Stress Responses to Hydrogen Peroxide and Hydric Stress-Related Acoustic Emissions (MHAF) in Capsicum annuum L. Applied in a Single or Combined Manner
Abstract
1. Introduction
2. Results
2.1. Biochemical Responses
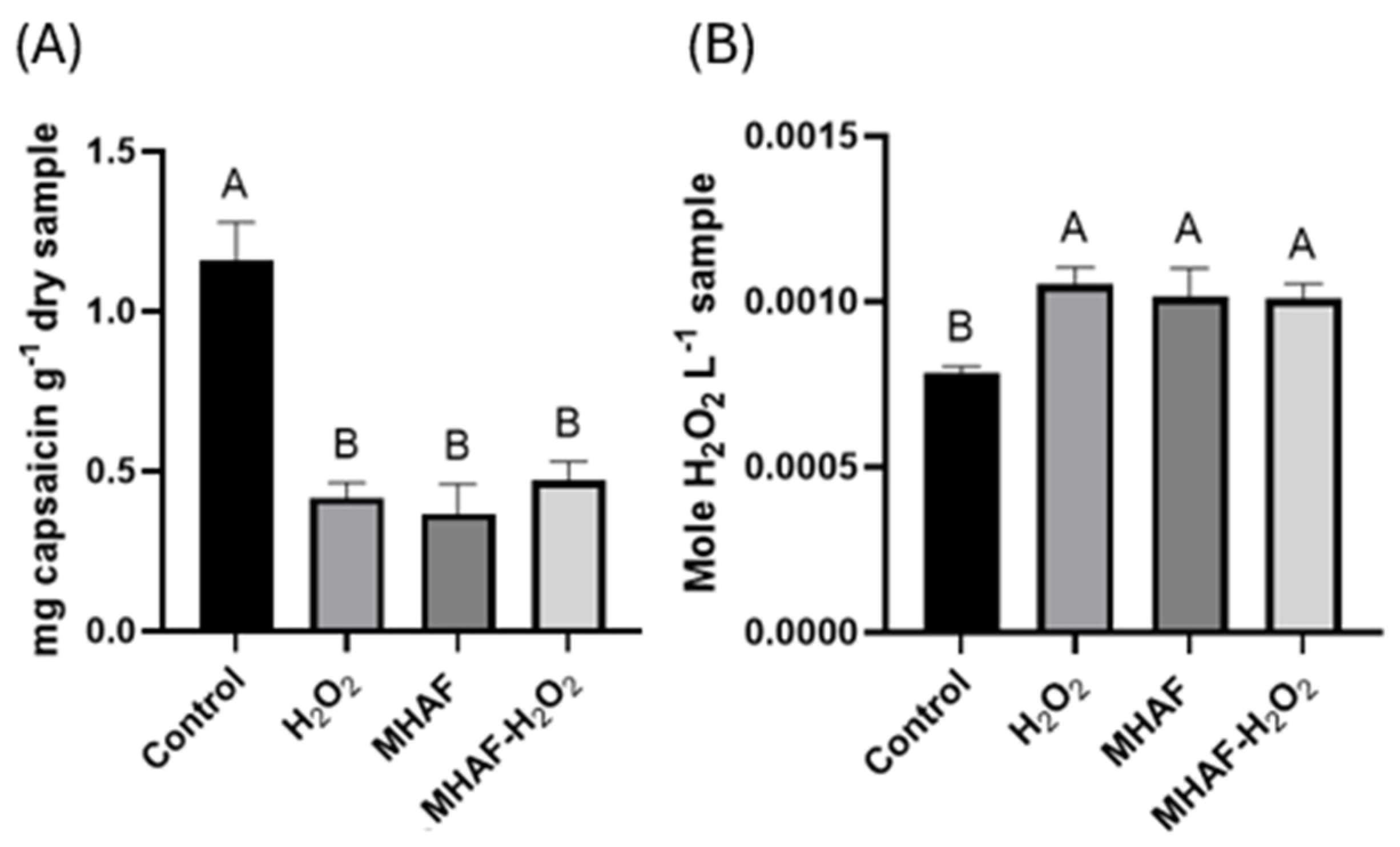
2.1.1. Capsaicin and Endogenous Hydrogen Peroxide
2.1.2. Antioxidant Enzymatic Activity
2.1.3. Total Phenolics, Total Flavonoids, and Antioxidant Capacity
2.2. Molecular Responses
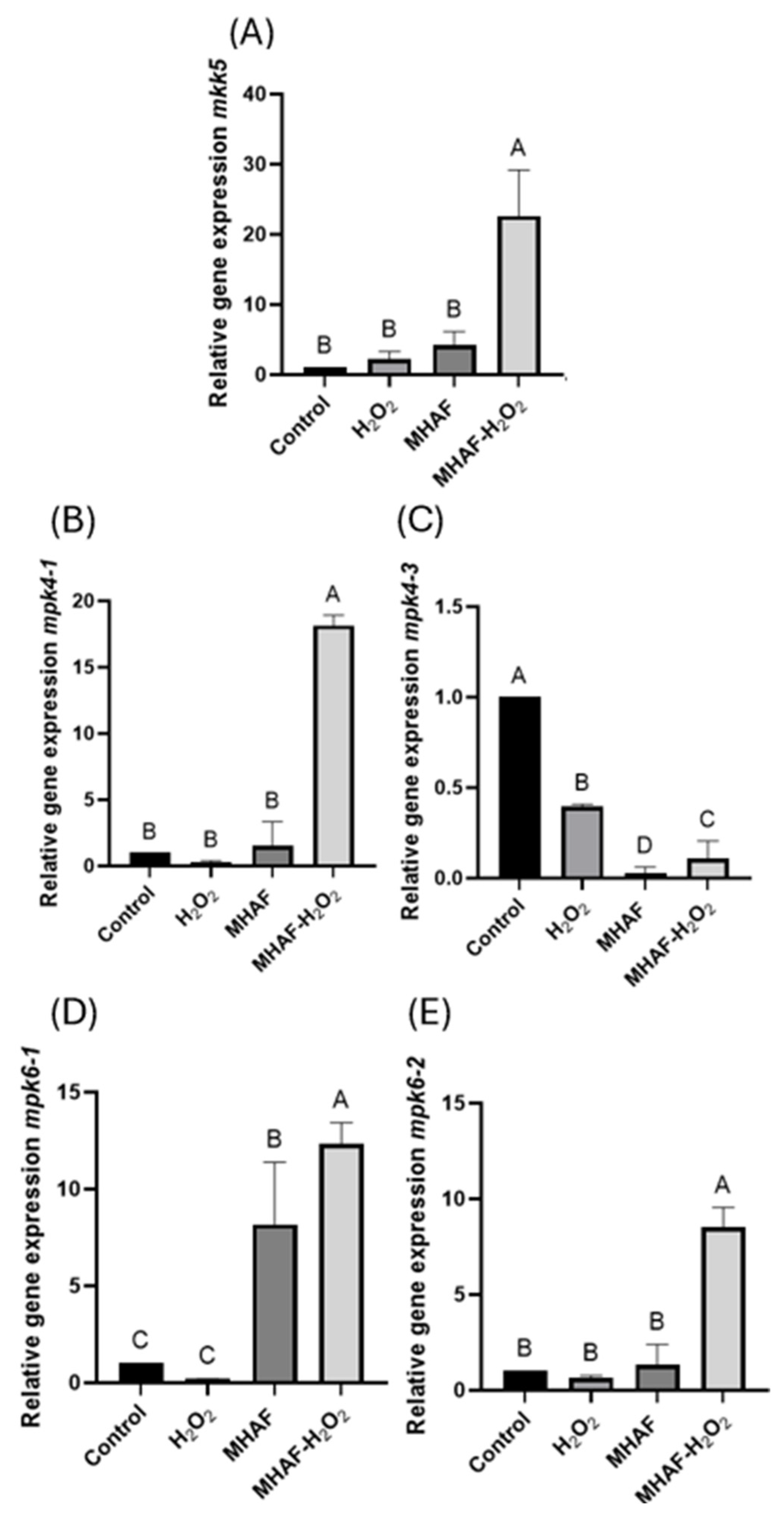
2.2.1. Expression of MAPkinases
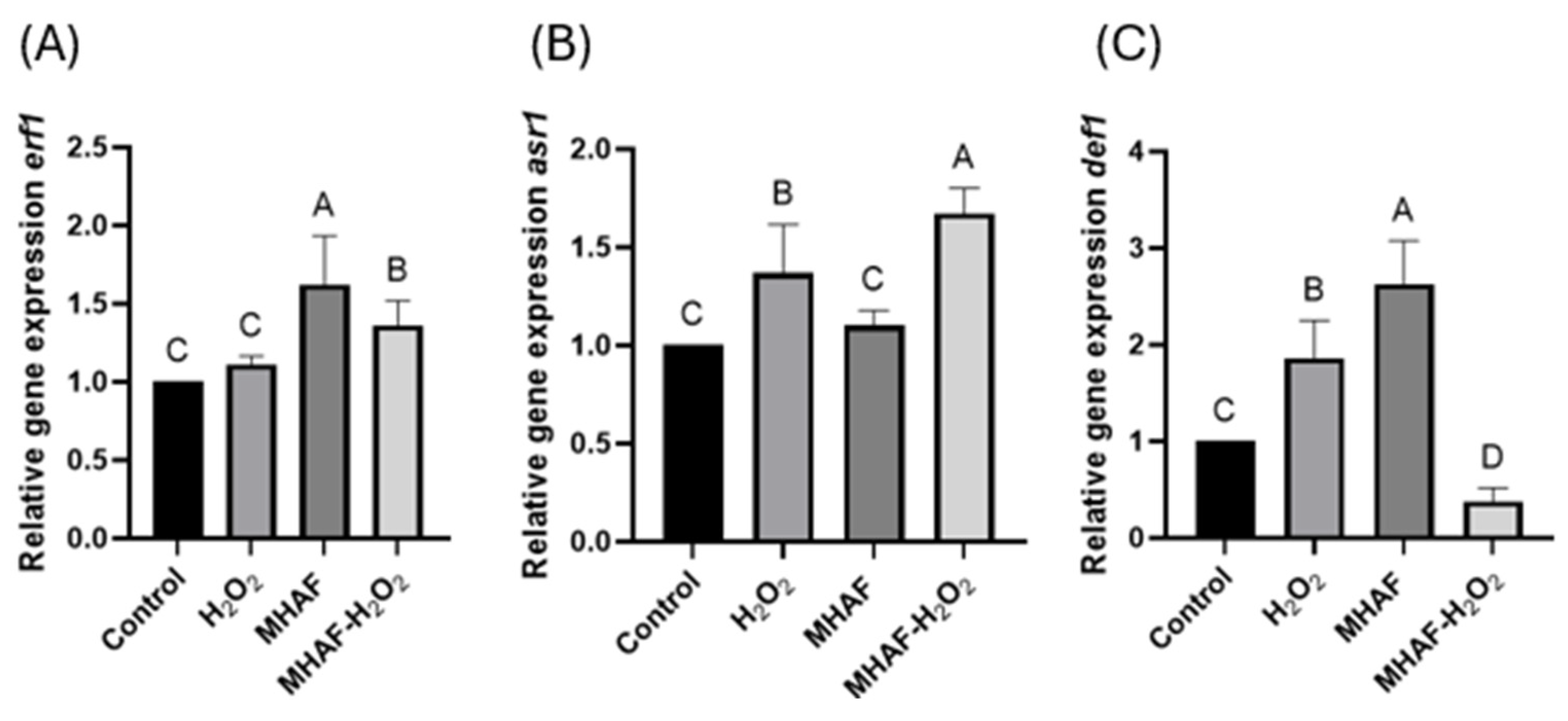
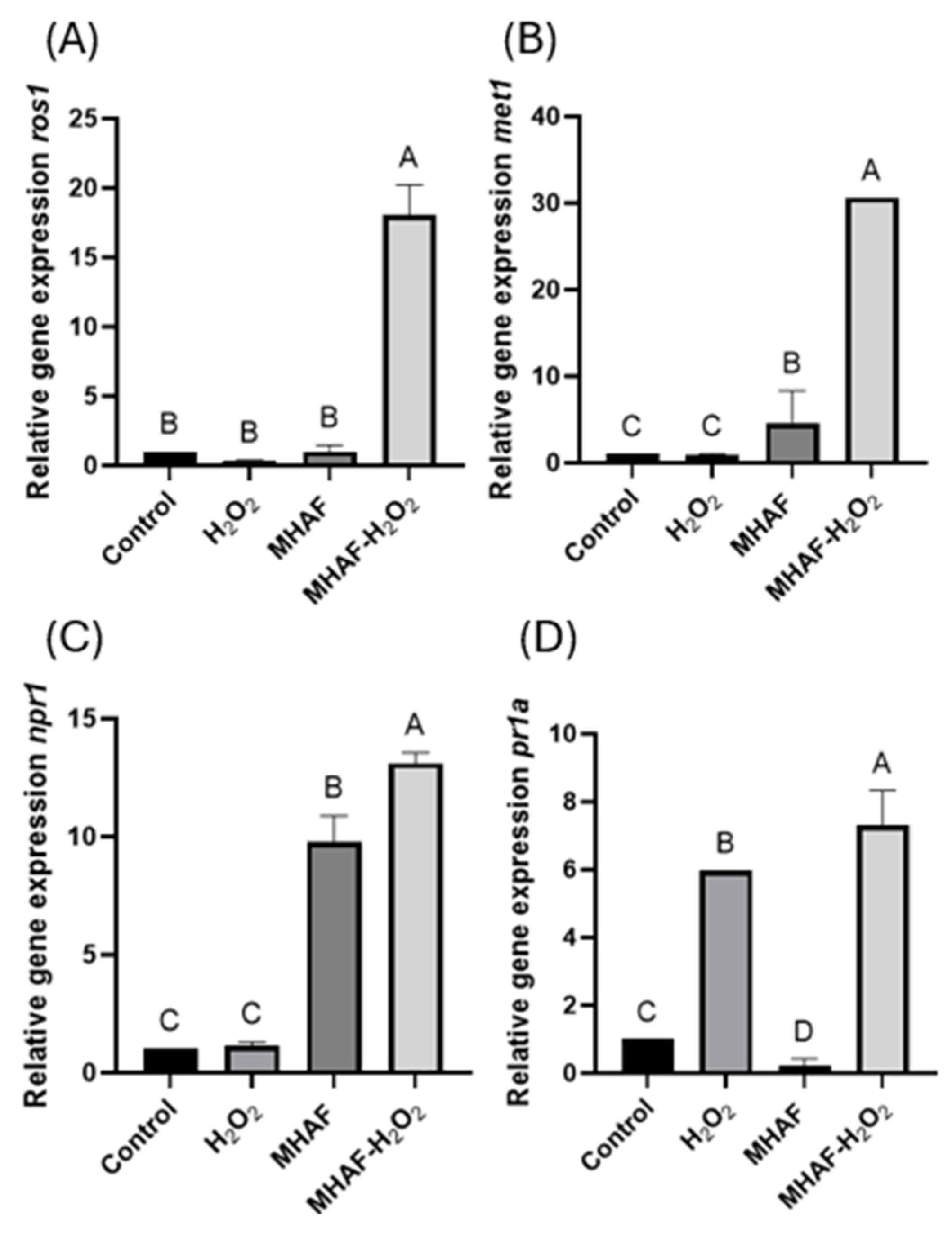
2.2.2. Expression of Induced Systemic Resistance (ISR) Gene Markers
2.2.3. Expression of Systemic Acquired Resistance (SAR) Gene Markers and Genes Related to DNA Methylation/Demethylation
2.3. Multivariable Analysis
3. Discussion
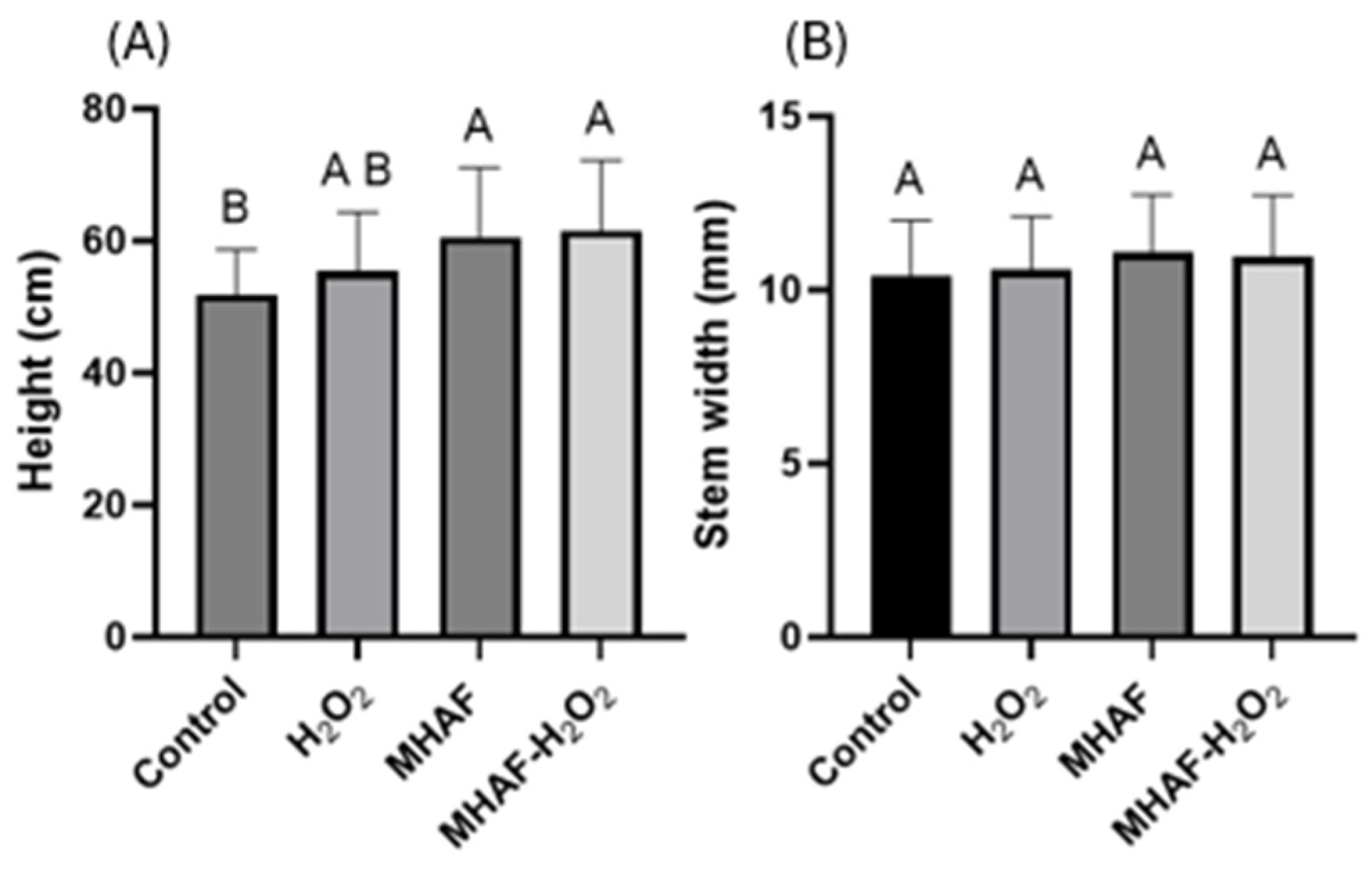
3.1. Morphological Response
3.2. Capsaicin and Hydrogen Peroxide
3.3. Antioxidant Enzymatic Assays
3.4. Antioxidant Non-Enzymatic Assays
3.5. Relative Gene Expression
3.6. PCA Interpretation
4. Materials and Methods
4.1. Chemical Reagents
4.2. Capsicum annuum L. Cultivation
4.3. MHAF Application
4.4. Hydrogen Peroxide Application
4.5. Morphological Variables Measurement
4.6. Enzymatic Assays
4.7. Capsaicin Quantification
4.8. Total Phenolic, Flavonoid Content, and Antioxidant Capacity Using DPPH and ABTS
4.9. Hydrogen Peroxide Quantification
4.10. Relative Gene Expression
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hernández-Pérez, T.; Gómez-García, M.R.; Elena Valverde, M.; Paredes-López, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, M.R.; Ochoa-Alejo, N. Biochemistry and Molecular Biology of Carotenoid Biosynthesis in Chili Peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef] [PubMed]
- Madala, N.; Wesly, C.; Nutakki, K.M.; Kolluri, S. Morphology, cultivation, diseases, importance, traditional breeding and advanced techniques in biotechnology in chilli (Capsicum annuum L.). Int. J. Chem. Stud. 2020, 8, 1132–1136. [Google Scholar] [CrossRef]
- Perry, L.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.M.; Piperno, D.R.; Zeidler, J.A. Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Contreras-Toledo, A.R.; Cortés-Cruz, M.A.; Costich, D.; Rico-Arce, M.L.; Brehm, J.M.; Maxted, N. A Crop Wild Relative Inventory for Mexico. Crop Sci. 2018, 58, 1292–1305. [Google Scholar] [CrossRef]
- Thakur, M.; Sohal, B. Role of elicitors in inducing resistance in plants against pathogen infection: A review. Int. Sch. Res. Not. 2013, 2013, 762412. [Google Scholar] [CrossRef]
- Godínez-Mendoza, P.L.; Rico-Chávez, A.K.; Ferrusquía-Jimenez, N.I.; Carbajal-Valenzuela, I.A.; Villagómez-Aranda, A.L.; Torres-Pacheco, I.; Guevara-González, R.G. Plant hormesis: Revising of the concepts of biostimulation, elicitation and their application in a sustainable agricultural production. Sci. Total Environ. 2023, 894, 164883. [Google Scholar] [CrossRef]
- Mejía-Teniente, L.; Durán-Flores, F.D.; Chapa-Oliver, A.M.; Torres-Pacheco, I.; Cruz-Hernández, A.; González-Chavira, M.M.; Ocampo-Velázquez, R.V.; Guevara-González, R.G. Oxidative and Molecular Responses in Capsicum annuum L. after Hydrogen Peroxide, Salicylic Acid and Chitosan Foliar Applications. Int. J. Mol. Sci. 2013, 14, 10178–10196. [Google Scholar] [CrossRef]
- Mejía-Teniente, L.; Durán-Flores, B.; Torres-Pacheco, I.; González-Chavira, M.; Rivera-Bustamante, R.; Feregrino-Pérez, A.; Pérez-Ramírez, I.; Rocha-Guzmán, N.; Reynoso-Camacho, R.; Guevara-González, R. Hydrogen peroxide protects pepper (Capsicum annuum L.) against pepper golden mosaic geminivirus (PepGMV) infections. Physiol. Mol. Plant Pathol. 2019, 106, 23–29. [Google Scholar] [CrossRef]
- Angole-Tierrablanca, J.A.; Jiménez-Hernández, A.; Aguilar-Rodríguez, P.; Feregrino-Perez, A.A.; Rico-Chávez, A.K.; Godínez-Mendoza, P.L.; Torres-Pacheco, I.; Guzman-Cruz, R.; Nuñez-Muñoz, L.; Guevara-González, R.G. Hydrogen Peroxide Application Based on a Hormetic Scheme Biostimulates Capsicum annuum L. Grown Under Two Fertigation Regimes. J. Soil Sci. Plant Nutr. 2025, 25, 693–709. [Google Scholar] [CrossRef]
- Chowdhury, E.K.; Lim, H.; Bae, H. Update on the Effects of Sound Wave on Plants. Res. Plant Dis. 2014, 20, 1–7. [Google Scholar] [CrossRef]
- Alvarado, A.M.; Aguirre-Becerra, H.; Vázquez-Hernández, M.C.; Magaña-Lopez, E.; Parola-Contreras, I.; Caicedo-Lopez, L.H.; Contreras-Medina, L.M.; Garcia-Trejo, F.; Guevara-Gonzalez, R.G.; Feregrino-Perez, A.A. Influence of Elicitors and Eustressors on the Production of Plant Secondary Metabolites. In Natural Bio-Active Compounds; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Kollasch, A.M.; Abdul-Kafi, A.R.; Body, M.J.A.; Pinto, C.F.; Appel, H.M.; Cocroft, R.B. Leaf vibrations produced by chewing provide a consistent acoustic target for plant recognition of herbivores. Oecologia 2020, 194, 1–13. [Google Scholar] [CrossRef]
- Caicedo-Lopez, L.H.; Contreras-Medina, L.M.; Guevara-Gonzalez, R.G.; Perez-Matzumoto, A.E.; Ruiz-Rueda, A. Effects of hydric stress on vibrational frequency patterns of Capsicum annuum plants. Plant Signal. Behav. 2020, 15, e1770489. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Lopez, L.H.; Guevara-Gonzalez, R.G.; Andrade, J.E.; Esquivel-Delgado, A.; Perez-Matzumoto, A.E.; Torres-Pacheco, I.; Contreras-Medina, L.M. Effect of hydric stress-related acoustic emission on transcriptional and biochemical changes associated with a water deficit in Capsicum annuum L. Plant Physiol. Biochem. 2021, 165, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sachdeva, S.; Bhat, K.V.; Vats, S. Plant Responses to Drought Stress: Physiological, Biochemical and Molecular Basis. In Biotic and Abiotic Stress Tolerance in Plants; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Smékalová, V.; Doskočilová, A.; Komis, G.; Samaj, J. Crosstalk between secondary messengers, hormones and MAPK modules during abiotic stress signaling in plants. Biotechnol. Adv. 2014, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, L.; Liu, Y.; Tang, Q.; Shen, L.; Yang, S.; Cai, J.; Yu, H.; Wang, R.; Wen, J.; et al. Genome-wide identification and transcriptional expression analysis of mitogen-activated protein kinase and mitogen-activated protein kinase kinase genes in Capsicum annuum. Front. Plant Sci. 2015, 6, 780. [Google Scholar] [CrossRef]
- Liu, R.; Lu, J.; Xing, J.; Xue, L.; Wu, Y.; Zhang, L. Characterization and functional analyses of wheat TaPR1 genes in response to stripe rust fungal infection. Sci. Rep. 2023, 13, 3362. [Google Scholar] [CrossRef]
- Montejano-Ramírez, V.; Valencia-Cantero, E. Cross-Talk between Iron Deficiency Response and Defense Establishment in Plants. Int. J. Mol. Sci. 2023, 24, 6236. [Google Scholar] [CrossRef]
- Yang, L.; Lang, C.; Wu, Y.; Meng, D.; Yang, T.; Li, D.; Jin, T.; Zhou, X. ROS1-mediated decrease in DNA methylation and increase in expression of defense genes and stress response genes in Arabidopsis thaliana due to abiotic stresses. BMC Plant Biol. 2022, 22, 104–114. [Google Scholar] [CrossRef]
- Mhamdi, A. CHAPTER Two—Hydrogen peroxide in plants. Adv. Bot. Res. 2023, 105, 43–75. [Google Scholar] [CrossRef]
- González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M.; Corpas, F.J. Class III Peroxidases (POD) in Pepper (Capsicum annuum L.): Genome-Wide Identification and Regulation during Nitric Oxide (NO)-Influenced Fruit Ripening. Antioxidants 2023, 12, 1013. [Google Scholar] [CrossRef]
- Jin, X.; Liu, Z.; Wu, W. POD, CAT, and SOD enzyme activity of corn kernels as affected by low plasma pretreatment. Int. J. Food Prop. 2023, 26, 38–48. [Google Scholar] [CrossRef]
- Vimala, R.; Suriachandraselvan, M. Induced resistance in bhendi against powdery mildew by foliar application of salicylic acid. J. Biopestic. 2009, 2, 111–114. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Parrilla, E.; De La Rosa, L.A.; Amarowicz, R.; Shahidi, F. Antioxidant Activity of Fresh and Processed Jalapeño and Serrano Peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef]
- Dominguez, P.G.; Carrari, F.O. ASR1 transcription factor and its role in metabolism. Plant Signal. Behav. 2014, 10, e992751. [Google Scholar] [CrossRef]
- Akinniyi, O.T.; Reese, J.C. DEF1: Much more than an RNA polymerase degradation factor. DNA Repair 2021, 107, 103202. [Google Scholar] [CrossRef]
- Liu, R.; Lang, Z.B. The mechanism and function of active DNA demethylation in plants. J. Integr. Plant Biol. 2019, 62, 148–159. [Google Scholar] [CrossRef]
- Buchmann, R.C.; Asad, S.; Wolf, J.N.; Mohannath, G.; Bisaro, D.M. Geminivirus AL2 and L2 proteins suppress transcriptional gene silencing and cause genome-wide reductions in cytosine methylation. J. Virol. 2009, 83, 5005–5013. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.K. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009, 12, 133–139. [Google Scholar] [CrossRef]
- Villagómez-Aranda, A.L.; Feregrino-Pérez, A.A.; García-Ortega, L.F.; Rivero-Montejo, S.J.; Torres-Pacheco, I.; Guevara-González, R.G. H2O2 priming: Biostimulation, drought tolerance and DNA methylation profile with intergenerational impact in tobacco plant. Environ. Exp. Bot. 2024, 226, 105859. [Google Scholar] [CrossRef]
- Cao, H.; Glazebrook, J.; Clarke, J.D.; Volko, S.; Dong, X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 1997, 88, 57–63. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil. 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, H.; Ali, M.; Ren, K.; Cheng, Z. Aqueous garlic extract stimulates growth and antioxidant enzymes activity of tomato (Solanum lycopersicum). Sci. Hortic. 2018, 240, 139–146. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Leonardi, C.; Romano, D. PAL activities in asparagus Spears during storage after ammonium sulfate treatments. Postharvest Biol. Technol. 2018, 140, 34–41. [Google Scholar] [CrossRef]
- Hadwan, M.H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018, 19, 7–14. [Google Scholar] [CrossRef]
- Abbas, A.A.; Abdulatif, M.O. Determination activities of optimal conditions of peroxidase in hot Pepper and Broccoli. Wasit J. Sci. Med. 2016, 8, 92–101. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef]
- Malm, L.; Palm, E.; Souihi, A.; Plassmann, M.; Liigand, J.; Kruve, A. Guide to Semi-Quantitative Non-Targeted Screening Using LC/ESI/HRMS. Molecules 2021, 26, 3524. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L.). J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of Scavenging Activity of Phenolic Compounds Using the ABTS+ Assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Huguette, S.; Lopez, F. Optimized Assay for Hydrogen Peroxide Determination in Plant Tissue Using Potassium Iodide. Am. J. Anal. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godínez-Mendoza, P.L.; Rico-Chávez, A.K.; Carbajal-Valenzuela, I.A.; Contreras-Medina, L.M.; Ocampo-Velázquez, R.V.; Rico-García, E.; Torres-Pacheco, I.; Guevara-González, R.G. Stress Responses to Hydrogen Peroxide and Hydric Stress-Related Acoustic Emissions (MHAF) in Capsicum annuum L. Applied in a Single or Combined Manner. Plants 2025, 14, 2591. https://doi.org/10.3390/plants14162591
Godínez-Mendoza PL, Rico-Chávez AK, Carbajal-Valenzuela IA, Contreras-Medina LM, Ocampo-Velázquez RV, Rico-García E, Torres-Pacheco I, Guevara-González RG. Stress Responses to Hydrogen Peroxide and Hydric Stress-Related Acoustic Emissions (MHAF) in Capsicum annuum L. Applied in a Single or Combined Manner. Plants. 2025; 14(16):2591. https://doi.org/10.3390/plants14162591
Chicago/Turabian StyleGodínez-Mendoza, Pablo L., Amanda K. Rico-Chávez, Ireri A. Carbajal-Valenzuela, Luis M. Contreras-Medina, Rosalía V. Ocampo-Velázquez, Enrique Rico-García, Irineo Torres-Pacheco, and Ramón G. Guevara-González. 2025. "Stress Responses to Hydrogen Peroxide and Hydric Stress-Related Acoustic Emissions (MHAF) in Capsicum annuum L. Applied in a Single or Combined Manner" Plants 14, no. 16: 2591. https://doi.org/10.3390/plants14162591
APA StyleGodínez-Mendoza, P. L., Rico-Chávez, A. K., Carbajal-Valenzuela, I. A., Contreras-Medina, L. M., Ocampo-Velázquez, R. V., Rico-García, E., Torres-Pacheco, I., & Guevara-González, R. G. (2025). Stress Responses to Hydrogen Peroxide and Hydric Stress-Related Acoustic Emissions (MHAF) in Capsicum annuum L. Applied in a Single or Combined Manner. Plants, 14(16), 2591. https://doi.org/10.3390/plants14162591








