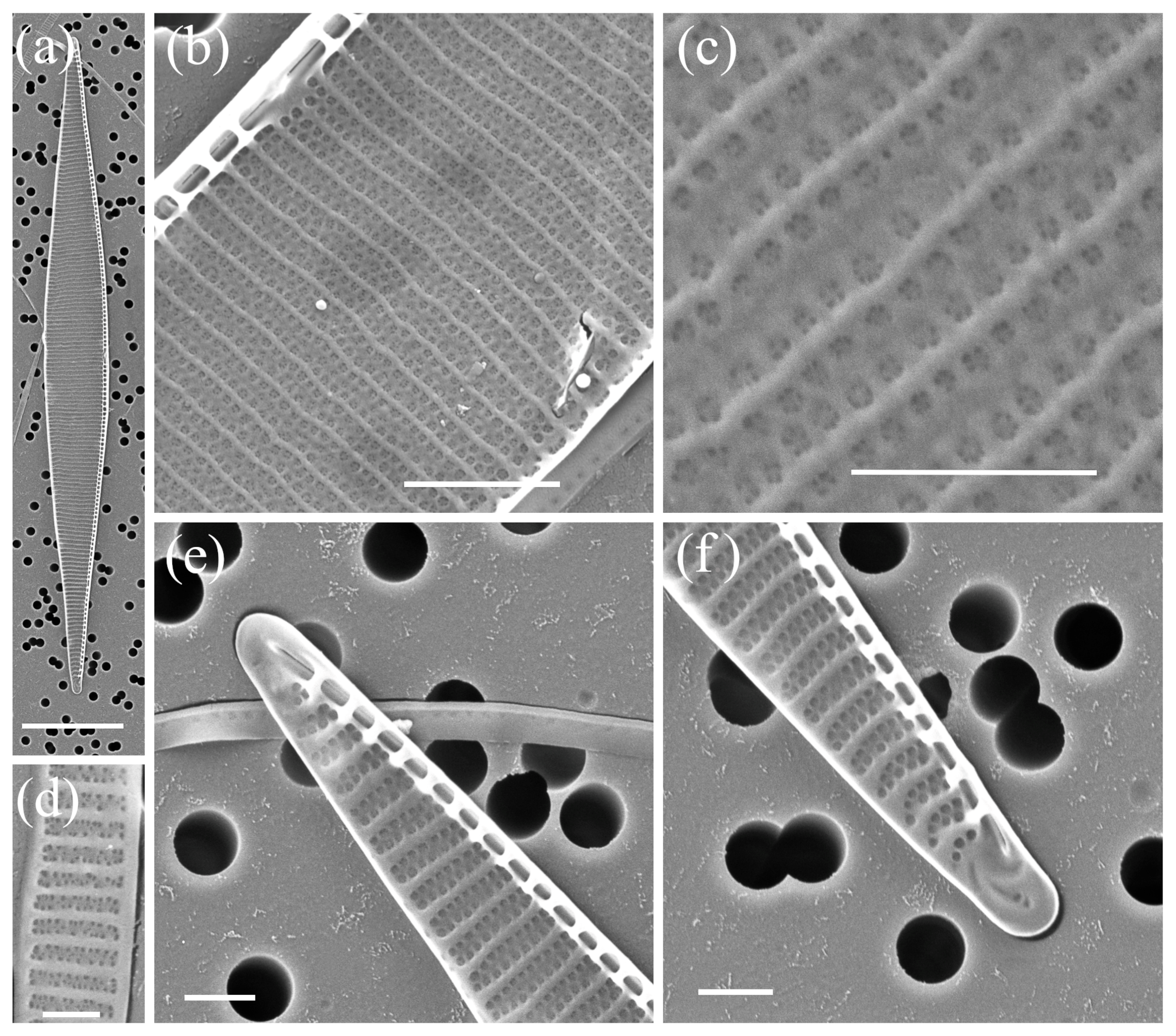
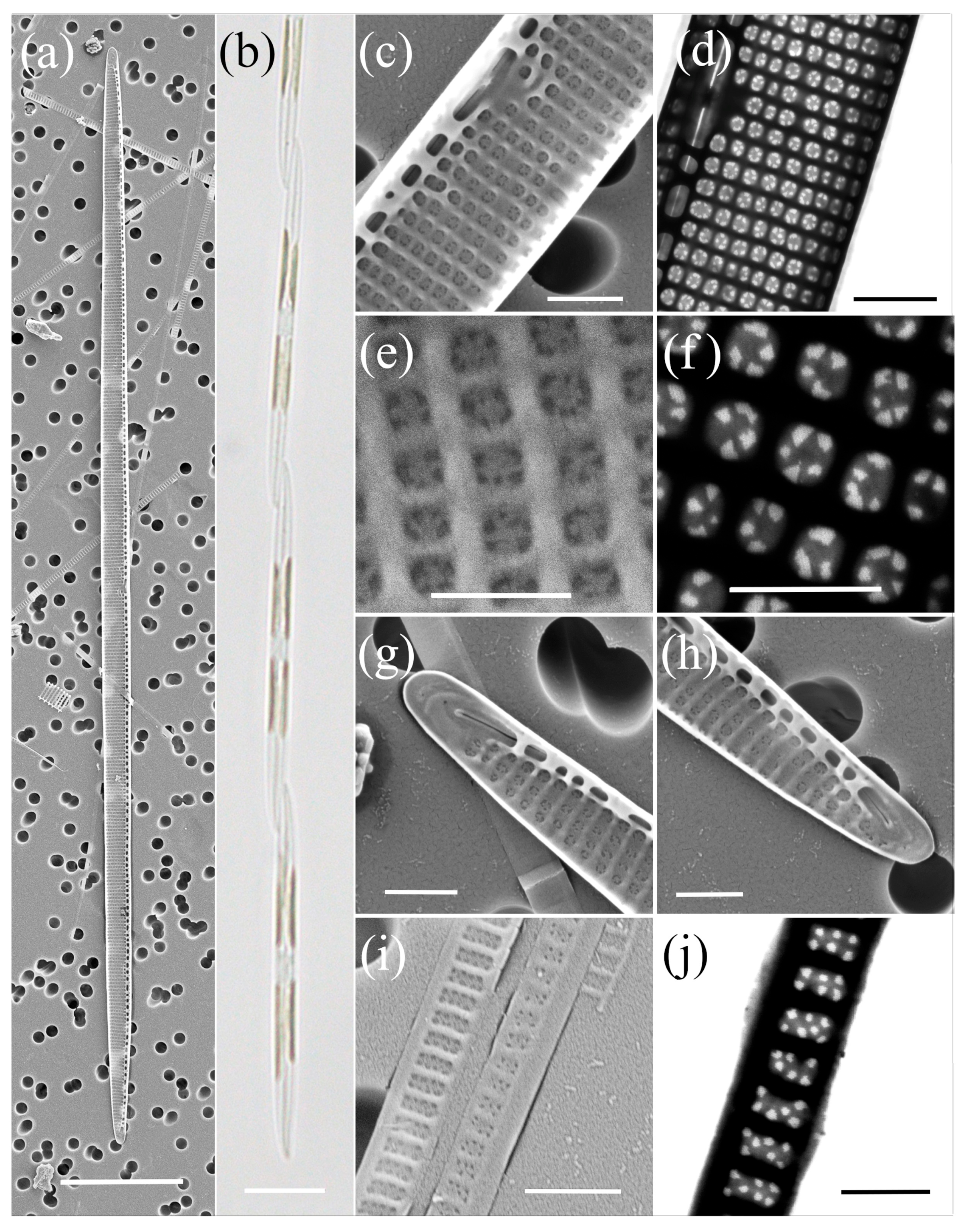
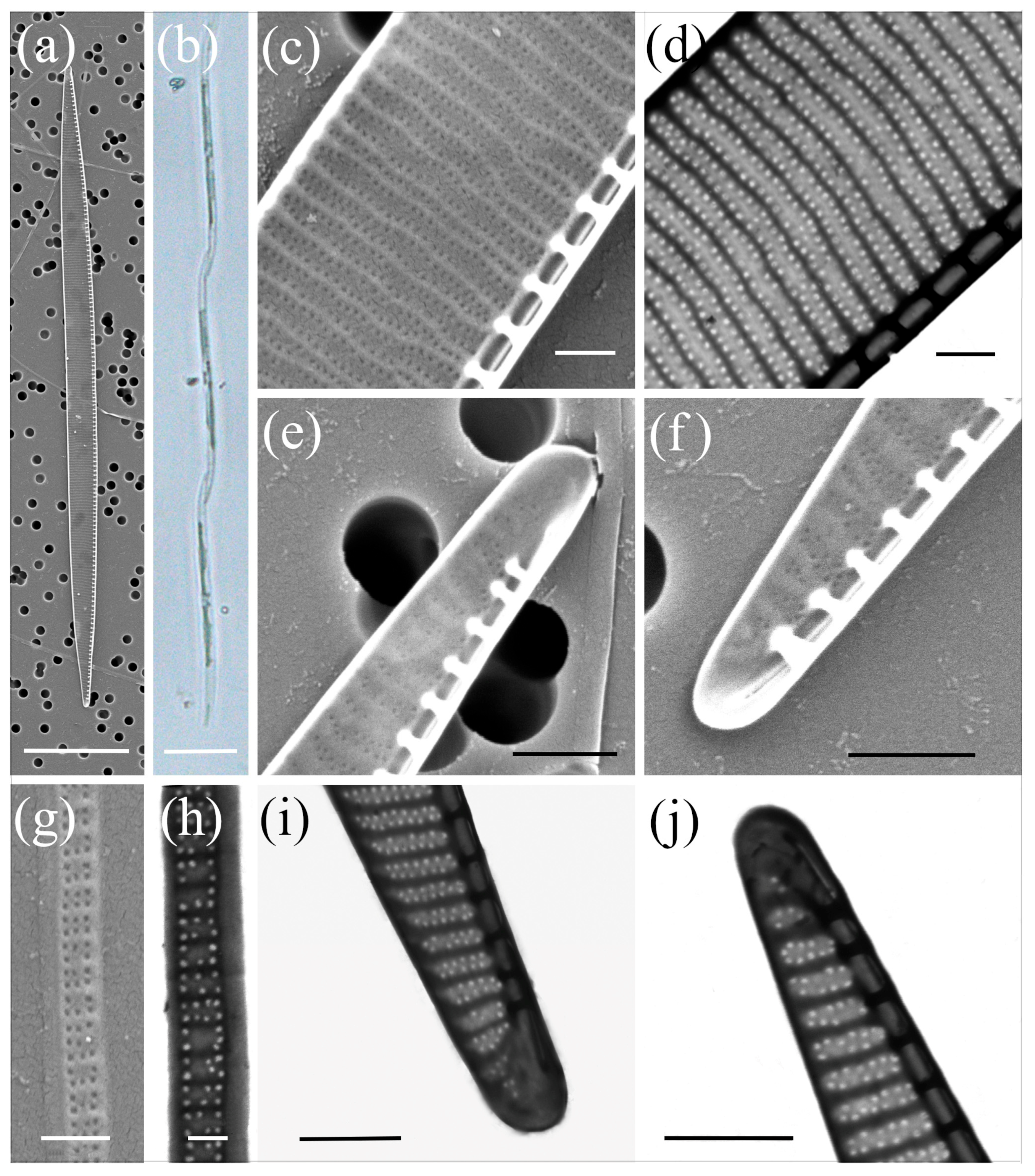



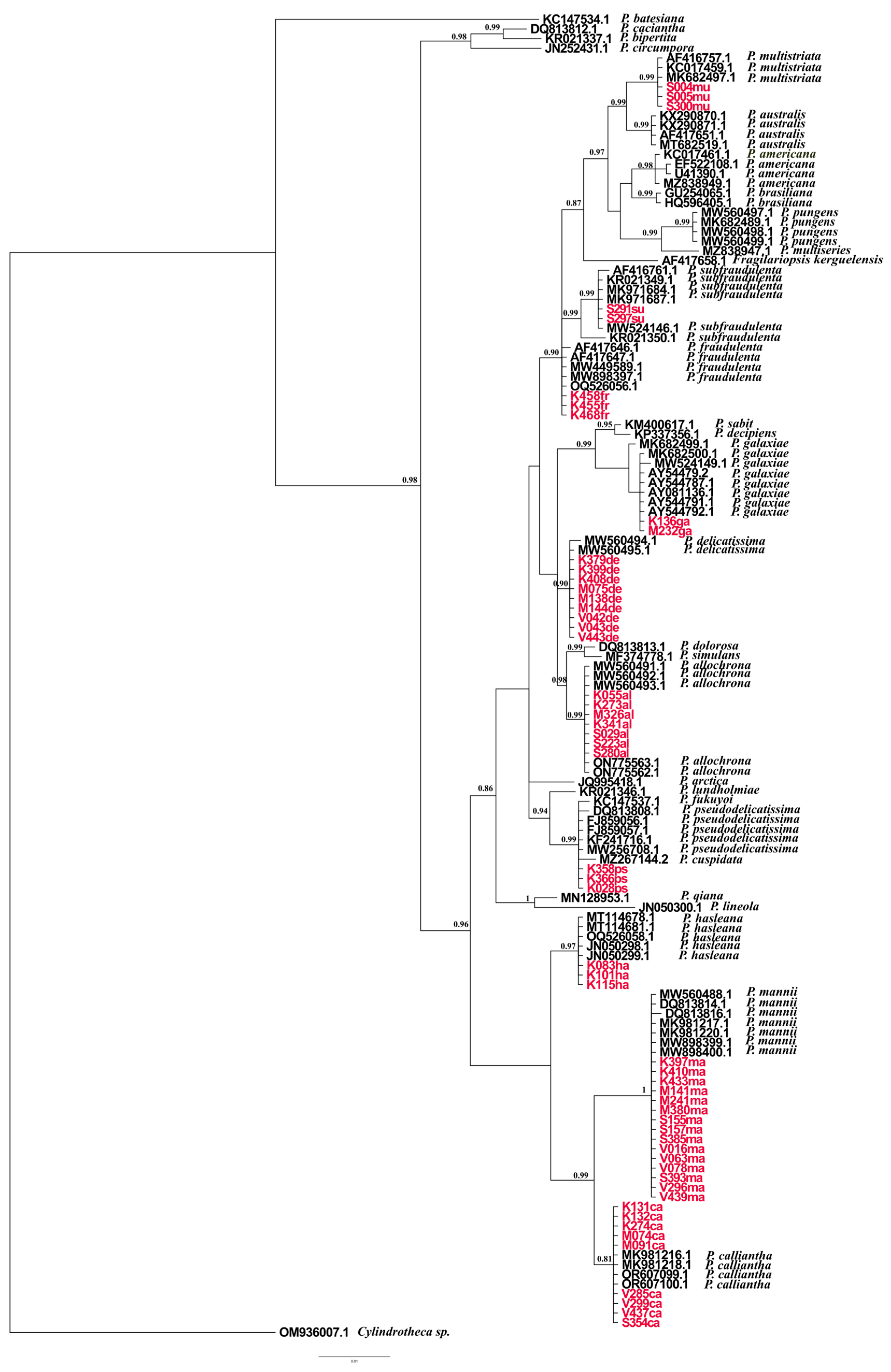



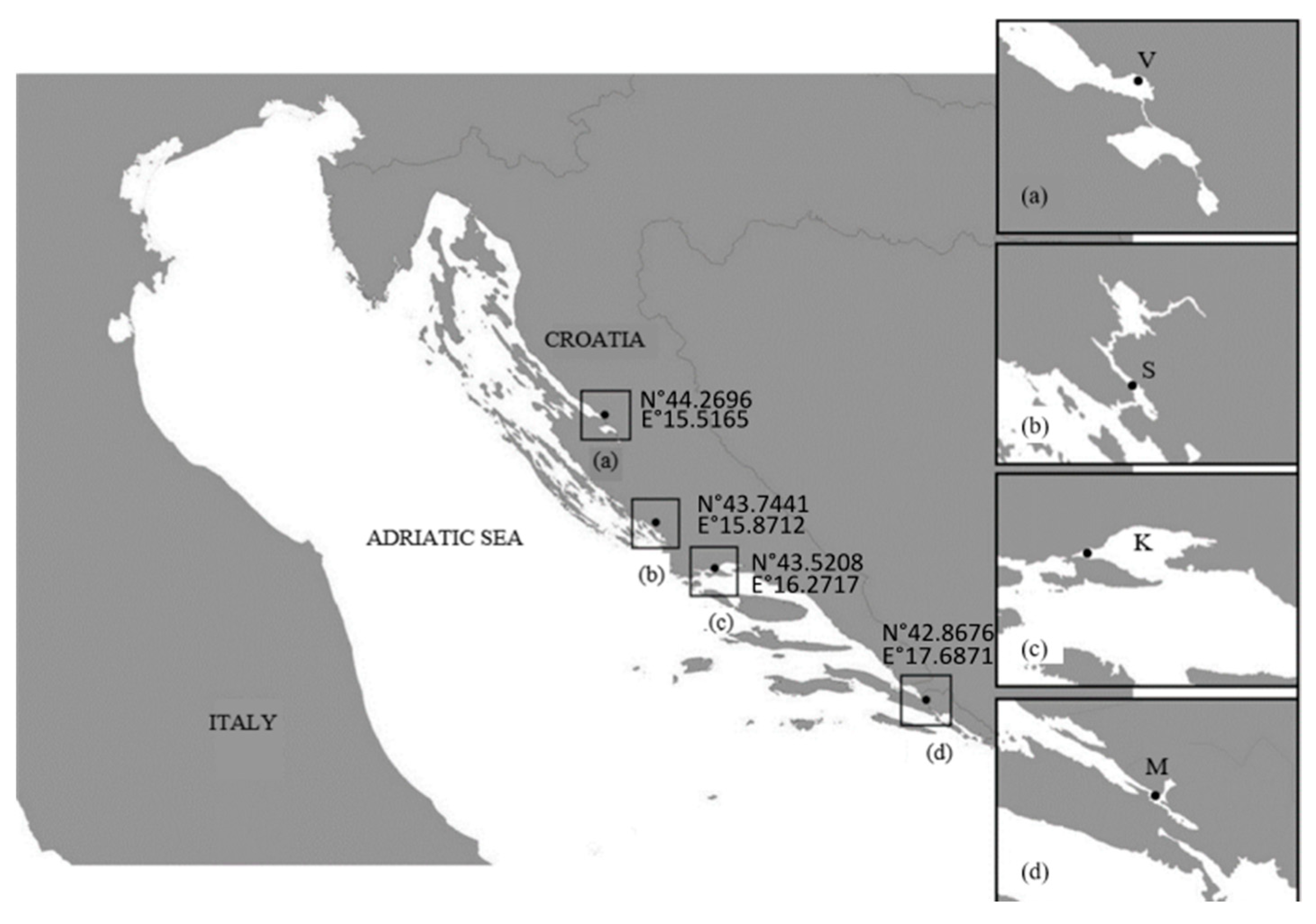
| Species | Number of Cultures | Location | Season | Methods |
|---|---|---|---|---|
| P. allochrona | 14 | K, M, S | A, SU | ITS, LSU, rbcL, SEM |
| P. calliantha | 24 | All locations | A, W, SU | ITS, LSU, rbcL, SEM/STEM |
| P. delicatissima | 47 | K, M, V | A, W, SP | ITS, LSU, rbcL, SEM/STEM |
| P. fraudulenta | 6 | K | A, W | ITS, LSU, rbcL, SEM |
| P. mannii | 51 | All locations | All seasons | ITS, LSU, rbcL, SEM/STEM |
| P. multistriata | 7 | S | A | ITS, LSU, rbcL, SEM/STEM |
| P. pseudodelicatissima | 16 | K | A, W, SU | ITS, LSU, rbcL, SEM/STEM |
| P. subfraudulenta | 6 | S, V | A, SU | ITS, LSU, rbcL, SEM/STEM |
| Species | Width (µm) | Length (µm) | Central Interspace | Fibulae in 10 µm | Interstriae in 10 µm | Poroids in 1 µm | Rows of Poroids | Poroid Sectors | Band Striae in 10 µm |
|---|---|---|---|---|---|---|---|---|---|
| P. allochrona (n = 50; 5) | 1.44–2.15 1.72 ± 0.18 | 51.06–63.01 58.12 ± 3.58 | + | 20–24 21.6 ± 0.9 | 36–39 37.1 ± 0.8 | 7–13 | (1)-2 | - | 43–45 43.9 ± 0.7 |
| P. calliantha (n = 97; 9) | 1.38–2.12 1.75 ± 0.17 | 64.08–107.14 83.53 ± 12.11 | + | 17–22 19.6 ± 1.1 | 34–38 36.2 ± 0.8 | 4–6 | 1 | 2–11 * 6 | 43–46 44.5 ± 0.8 |
| P. delicatissima (n = 126; 12) | 1.31–2.00 1.64 ± 0.15 | 31.05–94.21 57.43 ± 17.00 | + | 20–26 23.3 ± 1.2 | 37–42 40.4 ± 0.7 | 7–13 | (1)-2 | - | 48–51 49.6 ± 0.6 |
| P. fraudulenta (n = 56; 5) | 5.09–6.54 5.79 ± 0.37 | 60.22–67.42 65.04 ± 1.45 | + | 19–24 22.7 ± 0.9 | 23–25 23.8 ± 0.6 | 5–7 | (1)-2-(3) | 2–7 * 5 | 38–41 39.5 ± 0.6 |
| P. mannii (n = 148; 14) | 1.71–2.48 2.05 ± 0.16 | 69.08–123.56 93.37 ± 13.37 | + | 16–23 19.7 ± 1.2 | 33–37 35.3 ± 0.7 | 4–6 | 1 | 1–8 * 4 | 42–44 43.2 ± 0.6 |
| P. multistriata (n = 62; 6) | 2.33–3.51 2.91 ± 0.30 | 55.85–84.85 68.07 ± 7.94 | - | 23–26 24.3 ± 0.8 | 36–40 38.4 ± 0.9 | 9–12 | (1)-2 | - | 47–51 49.9 ± 0.9 |
| P. pseudodelicatissima (n = 84; 8) | 1.22–1.75 1.48 ± 0.12 | 59.14–90.99 72.76 ± 11.06 | + | 19–26 22.3 ± 1.2 | 38–41 39.3 ± 0.7 | 5–7 | 1 | 1–4 * 2 | 47–52 49.6 ± 1.0 |
| P. subfraudulenta (n = 60; 5) | 4.15–5.25 4.69 ± 0.22 | 96.19–140.33 118.42 ± 15.98 | + | 13–17 14.8 ± 0.9 | 23–25 24.6 ± 0.5 | 5–6 | (1)-2 | 2–10 * 5 | 39–41 40.1 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonačić, T.; Arapov, J.; Bušelić, I.; Lepen Pleić, I.; Milić Roje, B.; Tomašević, T.; Bužančić, M.; Mladinić, M.; Casabianca, S.; Penna, A.; et al. Advancing the Taxonomy of the Diatom Pseudo-nitzschia Through an Integrative Study Conducted in the Central and Southeastern Adriatic Sea. Plants 2025, 14, 245. https://doi.org/10.3390/plants14020245
Bonačić T, Arapov J, Bušelić I, Lepen Pleić I, Milić Roje B, Tomašević T, Bužančić M, Mladinić M, Casabianca S, Penna A, et al. Advancing the Taxonomy of the Diatom Pseudo-nitzschia Through an Integrative Study Conducted in the Central and Southeastern Adriatic Sea. Plants. 2025; 14(2):245. https://doi.org/10.3390/plants14020245
Chicago/Turabian StyleBonačić, Tina, Jasna Arapov, Ivana Bušelić, Ivana Lepen Pleić, Blanka Milić Roje, Tina Tomašević, Mia Bužančić, Marija Mladinić, Silvia Casabianca, Antonella Penna, and et al. 2025. "Advancing the Taxonomy of the Diatom Pseudo-nitzschia Through an Integrative Study Conducted in the Central and Southeastern Adriatic Sea" Plants 14, no. 2: 245. https://doi.org/10.3390/plants14020245
APA StyleBonačić, T., Arapov, J., Bušelić, I., Lepen Pleić, I., Milić Roje, B., Tomašević, T., Bužančić, M., Mladinić, M., Casabianca, S., Penna, A., Skejić, S., & Ninčević Gladan, Ž. (2025). Advancing the Taxonomy of the Diatom Pseudo-nitzschia Through an Integrative Study Conducted in the Central and Southeastern Adriatic Sea. Plants, 14(2), 245. https://doi.org/10.3390/plants14020245







