bra-miR9569 Targets the BrAHA6 Gene to Negatively Regulate H+-ATPases, Affecting Pollen Fertility in Chinese Cabbage (Brassica rapa L. ssp. pekinensis)
Abstract
1. Introduction
2. Results
2.1. Quality Control Analysis of Transcriptome Sequencing Data from Maintainer and Ogura CMS Chinese Cabbage Lines
2.2. Quality Control of miRNA Sequencing Data from Anther Samples of Both Lines
2.3. Sequencing Analysis of the Degradome of Two Chinese Cabbage Lines
2.4. Joint Analysis of miRNAs and Their Target Genes by miRNAomics
2.5. H+-ATPases Related Genes Are Significantly Downregulated During Anther Development in the Ogura CMS Sterile Line of Chinese Cabbage
2.6. Analysis of bra-miR9569 Targeting Relationship with Target Genes and Bioinformatics Analysis
2.7. qRT-PCR Validation of miR9569/AHA6 Regulation and Differential Expression Profile Reliability
2.8. Genetic Transformation of OE-miR9569 Arabidopsis Thaliana and Identification of Positive Seedlings
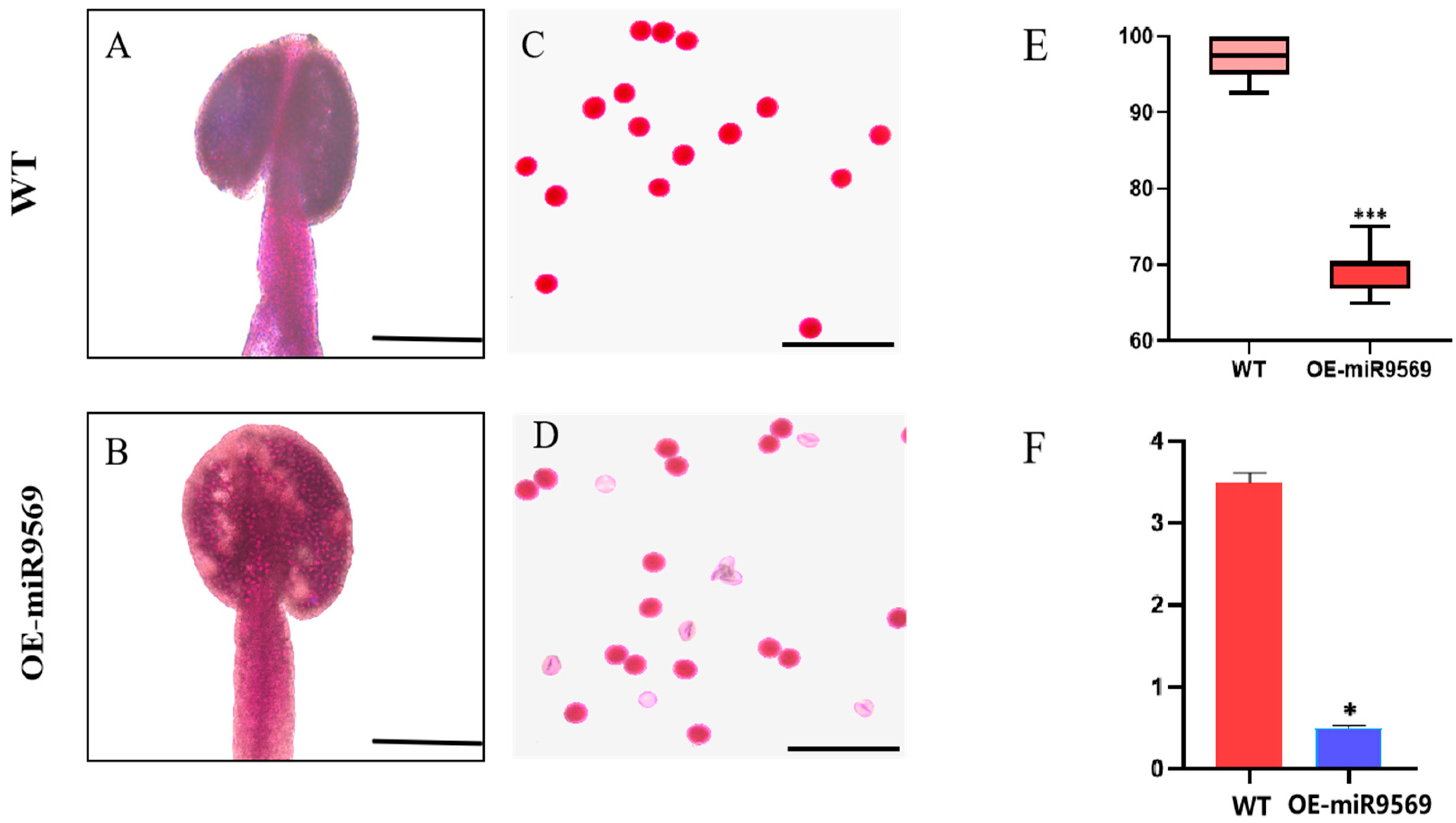
2.9. Phenotypic Analysis Reveals miR9569 Regulates Pollen Dispersal and Maturation in Arabidopsis
2.10. miR9569 Overexpression Compromises Pollen Viability and Anther ATP Levels via H+-ATPase Dysregulation
2.11. Anther Cavitation and ROS Toxicity: Key Phenotypes of Pollen Sterility in miR9569-Overexpressing Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. RNA Extraction and Library Construction
4.3. Construction of the cDNA Library and Data Processing
4.4. Construction of the Degradome Library and Statistics for the Sequencing Results
4.5. qRT-PCR Validation of miRNA and mRNA Sequencing Data
4.6. OE-miR9569 Arabidopsis Thaliana Transformation
4.7. Identification of Pollen Viability
4.8. Determination of the ATP Content and ROS Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Liu, Y.-G. Male Sterility and Fertility Restoration in Crops. Annu. Rev. Plant Biol. 2014, 65, 579–606. [Google Scholar] [CrossRef]
- Vedel, F.; Pla, M.; Vitart, V.; Gutierres, S.; Chétrit, P.; Paepe, R.d. Molecular basis of nuclear and cytoplasmic male sterility in higher plants. Plant Physiol. Biochem. 1994, 32, 601–608. [Google Scholar]
- Wang, F.; Feng, C.; O’Connell, M.A.; Stewart, J.M.; Zhang, J. RFLP analysis of mitochondrial DNA in two cytoplasmic male sterility systems (CMS-D2 and CMS-D8) of cotton. Euphytica 2010, 172, 93–99. [Google Scholar] [CrossRef]
- Dong, X.; Kim, W.K.; Lim, Y.-P.; Kim, Y.-K.; Hur, Y. Ogura-CMS in Chinese cabbage (Brassica rapa ssp. pekinensis) causes delayed expression of many nuclear genes. Plant Sci. 2013, 199–200, 7–17. [Google Scholar] [CrossRef]
- Su, T.; Wang, W.; Li, P.; Zhang, B.; Li, P.; Xin, X.; Sun, H.; Yu, Y.; Zhang, D.; Zhao, X. A genomic variation map provides insights into the genetic basis of spring Chinese cabbage (Brassica rapa ssp. pekinensis) selection. Mol. Plant 2018, 11, 1360–1376. [Google Scholar] [CrossRef]
- Pokluda, R. Nutritional quality of Chinese cabbage from integrated culture. Hortic. Sci. 2008, 35, 145–150. [Google Scholar] [CrossRef]
- Seong, G.-U.; Hwang, I.-W.; Chung, S.-K. Antioxidant capacities and polyphenolics of Chinese cabbage (Brassica rapa L. ssp. Pekinensis) leaves. Food Chem. 2016, 199, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Musumi, K.; Ayugase, J. Carotenoid pigment composition, polyphenol content, and antioxidant activities of extracts from orange-colored Chinese cabbage. LWT—Food Sci. Technol. 2011, 44, 1971–1975. [Google Scholar] [CrossRef]
- Ren, W.; Si, J.; Chen, L.; Fang, Z.; Zhuang, M.; Lv, H.; Wang, Y.; Ji, J.; Yu, H.; Zhang, Y. Mechanism and utilization of ogura cytoplasmic male sterility in cruciferae crops. Int. J. Mol. Sci. 2022, 23, 9099. [Google Scholar] [CrossRef]
- Lin, S.; Miao, Y.; Su, S.; Xu, J.; Jin, L.; Sun, D.; Peng, R.; Huang, L.; Cao, J. Comprehensive analysis of Ogura cytoplasmic male sterility-related genes in turnip (Brassica rapa ssp. rapifera) using RNA sequencing analysis and bioinformatics. PLoS ONE 2019, 14, e0218029. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, X.; Yao, Q.; Yuan, Y.; Li, X.; Wei, F.; Zhao, Y.; Zhang, Q.; Wang, Z.; Jiang, W.; et al. The miRNAs and their regulatory networks responsible for pollen abortion in Ogura-CMS Chinese cabbage revealed by high-throughput sequencing of miRNAs, degradomes, and transcriptomes. Front. Plant Sci. 2015, 6, 894. [Google Scholar] [CrossRef]
- Chase, C.D. Cytoplasmic male sterility: A window to the world of plant mitochondrial-nuclear interactions. Trends Genet. 2007, 23, 81–90. [Google Scholar] [CrossRef]
- Takatsuka, A.; Kazama, T.; Toriyama, K. Cytoplasmic Male Sterility-Associated Mitochondrial Gene orf312 Derived from Rice (Oryza sativa L.) Cultivar Tadukan. Rice 2020, 14, 46. [Google Scholar] [CrossRef]
- Rogers, K.; Chen, X. Biogenesis, Turnover, and Mode of Action of Plant MicroRNAs. Plant Cell 2013, 25, 2383–2399. [Google Scholar] [CrossRef] [PubMed]
- Sun, G. MicroRNAs and their diverse functions in plants. Plant Mol. Biol. 2012, 80, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Grant-Downton, R.; Le Trionnaire, G.; Schmid, R.; Rodriguez-Enriquez, J.; Hafidh, S.; Mehdi, S.; Twell, D.; Dickinson, H. MicroRNA and tasiRNA diversity in mature pollen of Arabidopsis thaliana. BMC Genom. 2009, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Lv, M.; Liang, Y.; Ma, Z.; Cao, J. Identification of novel and conserved miRNAs involved in pollen development in Brassica campestris ssp. chinensis by high-throughput sequencing and degradome analysis. BMC Genom. 2014, 15, 146. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Wu, M.; Li, L.; Jin, C.; Zhang, Q.; Chen, C.; Song, W.; Wang, C. Small RNA sequencing reveals differential miRNA expression in the early development of broccoli (Brassica oleracea var. italica) pollen. Front. Plant Sci. 2017, 8, 404. [Google Scholar] [CrossRef]
- Chambers, C.; Shuai, B. Profiling microRNA expression in Arabidopsis pollen using microRNA array and real-time PCR. BMC Plant Biol. 2009, 9, 87. [Google Scholar] [CrossRef]
- Quilichini, T.D.; Friedmann, M.C.; Samuels, A.L.; Douglas, C.J. ATP-binding cassette transporter G26 is required for male fertility and pollen exine formation in Arabidopsis. Plant Physiol. 2010, 154, 678–690. [Google Scholar] [CrossRef]
- Mizelle, M.; Sethi, R.; Ashton, M.; Jemen, W. Development of the pollen grain and tapetum of wheat (Triticum aestivum) in untreated plants and plants treated with chemical hybridizing agent RH0007. Sex. Plant Reprod. 1989, 2, 231–253. [Google Scholar] [CrossRef]
- Liu, J.; Lim, S.-L.; Zhong, J.Y.; Lim, B.L. Bioenergetics of pollen tube growth in Arabidopsis thaliana revealed by ratiometric genetically encoded biosensors. Nat. Commun. 2022, 13, 7822. [Google Scholar] [CrossRef]
- Harper, J.F.; Manney, L.; Sussman, M.R. The plasma membrane H+-ATPase gene family in Arabidopsis: Genomic sequence of AHA10 which is expressed primarily in developing seeds. Mol. Gen. Genet. MGG 1994, 244, 572–587. [Google Scholar] [CrossRef]
- Haruta, M.; Gray, W.M.; Sussman, M.R. Regulation of the plasma membrane proton pump (H+-ATPase) by phosphorylation. Curr. Opin. Plant Biol. 2015, 28, 68–75. [Google Scholar] [CrossRef]
- Morsomme, P.; Boutry, M. The plant plasma membrane H+-ATPase: Structure, function and regulation. BBA Biomembr. 2000, 1465, 1–16. [Google Scholar] [CrossRef]
- Palmgren, M. Plant Plasma Membrane H+-ATPases: Powerhouses for Nutrient Uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 817–845. [Google Scholar] [CrossRef]
- Duby, G.; Boutry, M. The plant plasma membrane proton pump ATPase: A highly regulated P-type ATPase with multiple physiological roles. Pflügers Arch. Eur. J. Physiol. 2009, 457, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Selinski, J.; Scheibe, R. Pollen tube growth: Where does the energy come from? Plant Signal. Behav. 2014, 9, e977200. [Google Scholar] [CrossRef]
- Hoffmann, R.D.; Portes, M.T.; Olsen, L.I.; Damineli, D.S.C.; Hayashi, M.; Nunes, C.O.; Pedersen, J.T.; Lima, P.T.; Campos, C.; Feijó, J.A.; et al. Plasma membrane H+-ATPases sustain pollen tube growth and fertilization. Nat. Commun. 2020, 11, 2395. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jia, P.-F.; Yang, W.-C.; Li, H.-J. Plasma membrane H+-ATPases-mediated cytosolic proton gradient regulates pollen tube growth. J. Integr. Plant Biol. 2020, 62, 1817–1822. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Y.; Zhao, Y.; Chen, W.; Nath, U.K.; Yang, S.; Su, H.; Wang, Z.; Zhang, W.; Tian, B.; et al. Mitotic Pollen Abnormalities Linked to Ogura Cytoplasmic Male Sterility in Chinese cabbage (Brassica rapa L. ssp. pekinensis). J. Integr. Agric. 2024, 24, 1092–1107. [Google Scholar] [CrossRef]
- Yang, H.; Xue, Y.; Li, B.; Lin, Y.; Li, H.; Guo, Z.; Li, W.; Fu, Z.; Ding, D.; Tang, J. The chimeric gene atp6c confers cytoplasmic male sterility in maize by impairing the assembly of mitochondrial ATP synthase complex. Mol. Plant 2022, 15, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Amanda, D.; Frey, F.P.; Neumann, U.; Przybyl, M.; Šimura, J.; Zhang, Y.; Chen, Z.; Gallavotti, A.; Fernie, A.R.; Ljung, K.; et al. Auxin boosts energy generation pathways to fuel pollen maturation in barley. Curr. Biol. 2022, 32, 1798–1811.e1798. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dong, X.; Yang, H.; Chai, Y.; Xia, Y.; Tian, L.; Qu, L.Q. Cytosolic disproportionating enzyme2 is essential for pollen germination and pollen tube elongation in rice. Plant Physiol. 2022, 191, 96–109. [Google Scholar] [CrossRef]
- Ji, J.-J.; Huang, W.; Li, D.-W.; Yin, Y.-X.; Chai, W.-G.; Gong, Z.-H. A CMS-related gene, ψatp6-2, causes increased ATP hydrolysis activity of the mitochondrial F1 Fo-ATP synthase and induces male sterility in pepper (Capsicum annuum L.). Plant Mol. Biol. Report. 2014, 32, 888–899. [Google Scholar] [CrossRef]
- Linke, B.; Börner, T. Mitochondrial effects on flower and pollen development. Mitochondrion 2005, 5, 389–402. [Google Scholar] [CrossRef]
- Bock, K.W.; Honys, D.; Ward, J.M.; Padmanaban, S.; Nawrocki, E.P.; Hirschi, K.D.; Twell, D.; Sze, H. Integrating Membrane Transport with Male Gametophyte Development and Function through Transcriptomics. Plant Physiol. 2006, 140, 1151–1168. [Google Scholar] [CrossRef]
- Ewing, B.; Green, P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998, 8, 175–185. [Google Scholar] [CrossRef]
- Langmead, B. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. J. Genome Biol. 2009, 25, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Burge, S.W.; Daub, J.; Eberhardt, R.; Tate, J.; Barquist, L.; Nawrocki, E.P.; Eddy, S.R.; Gardner, P.P.; Bateman, A. Rfam 11.0: 10 years of RNA families. Nucleic Acids Res. 2012, 41, D226–D232. [Google Scholar] [CrossRef] [PubMed]
- Addo-Quaye, C.; Miller, W.; Axtell, M.J. CleaveLand: A pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics 2009, 25, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
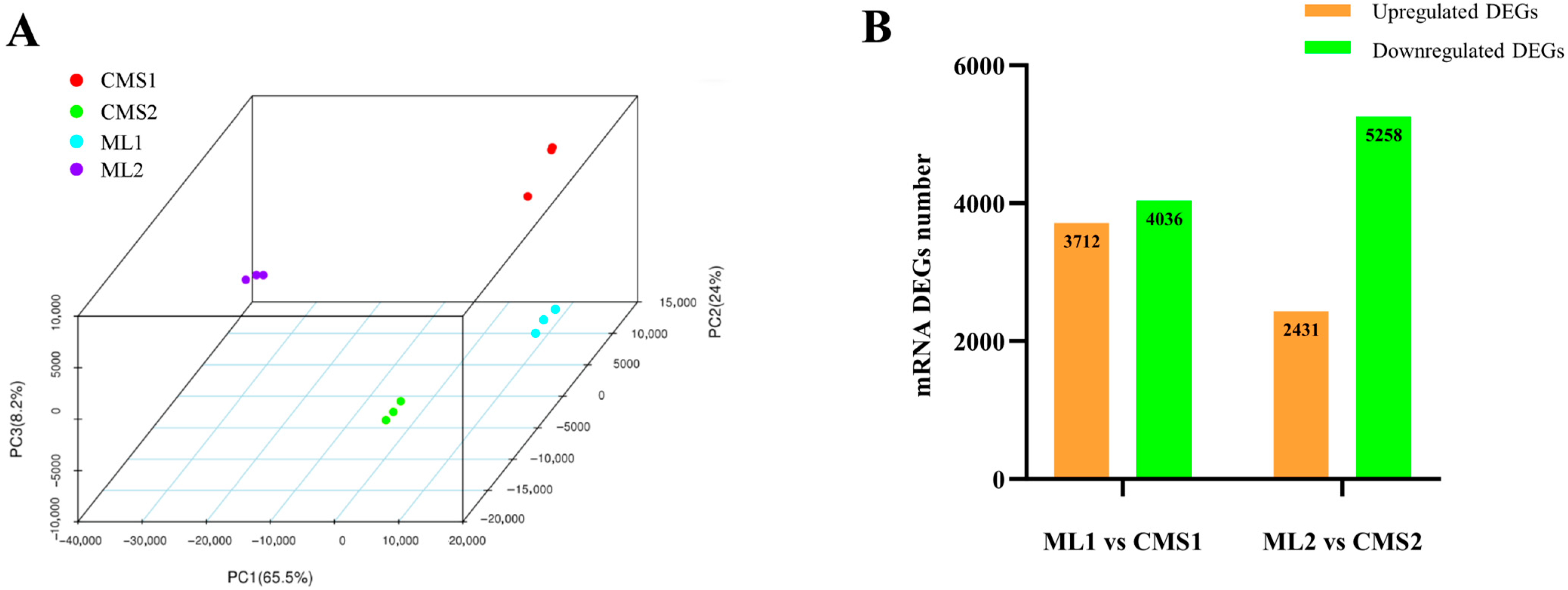

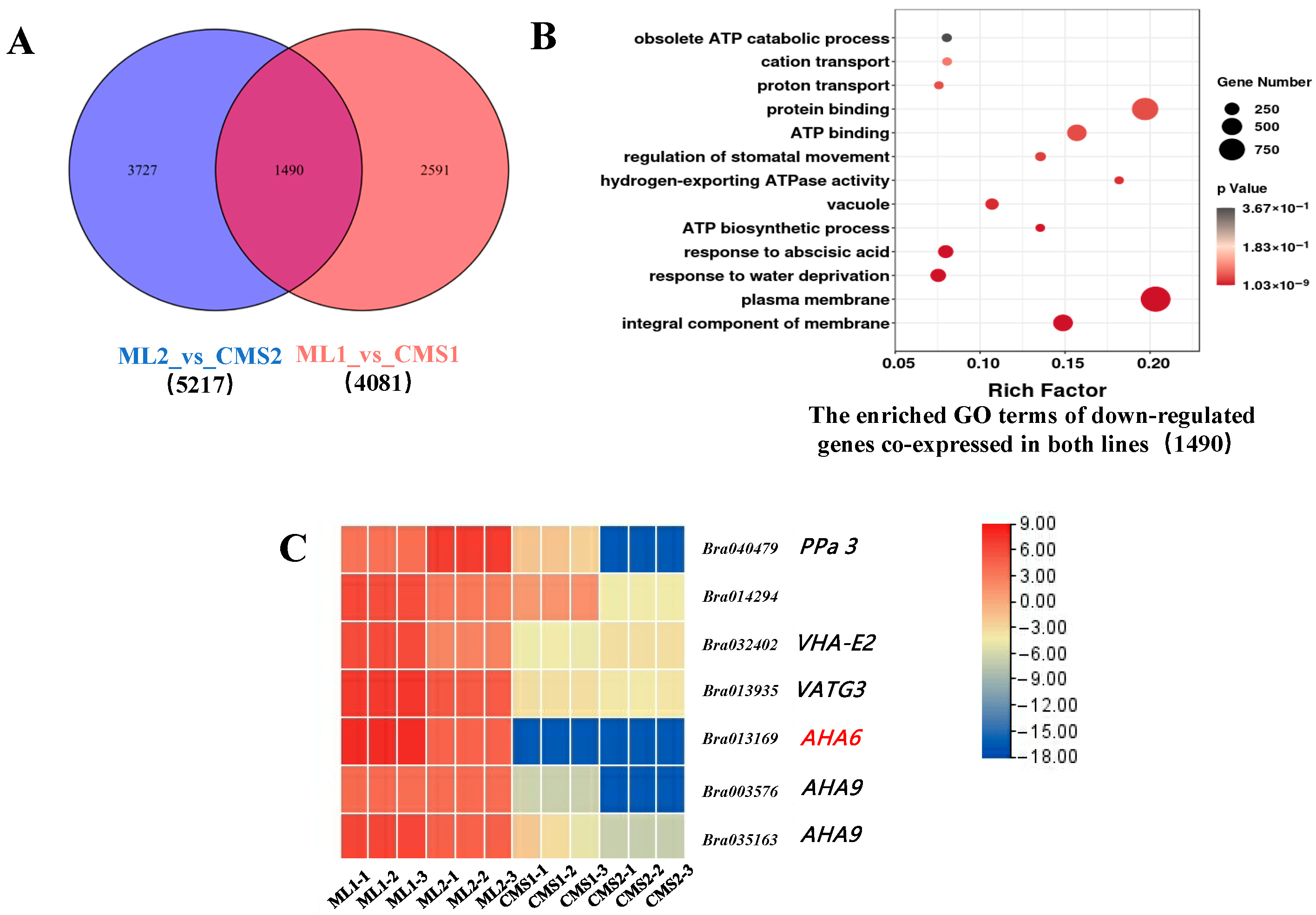
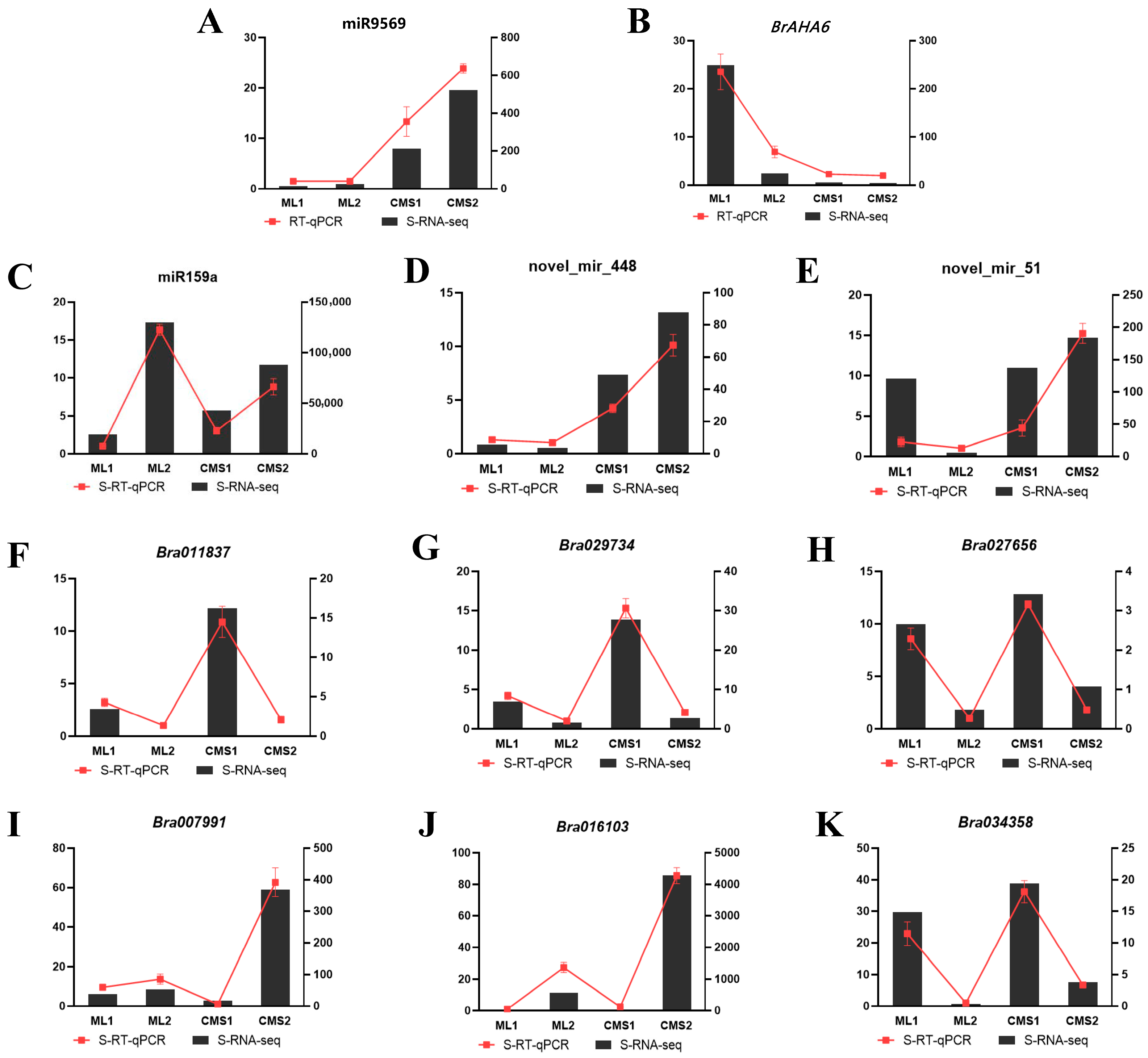
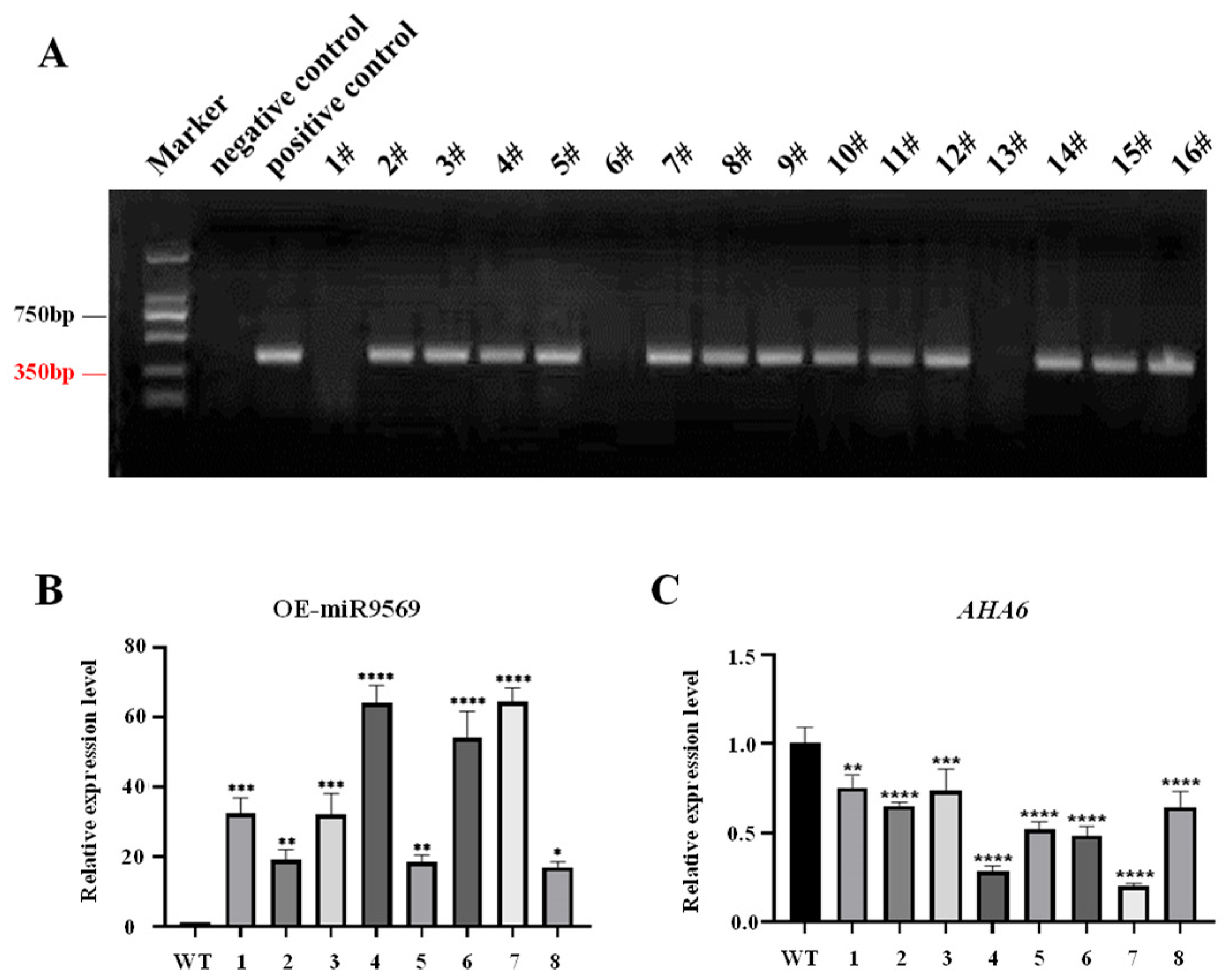



| Sample ID | Samples | Clean Reads | GC (%) | Q30 (%) | Mapped Reads |
|---|---|---|---|---|---|
| S07 | ML1-1 | 68,768,532 | 43.85 | 95.99 | 51,673,715 (75.14%) |
| S08 | ML1-2 | 85,298,784 | 44.47 | 96.03 | 64,307,383 (75.39%) |
| S09 | ML1-3 | 78,197,288 | 45.19 | 96.02 | 60,180,391 (76.96%) |
| ML1-average | 77,421,535 | 44.50 | 96.01 | 58,720,496 (75.83%) | |
| S10 | CMS1-1 | 73,943,440 | 43.46 | 95.80 | 52,303,161 (70.73%) |
| S11 | CMS1-2 | 80,664,306 | 45.57 | 96.16 | 58,978,727 (73.12%) |
| S12 | CMS1-3 | 74,097,366 | 45.38 | 95.99 | 53,966,421 (72.83%) |
| CMS1-average | 76,235,037 | 44.80 | 95.98 | 55,082,768 (72.23%) | |
| S01 | ML2-1 | 83,770,774 | 45.19 | 95.64 | 77,887,958 (89.76%) |
| S02 | ML2-2 | 77,257,468 | 44.90 | 96.01 | 69,431,461 (89.87%) |
| S03 | ML2-3 | 75,009,460 | 44.93 | 95.74 | 64,617,111 (86.15%) |
| ML2-average | 78,679,234 | 45.01 | 95.80 | 70,645,510 (88.59%) | |
| S04 | CMS2-1 | 77,760,428 | 45.07 | 95.99 | 60,702,156 (78.06%) |
| S05 | CMS2-2 | 69,712,214 | 44.69 | 96.06 | 52,983,713 (76.00%) |
| S06 | CMS2-3 | 80,281,016 | 45.42 | 95.96 | 63,100,530 (78.60%) |
| CMS2-average | 75,917,886 | 45.06 | 96.00 | 58,928,800 (77.55%) |
| Sample ID | Sample | SNP Number | Genic SNPs | Transition | Transversion | Heterozygosity |
|---|---|---|---|---|---|---|
| S07 | ML1-1 | 216,700 | 170,197 | 57.24% | 42.76% | 29.14% |
| S08 | ML1-2 | 237,398 | 184,124 | 57.04% | 42.96% | 29.68% |
| S09 | ML1-3 | 208,136 | 166,036 | 57.31% | 42.69% | 29.24% |
| ML1-average | 220,745 | 173,452 | 57.20% | 42.80% | 29.35% | |
| S10 | CMS1-1 | 227,465 | 179,713 | 57.09% | 42.91% | 45.29% |
| S11 | CMS1-2 | 230,515 | 182,973 | 57.25% | 42.75% | 44.86% |
| S12 | CMS1-3 | 225,547 | 179,233 | 57.32% | 42.68% | 44.34% |
| CMS1-average | 227,842 | 180,640 | 57.22% | 42.78% | 44.83% | |
| S01 | ML2-1 | 176,283 | 140,122 | 57.69% | 42.31% | 28.57% |
| S02 | ML2-2 | 172,702 | 137,363 | 57.68% | 42.32% | 28.27% |
| S03 | ML2-3 | 173,190 | 138,322 | 57.66% | 42.34% | 28.53% |
| ML2-average | 174,058 | 138,602 | 57.68% | 42.32% | 28.46% | |
| S04 | CMS2-1 | 199,387 | 157,597 | 57.35% | 42.65% | 39.67% |
| S05 | CMS2-2 | 188,110 | 148,740 | 57.42% | 42.58% | 38.77% |
| S06 | CMS2-3 | 197,367 | 156,199 | 57.38% | 42.62% | 39.18% |
| CMS2-average | 194,955 | 154,179 | 57.38% | 42.62% | 39.21% |
| Samples | Sample ID | Raw_Reads | Containing ‘N’ Reads | Length < 18 | Length > 30 | Clean Reads | Q30 (%) |
|---|---|---|---|---|---|---|---|
| ML1 | S07 | 15,893,277 | 144 | 3,955,883 | 1,122,051 | 10,815,199 | 96.87 |
| ML1 | S08 | 20,103,763 | 78 | 5,518,188 | 1,542,877 | 13,042,620 | 96.11 |
| ML1 | S09 | 19,280,153 | 70 | 3,545,553 | 2,318,058 | 13,416,472 | 96.73 |
| CMS1 | S10 | 15,264,422 | 93 | 2,935,708 | 907,131 | 11,421,490 | 96.70 |
| CMS1 | S11 | 41,575,041 | 67 | 22,906,812 | 1,512,066 | 17,156,096 | 96.59 |
| CMS1 | S12 | 15,261,148 | 116 | 1,457,723 | 2,035,911 | 11,767,398 | 96.36 |
| ML2 | S01 | 13,467,807 | 42 | 2,319,292 | 911,338 | 10,237,135 | 97.48 |
| ML2 | S02 | 17,142,925 | 41 | 4,425,026 | 868,529 | 11,849,329 | 97.04 |
| ML2 | S03 | 13,400,654 | 40 | 850,586 | 1,322,760 | 11,227,268 | 97.27 |
| CMS2 | S04 | 20,246,038 | 111 | 9,026,132 | 1,174,822 | 10,044,973 | 96.86 |
| CMS2 | S05 | 20,265,519 | 111 | 7,341,508 | 1,495,005 | 11,428,895 | 96.93 |
| CMS2 | S06 | 21,509,385 | 124 | 9,318,264 | 960,142 | 11,230,855 | 96.88 |
| Type | Tag Number | Percent |
|---|---|---|
| Total | 6,944,263 | 100% |
| Mapped | 4,618,210 | 66.50% |
| Perfect map | 3,657,820 | 79.20% |
| Imperfect map | 960,390 | 20.80% |
| Unmapped | 2,326,053 | 32.50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, S.; Wei, X.; Zhang, W.; Zhao, Y.; Yang, S.; Su, H.; Tian, B.; Wei, F.; Zhang, X.; Yuan, Y. bra-miR9569 Targets the BrAHA6 Gene to Negatively Regulate H+-ATPases, Affecting Pollen Fertility in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Plants 2025, 14, 2604. https://doi.org/10.3390/plants14162604
Xiong S, Wei X, Zhang W, Zhao Y, Yang S, Su H, Tian B, Wei F, Zhang X, Yuan Y. bra-miR9569 Targets the BrAHA6 Gene to Negatively Regulate H+-ATPases, Affecting Pollen Fertility in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Plants. 2025; 14(16):2604. https://doi.org/10.3390/plants14162604
Chicago/Turabian StyleXiong, Siyu, Xiaochun Wei, Wenjing Zhang, Yanyan Zhao, Shuangjuan Yang, Henan Su, Baoming Tian, Fang Wei, Xiaowei Zhang, and Yuxiang Yuan. 2025. "bra-miR9569 Targets the BrAHA6 Gene to Negatively Regulate H+-ATPases, Affecting Pollen Fertility in Chinese Cabbage (Brassica rapa L. ssp. pekinensis)" Plants 14, no. 16: 2604. https://doi.org/10.3390/plants14162604
APA StyleXiong, S., Wei, X., Zhang, W., Zhao, Y., Yang, S., Su, H., Tian, B., Wei, F., Zhang, X., & Yuan, Y. (2025). bra-miR9569 Targets the BrAHA6 Gene to Negatively Regulate H+-ATPases, Affecting Pollen Fertility in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Plants, 14(16), 2604. https://doi.org/10.3390/plants14162604







