The Effect of Foliar Spraying of Different Selenium Fertilizers on the Growth, Yield, and Quality of Garlic (Allium sativum L.)
Abstract
1. Introduction
2. Results
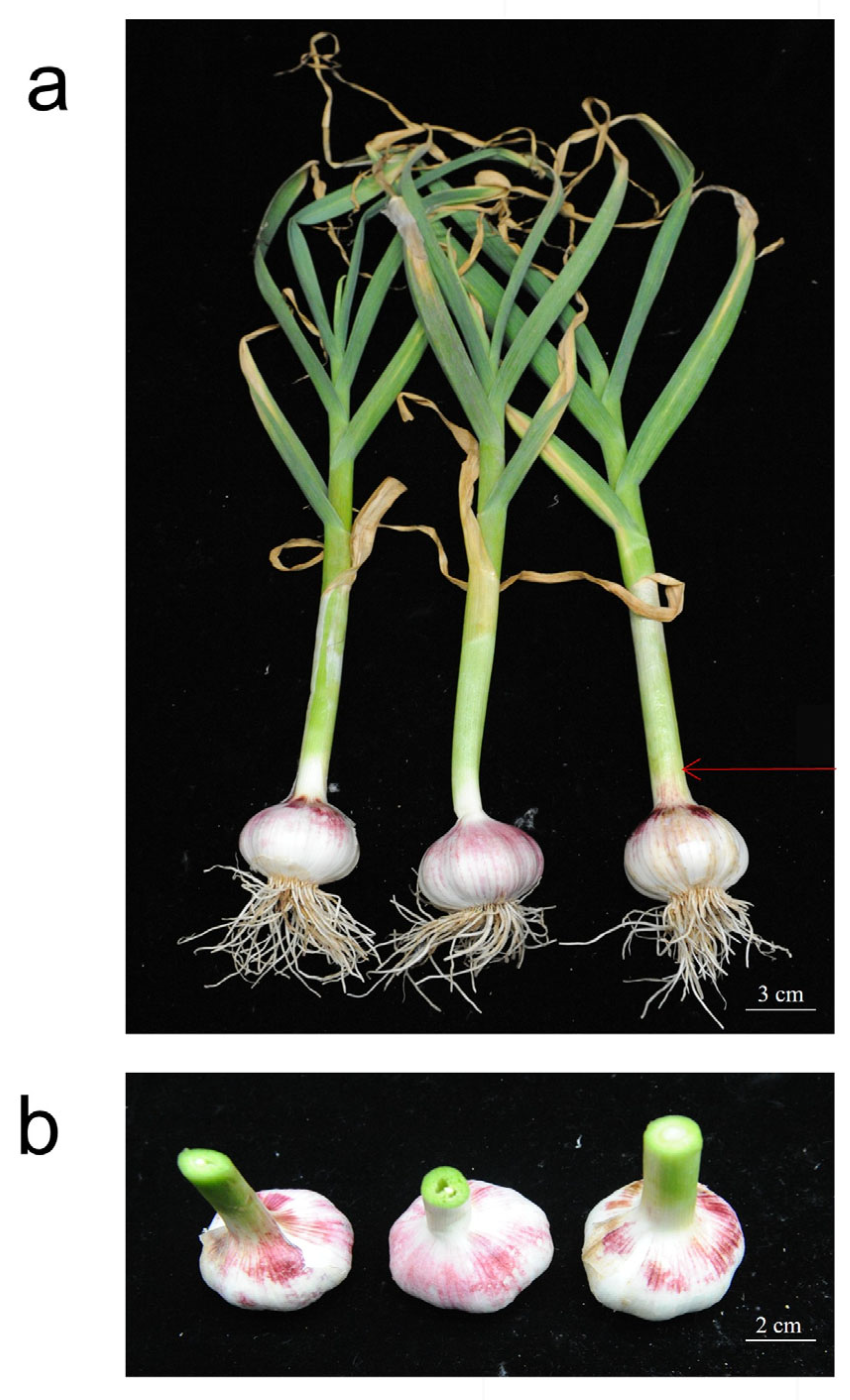
2.1. Growth Status of Plant Shoots Post-Fertilization Treatment
2.2. Effect of Selenium Fertilizer Application on Garlic Bulb Yield
2.3. Effects of Different Selenium Fertilizer Treatments on Garlic Bulb Nutritional Components
2.4. Selenium Accumulation in Garlic Under Different Treatments
2.5. Comprehensive Evaluation of Fertilization Efficacy Under Different Treatments
3. Discussion
3.1. Application of Fertilizers Promotes the Growth of Garlic Aboveground Parts and Bulbs
3.2. Application of Selenium Fertilizer Can Increase the Selenium Content and Other Nutrient Contents in Garlic Bulbs
3.3. Determining the Appropriate Selenium Application Method Using Principal Component Analysis and Membership Function Method
4. Materials and Methods
4.1. Plant Materials and Growth Environment
4.2. Experimental Treatments
4.3. Morphological Indicators
4.4. Bulb Yield Indicators
4.5. Bulb Quality Indicators
4.6. Determination of Selenium Content
4.7. Comprehensive Evaluation
4.7.1. Data Standardization
4.7.2. Principal Component Scores
4.7.3. Weights of Each Principal Component
4.7.4. Membership Function Values
4.7.5. Evaluation of Fertilization Effects for Each Treatment
4.8. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Yang, F.; Fan, J. Research progress on extraction technology of garlic organosulfur compounds. China Condiment 2020, 45, 197–200. [Google Scholar] [CrossRef]
- China Garlic Annual Supply and Demand Balance Sheet (August 2024 Consultation Release). [R/OL.]. Available online: http://www.scs.moa.gov.cn/jcyj/202409/t20240902_6461617.htm (accessed on 2 September 2024).
- You, S.; Du, S.; Ge, G.; Wan, T.; Jia, Y. Selection of lactic acid bacteria from native grass silage and its effects as inoculant on silage fermentation. Agron. J. 2021, 113, 3169–3177. [Google Scholar] [CrossRef]
- Li, T.; Xu, H. Selenium-containing nanomaterials for cancer treatment. Cell Rep. Phys. Sci. 2020, 1, 1038. [Google Scholar] [CrossRef]
- Handa, E.; Puspitasari, I.M.; Abdulah, R.; Yamazaki, C.; Kameo, S.; Nakano, T.; Koyama, H. Recent advances in clinical studies of selenium supplementation in radiotherapy. J. Trace Elem. Med. Biol. 2020, 62, 126653. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Qiu, Y.; Zhao, Y.; Fu, L.; Xia, D.; Ying, J. Selenium protects against Pb-induced renal oxidative injury in weaning rats and human renal tubular epithelial cells through activating NRF2. J. Trace Elem. Med. Biol. 2024, 1, 127420. [Google Scholar] [CrossRef]
- Xin, T.; Fu, Y.; Wang, X.; Jiang, N.; Zhai, D.; Shang, X.; Dong, H.; Luan, T.; Tang, G.; Yu, H. Research Progress of Selenium-Enriched Edible Fungi. Horticulturae 2025, 11, 531. [Google Scholar] [CrossRef]
- Fernandes, K.F.M.; Berton, R.; Coscione, A. Selenium biofortiffcation of rice and radish: Effect of soil texture and efffciency of two extractants. Plant Soil. Environ. 2014, 60, 105–110. [Google Scholar] [CrossRef]
- Yuan, J.Y.; Wang, P.; Liu, Z.X.; Guo, F.; Hu, F.; Shi, S. Effects of Selenium Application on Physiological Characteristics, Selenium Content, and Yield of Garlic. J. Xinjiang Agric. Univ. 2010, 33, 19–22. [Google Scholar]
- Larsen, E.H.; Lobinski, R.; Burger-Meÿer, K.; Hansen, M.; Ruzik, R.; Mazurowska, L.; Rasmussen, P.H.; Sloth, J.J.; Scholten, O.; Kik, C. Uptake and speciation of selenium in garlic cultivated in soil amended with symbiotic fungi (mycorrhiza) and selenate. Anal. Bioanal. Chem. 2006, 385, 1098–1108. [Google Scholar] [CrossRef]
- Fang, Y.; Luo, P.; Hu, Y.; Ma, N.; Yang, W.; Xin, Z.; Zhao, L.; Hu, Q. Bioaccumulation and Speciation Analysis of Selenium in Garlic (Allium sativum L.). Food Sci. 2012, 33, 1–5. [Google Scholar]
- Jiang, Y.; Zh, Z.; Bu, Y.; Cz, R.; Jz, L.; Jj, H.; Tao, C.; Zhang, K.; Xx, W.; Gx, L.; et al. Effects of selenium fertilizer on grain yield, Se uptake and distribution in common buckwheat (Fagopyrum esculentum Moench). Plant Soil. Environ. 2016, 61, 371–377. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium enrichment enhances the quality and shelf life of Basil leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef]
- Beyene, T.M. Application of Bio-fortiffcation through Plant Breeding to Improve the Value of Staple Crops. J. Biomed. Biotechnol. 2015, 3, 11–19. [Google Scholar]
- Rocha, L.; Silva, E.; Gonçalves, A.; Brito, C.; Ferreira, H.; Matos, C.; Malheiro, A.C.; Araújo, S.; Lima-Brito, J.; Moutinho-Pereira, J. Biomass, Physiological, and Antioxidant Activity Responses of Wheat Plants After Selenium Foliar Spray Under Water Deficit. Agriculture 2025, 15, 1086. [Google Scholar] [CrossRef]
- Wang, S.; Mao, J.; Xu, Y.; Liu, S.; Qiao, Z.; Cao, X. Optimal Selenium Fertilizer Affects the Formation of Foxtail Millet (Setaria italica L.) Quality by Regulating Flavonoid Metabolism and Amino Acid Metabolism. Food Sci. Nutr. 2025, 13, e70362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, M.; Chen, Y.; Liu, H.; Xia, J.; Sun, H. Research and Application of Selenium Rich Fertilizer. Chin. J. Soil. Fertil. 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Ros, G.H.; van Rotterdam, A.M.D.; Bussink, D.W.; Bindraban, P.S. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Foliar application of nutrients on medicinal and aromatic plants, the sustainableapproaches for higher and better production. Beni-Suef Univ. J. Basic. Appl. Sci. 2022, 11, 26. [Google Scholar] [CrossRef]
- Boldrin, P.F.; Faquin, V.; Ramos, S.J.; Boldrin, K.V.F.; Ávila, F.W.; Guilmaraes Guilherme, L.R. Soil and foliar application of selenium in rice biofortification. J. Food Compost. Anal. 2013, 31, 238–244. [Google Scholar] [CrossRef]
- Liu, M. Effects of Two Different Selenium Fertilizers on Selenium Accumulation in Rice Grains and Quality-Related Traits. Master’s Dissertation, Yangzhou University, Yangzhou, China, 2021. [Google Scholar] [CrossRef]
- Wang, W.; Yang, W.; Wang, R. Effects of Nano-Selenium on Rice Yield and Quality. Anhui Agric. Sci. Bull. 2022, 28, 77–78. [Google Scholar] [CrossRef]
- Fang, Q.; Liu, Z.; Wang, K. Selenium Nanoparticles vs Selenite Fertilizers: Implications for Toxicological Profiles, Antioxidant Defense, and Ferroptosis Pathways. J. Agric. Food Chem. 2025, 73, 11634–11646. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef]
- Ramkissoon, C.; Degryse, F.; da Silva, R.; Baird, R.; Young, S.; Bailey, E.; McLaughlin, M. Improving the efffcacy of selenium fertilizers for wheat biofortiffcation. Sci. Rep. 2019, 9, 19520. [Google Scholar] [CrossRef] [PubMed]
- Radawiec, A.; Szulc, W.; Rutkowska, B. Selenium biofortiffcation of wheat as a strategy to improve Human nutrition. Agriculture 2021, 11, 144. [Google Scholar] [CrossRef]
- Wang, X.; Tian, X.; Zhang, X.; Zhang, L.; Si, D.; Lv, F. Effects of Optimized Fertilization on Growth, Yield, and Quality of Chinese Cabbage. J. Anhui Agric. Sci. 2022, 50, 38–41. [Google Scholar]
- Ge, J.; Fan, J.; Zhao, Y.; Lu, X.; Liu, C.; Zhang, B.; Yang, Q.; Li, M.; Yang, Y.; Yang, F. Comparative transcriptomics analysis reveals stage-specific gene expression profiles associated with gamete formation in Garlic. Hortic. Plant J. 2025, 112, 839–853. [Google Scholar] [CrossRef]
- Ge, J.; Liu, G.; Yang, Q.; Fan, J.; Zhao, Y.; Lu, X.; Liu, C.; Zhang, B.; Yang, Q.; Li, M.; et al. Gene Co-Expression Network Analysis Reveals AsAMS as a Key Regulator of Gametophyte Fertility in Garlic. Plant Cell Environ. 2025. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Fan, J.; Liu, C.; Zhao, Y.; Xu, Z.; Lu, X.; Ge, J.; Zhang, B.; Li, M.; Yang, Y.; et al. Physiological and transcriptome analysis of changes in endogenous hormone contents and related synthesis and signaling genes during the heat stress in garlic (Allium sativum L.). BMC Plant Biol. 2025, 25, 464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Q.; Yang, F.; Liu, C.; Zhao, Y.; Lu, X.; Ge, J.; Zhang, B.; Li, M.; Yang, Y.; Fan, J. Genome-wide analysis of the HSF family in Allium sativum L. and AsHSFB1 overexpression in Arabidopsis under heat stress. BMC Genom. 2024, 25, 1072. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, B.; Fan, J.; Lu, X.; Liu, C.; Zhao, Y.; Yang, F. The effect of foliar sulfur application on the growth, yield, and quality of garlic. Jiangsu Agric. Sci. 2022, 50, 96–100. [Google Scholar] [CrossRef]
- Tian, J.; Zhou, Q.; Tie, Y.; Sun, H.; Huang, S. Metabolomics analysis of garlic seedlings under drought stress. Acta Hortic. Sin. 2024, 51, 133–144. [Google Scholar] [CrossRef]
- Dias, M.C.; Correia, S.; Serôdio, J.; Silva, A.M.S.; Freitas, H.; Santos, C. Chlorophyll ffuorescence and oxidative stress endpoints to discriminate olive cultivars tolerance to drought and heat episodes. Sci. Hortic. 2018, 231, 31–35. [Google Scholar] [CrossRef]
- Wang, M.; Dinh, T.; Qi, M.; Min, W.; Yang, W.; Fei, Z.; Liang, D. Radicular and foliar uptake, and xylem- and phloem-mediated transport of selenium in maize (Zea mays L.): A comparison of ffve Se exogenous species. Plant Soil 2019, 446, 111–123. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its inffuence on photosynthesis and chlorophyll ffuorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Proietti, P.; Nasini, L.; Del Buono, D.; D’Amato, R.; Tedeschini, E.; Businelli, D. Selenium protects olive (Olea europaea L.) from drought stress. Sci. Hortic. 2013, 164, 165–171. [Google Scholar] [CrossRef]
- Su, S. Xusuan 918. Farmers Get. Rich 2018, 17, 24. [Google Scholar]
- Wang, Y.-D.; Wang, X.; Wong, Y.-S. Proteomics analysis reveals multiple regulatory mechanisms in response to selenium in rice. J. Proteom. 2012, 75, 1849–1866. [Google Scholar] [CrossRef]
- Andrade, F.R.; Da Silva, G.N.; Guimarães, K.C.; Barreto, H.B.F.; De Souza, K.R.D.; Guilherme, L.R.G.; Faquin, V.; Reis, A.R.D. Selenium protects rice plants from water deffcit stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef]
- Feng, T.; Chen, S.; Gao, D.; Liu, G.; Bai, H.; Li, A.; Peng, L.; Ren, Z. Selenium improves photosynthesis and protects photosystemII in pear (Pyrus bretschneideri), grape (Vitis vinifera), and peach (Prunus persica). Photosynthetica 2015, 53, 609–612. [Google Scholar] [CrossRef]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bose, B.; Md, P.; Gupta, N.K.; Mehta, B.K.; Kumar, P.; Pandey, S.; et al. Drought stress responses and inducing tolerance by seed priming approach in plants. Plant Stress. 2022, 4, 100066. [Google Scholar] [CrossRef]
- Nakazawa, S.; Hou, J.; Kato, M.; Togo, S.; Arai, Y.; Motomura, H.; Kurata, K.; Sueyasu, T.; Hirakawa, H.; Ochi, Y.; et al. Allicin induced AMPK signaling attenuated Smad3 pathway mediated lung fibrosis. Sci. Rep. 2025, 15, 19060. [Google Scholar] [CrossRef]
- Wessjohann, L.A.; Schneider, A.; Abbas, M.; Brandt, W. Selenium in chemistry and biochemistry in comparison to sulfur. Biol. Chem. 2007, 388, 997–1006. [Google Scholar] [CrossRef]
- Huang, C.; Ying, H.; Yang, X.; Gao, Y.; Li, T.; Wu, B.; Ren, M.; Zhang, Z.; Ding, J.; Gao, J.; et al. The Cardamine enshiensis genome reveals whole genome duplication and insight into selenium hyperaccumulation and tolerance. Cell Discov. 2021, 7, 62. [Google Scholar] [CrossRef]
- El Kassis, E.; Cathala, N.; Rouached, H.; Fourcroy, P.; Berthomieu, P.; Terry, N.; Davidian, J.-C. Characterization of a selenate-resistant Arabidopsis mutant. root growth as a potential target for selenate toxicity. Plant Physiol. 2007, 143, 1231–1241. [Google Scholar] [CrossRef]
- Hou, P.; Wang, F.; Luo, B.; Li, A.; Wang, C.; Shabala, L.; Ahmed, H.A.I.; Deng, S.; Zhang, H.; Song, P.; et al. Antioxidant Enzymatic Activity and Osmotic Adjustment as Components of the Drought Tolerance Mechanism in Carex duriuscula. Plants 2021, 10, 436. [Google Scholar] [CrossRef]
- Mahmood, T.; Abdullah, M.; Ahmar, S.; Yasir, M.; Iqbal, M.S.; Yasir, M.; Ur Rehman, S.; Ahmed, S.; Rana, R.M.; Ghafoor, A.; et al. Incredible Role of Osmotic Adjustment in Grain Yield Sustainability under Water Scarcity Conditions in Wheat (Triticum aestivum L.). Plants 2020, 9, 1208. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tao, F.; Hao, Y.; Tong, J.; Xiao, Y.; He, Z.; Reynolds, M. Unfolding the leaf economics spectrum for wheat: Trait analysis and genomic associations across cultivars. Environ. Exp. Bot. 2024, 226, 105928. [Google Scholar] [CrossRef]
- Fan, X.; Cui, Y.; Song, J.; Fan, H.; Tang, L.; Wang, J. Preliminary Exploration of Physiology and Genetic Basis Underlying High Yield in Indica–Japonica Hybrid Rice. Agriculture 2024, 14, 607. [Google Scholar] [CrossRef]
- Chen, Y.; Bao, W.; Hong, W.; Dong, X.; Gong, M.; Cheng, Q.; Mao, K.; Yao, C.; Liu, Z.; Wang, N. Evaluation of eleven kiwifruit genotypes for bicarbonate tolerance and characterization of two tolerance-contrasting genotypes. Plant Physiol. Biochem. 2023, 194, 202–213. [Google Scholar] [CrossRef]
- Wang, H.; Yang, A.; Yang, G.; Zhao, H.; Xie, F.; Zhang, H.; Wang, H.; Ao, X. Screening and identification of soybean varieties with high phosphorus efficiency at seedling stage. Oil Crop Sci. 2021, 6, 41–49. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, D.; Zou, F.; Fan, X.; Tang, J.; Zhu, Z. A Study on the Xenia Effect in Castanea henryi. Hortic. Plant J. 2017, 2, 301–308. [Google Scholar] [CrossRef]
- Wei, W.; Li, P.; Zhou, Z.-G.; Wang, X.-X.; Ding, C.-F. Changes in the availability of different exogenous selenium sources in soil and their effects on selenium accumulation in wheat. Environ. Sci. 2023, 44, 1003–1011. [Google Scholar] [CrossRef]
- Kong, Q.H.; Li, F.F.; Qin, L.; Chen, E.Y.; Yang, Y.B.; Zhang, H.D.; Guan, Y.A. Screening and analysis of Se responsive genes in leaves of foxtail millet. Mol. Plant Breed. 2021, 19, 2798–2810. [Google Scholar]
- Wang, Y.; Tan, G.; Chen, J.; Wu, J.; Liu, S.; He, X. Effects of Foliar Spraying of Organic Selenium and Nano-Selenium Fertilizer on Pak Choi (Brassica chinensis var. pekinensis. cv. ‘Suzhouqing’) under Low Temperature Stress. Agriculture 2023, 13, 2140. [Google Scholar] [CrossRef]
- Li, X.; Zhu, D.; Du, Y.; Wang, H.; Shen, H.; Shan, X. Descriptive Specifications and Data Standards for Garlic Germplasm Resources; China Agriculture Press: Beijing, China, 2006; pp. 10–26. [Google Scholar]
- Feng, Y.; Zhou, C.; Yagoub, A.E.A.; Sun, Y.; Owusu-Ansah, P.; Yu, X.; Wang, X.; Xu, X.; Zhang, J.; Ren, Z. Improvement of the catalytic infrared drying process and quality characteristics of the dried garlic slices by ultrasound-assisted alcohol pretreatment. LWT 2019, 116, 108577. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Treatment | Plant Height (cm) | Plant Width (cm) | Leaf Length (cm) | Leaf Width (cm) | Number of Functional Leaves per Plant | Height of Above-Ground Pseudostem (cm) | Thickness of Above-Ground Pseudostem (mm) |
|---|---|---|---|---|---|---|---|
| CK | 60.40 ± 5.04 bc | 51.10 ± 4.75 d | 50.76 ± 3.12 d | 3.35 ± 0.20 c | 7.03 ± 0.05 a | 34.47 ± 0.67 b | 15.25 ± 1.37 c |
| N1 | 63.14 ± 3.55 abc | 62.26 ± 1.72 a | 55.80 ± 3.37 abc | 3.73 ± 0.39 abc | 7.22 ± 0.16 a | 38.57 ± 0.88 a | 19.37 ± 0.78 a |
| N2 | 57.79 ± 6.10 c | 56.81 ± 4.19 bc | 55.21 ± 3.63 c | 3.83 ± 0.36 ab | 7.17 ± 0.19 a | 36.91 ± 1.83 a | 17.14 ± 1.34 b |
| N3 | 65.35 ± 1.66 ab | 60.59 ± 1.18 abc | 59.07 ± 0.97 ab | 3.69 ± 0.32 abc | 7.31 ± 0.47 a | 38.15 ± 0.46 a | 18.48 ± 0.44 ab |
| E1 | 66.03 ± 4.79 ab | 62.15 ± 1.91 a | 59.23 ± 1.26 ab | 3.79 ± 0.38 abc | 7.18 ± 0.33 a | 36.32 ± 3.15 ab | 18.22 ± 1.01 ab |
| E2 | 65.04 ± 4.24 ab | 60.37 ± 2.77 abc | 55.64 ± 3.58 bcd | 3.61 ± 0.30 abc | 7.08 ± 0.17 a | 37.18 ± 1.63 a | 17.69 ± 0.83 ab |
| E3 | 67.65 ± 4.16 a | 61.83 ± 2.77 a | 58.95 ± 1.65 ab | 3.53 ± 0.10 abc | 7.17 ± 0.34 a | 37.63 ± 1.81 a | 18.40 ± 1.14 ab |
| O1 | 64.79 ± 2.80 ab | 63.45 ± 1.90 a | 60.15 ± 2.52 a | 3.88 ± 0.40 a | 7.38 ± 0.34 a | 38.93 ± 1.05 a | 18.62 ± 1.54 ab |
| O2 | 61.17 ± 3.25 abc | 55.42 ± 4.53 d | 52.95 ± 4.67 d | 3.40 ± 0.09 bc | 7.05 ± 0.10 a | 37.48 ± 0.83 a | 19.48 ± 1.93 a |
| O3 | 62.78 ± 4.95 abc | 56.64 ± 4.02 c | 57.49 ± 2.20 ab | 3.51 ± 0.14 abc | 7.10 ± 0.20 a | 38.36 ± 1.45 a | 17.71 ± 0.91 ab |
| M1 | 65.38 ± 3.94 ab | 60.43 ± 1.66 abc | 59.22 ± 1.03 ab | 3.64 ± 0.22 abc | 7.05 ± 0.10 a | 37.19 ± 1.27 a | 19.28 ± 0.75 a |
| M2 | 63.08 ± 3.69 abc | 58.68 ± 3.42 abc | 59.53 ± 1.53 ab | 3.65 ± 0.14 abc | 7.07 ± 0.14 a | 37.58 ± 1.87 a | 18.25 ± 0.37 ab |
| M3 | 64.92 ± 3.21 ab | 58.33 ± 3.56 abc | 59.50 ± 2.35 ab | 3.50 ± 0.23 abc | 7.11 ± 0.16 a | 37.38 ± 1.86 a | 19.29 ± 1.09 a |
| Treatment | Fresh Weight (t ha−1) | Dry Weight (t ha−1) | DW/FW Ratio (%) | Bulb Height (mm) | Bulb Width (mm) | Single Bulb Weight (g) |
|---|---|---|---|---|---|---|
| CK | 24.10 ± 1.29 e | 17.64 ± 0.21 c | 73.18 ± 2.21 c | 35.42 ± 0.77 b | 56.80 ± 2.99 c | 60.63 ± 1.53 d |
| N1 | 25.07 ± 0.53 cde | 19.40 ± 0.43 ab | 77.39 ± 1.40 ab | 38.07 ± 1.38 a | 62.02 ± 1.58 ab | 68.91 ± 2.71 ab |
| N2 | 23.29 ± 1.01 e | 18.61 ± 0.47 bc | 79.91 ± 2.62 a | 35.84 ± 1.28 b | 59.59 ± 1.38 b | 63.87 ± 2.05 cd |
| N3 | 26.66 ± 1.40 abcd | 19.89 ± 1.27 ab | 74.58 ± 5.96 bc | 36.90 ± 0.65 ab | 61.55 ± 2.49 ab | 67.08 ± 1.99 abc |
| E1 | 25.30 ± 1.83 bcde | 19.50 ± 1.73 ab | 77.07 ± 1.77 ab | 35.93 ± 2.15 b | 60.06 ± 2.12 ab | 68.26 ± 2.85 abc |
| E2 | 25.82 ± 2.00 abcd | 19.59 ± 1.16 ab | 75.86 ± 2.77 bc | 35.92 ± 1.11 b | 60.51 ± 1.42 ab | 67.44 ± 2.21 abc |
| E3 | 25.84 ± 2.30 abcd | 19.49 ± 1.41 ab | 75.45 ± 1.88 bc | 36.96 ± 0.28 ab | 62.83 ± 0.69 ab | 67.81 ± 2.19 abc |
| O1 | 26.45 ± 1.56 abcd | 19.72 ± 0.89 ab | 74.58 ± 0.78 bc | 36.80 ± 0.54 ab | 61.73 ± 1.30 ab | 66.57 ± 2.51 bc |
| O2 | 24.81 ± 0.17 cde | 18.99 ± 0.31 bc | 76.55 ± 1.79 ab | 36.61 ± 1.06 ab | 61.60 ± 1.74 ab | 65.37 ± 3.54 bc |
| O3 | 26.89 ± 2.16 abc | 20.34 ± 1.58 ab | 75.64 ± 1.48 bc | 37.20 ± 0.74 ab | 63.29 ± 1.36 a | 68.94 ± 2.47 ab |
| M1 | 27.11 ± 1.15 abc | 20.20 ± 0.57 ab | 74.51 ± 1.39 bc | 36.95 ± 1.41 ab | 61.29 ± 3.03 ab | 69.21 ± 6.07 ab |
| M2 | 28.39 ± 2.05 a | 20.95 ± 1.61 a | 73.79 ± 2.82 c | 36.03 ± 0.95 ab | 60.97 ± 1.72 ab | 71.53 ± 2.59 a |
| M3 | 27.73 ± 1.15 ab | 20.32 ± 0.86 ab | 73.27 ± 1.54 c | 36.42 ± 1.21 ab | 61.06 ± 1.72 ab | 69.57 ± 2.81 ab |
| Treatment | Allicin (mg g−1) | Vc (mg g−1) | TSS (%) | TSP (mg g−1) |
|---|---|---|---|---|
| CK | 2.41 ± 0.08 e | 0.86 ± 0.01 cd | 17.21 ± 0.22 e | 7.30 ± 0.08 e |
| N1 | 2.70 ± 0.09 a | 0.93 ± 0.02 a | 19.35 ± 0.24 a | 7.72 ± 0.03 a |
| N2 | 2.61 ± 0.05 ab | 0.91 ± 0.03 ab | 19.00 ± 0.11 ab | 7.68 ± 0.07 b |
| N3 | 2.54 ± 0.01 bcd | 0.88 ± 0.01 ab | 18.95 ± 0.18 ab | 7.62 ± 0.04 b |
| E1 | 2.48 ± 0.10 cde | 0.87 ± 0.02 bcd | 18.65 ± 0.39 bc | 7.48 ± 0.05 c |
| E2 | 2.48 ± 0.06 cde | 0.85 ± 0.03 cd | 18.34 ± 0.19 c | 7.62 ± 0.02 b |
| E3 | 2.49 ± 0.04 cde | 0.85 ± 0.03 cd | 18.36 ± 0.31 c | 7.35 ± 0.04 de |
| O1 | 2.59 ± 0.03 abc | 0.83 ± 0.02 d | 19.46 ± 0.05 a | 7.73 ± 0.04 a |
| O2 | 2.62 ± 0.12 ab | 0.86 ± 0.03 bcd | 18.90 ± 0.06 ab | 7.62 ± 0.04 b |
| O3 | 2.53 ± 0.06 bcd | 0.83 ± 0.02 d | 18.87 ± 0.11 ab | 7.43 ± 0.01 cd |
| M1 | 2.54 ± 0.02 bcd | 0.85 ± 0.03 cd | 18.58 ± 0.34 bc | 7.41 ± 0.06 cd |
| M2 | 2.44 ± 0.01 de | 0.87 ± 0.04 bcd | 17.80 ± 0.48 d | 7.38 ± 0.06 d |
| M3 | 2.43 ± 0.10 de | 0.87 ± 0.02 bcd | 17.48 ± 0.35 de | 7.39 ± 0.04 d |
| Treatment | Selenium Content in Leaves (mg kg−1) | Selenium Content in Bulbs (mg kg−1) | Bulb-to-Leaf Ratio (%) |
|---|---|---|---|
| CK | 0.02 ± 0.02 e | 0.01 ± 0.01 e | 50.32 ± 3.16 c |
| N1 | 0.50 ± 0.10 a | 0.29 ± 0.05 a | 58.48 ± 5.14 ab |
| N2 | 0.29 ± 0.06 b | 0.16 ± 0.04 b | 55.25 ± 5.38 b |
| N3 | 0.29 ± 0.11 b | 0.12 ± 0.06 bc | 41.33 ± 3.94 c |
| E1 | 0.15 ± 0.06 cde | 0.09 ± 0.02 bcd | 60.78 ± 5.71 a |
| E2 | 0.11 ± 0.04 cde | 0.05 ± 0.02 de | 45.24 ± 4.28 c |
| E3 | 0.08 ± 0.05 cde | 0.04 ± 0.01 de | 50.33 ± 3.78 bc |
| O1 | 0.22 ± 0.07 bc | 0.10 ± 0.04 bcd | 45.44 ± 5.48 c |
| O2 | 0.21 ± 0.02 bcd | 0.09 ± 0.02 bcd | 43.22 ± 1.33 c |
| O3 | 0.18 ± 0.10 cd | 0.09 ± 0.03 bcd | 50.65 ± 6.64 bc |
| M1 | 0.13 ± 0.04 cde | 0.08 ± 0.03 cde | 62.89 ± 1.46 a |
| M2 | 0.08 ± 0.02 de | 0.05 ± 0.01 cde | 63.47 ± 4.69 a |
| M3 | 0.06 ± 0.01 de | 0.03 ± 0.01 de | 50.74 ± 2.30 bc |
| Treatment | Z (1) | Z (2) | Z (3) | Z (4) | μ (1) | μ (2) | μ (3) | μ (4) | D | Ranking |
|---|---|---|---|---|---|---|---|---|---|---|
| Ck | 11.578 | 0.385 | 0.187 | 0.733 | 0 | 1 | 1 | 0 | 0.429 | 13 |
| N1 | 48.188 | −34.172 | −4.552 | 11.291 | 1 | 0 | 0 | 1 | 0.571 | 1 |
| N2 | 31.671 | −18.704 | −2.333 | 6.486 | 0.549 | 0.448 | 0.468 | 0.545 | 0.507 | 2 |
| N3 | 29.386 | −15.605 | −1.989 | 5.453 | 0.486 | 0.537 | 0.541 | 0.447 | 0.506 | 3 |
| E1 | 22.375 | −8.897 | −1.01 | 3.754 | 0.295 | 0.731 | 0.747 | 0.286 | 0.483 | 5 |
| E2 | 18.186 | −4.939 | −0.538 | 2.41 | 0.181 | 0.846 | 0.847 | 0.159 | 0.464 | 10 |
| E3 | 16.633 | −3.138 | −0.332 | 1.933 | 0.138 | 0.898 | 0.89 | 0.114 | 0.461 | 11 |
| O1 | 25.761 | −11.931 | −1.427 | 4.413 | 0.387 | 0.644 | 0.659 | 0.349 | 0.496 | 4 |
| O2 | 24.298 | −11.169 | −1.534 | 4.09 | 0.347 | 0.666 | 0.637 | 0.318 | 0.479 | 7 |
| O3 | 23.397 | −9.871 | −1.323 | 3.906 | 0.323 | 0.703 | 0.681 | 0.301 | 0.482 | 6 |
| M1 | 21.106 | −7.446 | −0.948 | 3.362 | 0.26 | 0.773 | 0.761 | 0.249 | 0.478 | 8 |
| M2 | 17.265 | −3.676 | −0.441 | 2.319 | 0.155 | 0.882 | 0.868 | 0.15 | 0.465 | 9 |
| M3 | 15.204 | −1.702 | −0.205 | 1.641 | 0.099 | 0.94 | 0.917 | 0.086 | 0.456 | 12 |
| Weight | 0.4986 | 0.3259 | 0.1028 | 0.0726 |
| Types of Fertilizers | Treatment | Selenium Concentration (mg·L−1) |
|---|---|---|
| N1 | 50 | |
| Nano-Se | N2 | 25 |
| N3 | 12.5 | |
| E1 | 50 | |
| EDTA-Se | E2 | 25 |
| E3 | 12.5 | |
| O1 | 50 | |
| Organic-Se | O2 | 25 |
| O3 | 12.5 | |
| M1 | 50 | |
| Microbial-Se | M2 | 25 |
| M3 | 12.5 | |
| CK | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Ge, J.; Fan, J.; Zhao, Y.; Lu, X.; Liu, C.; Zhang, B.; Yang, Q.; Li, M.; Yang, Y.; et al. The Effect of Foliar Spraying of Different Selenium Fertilizers on the Growth, Yield, and Quality of Garlic (Allium sativum L.). Plants 2025, 14, 2505. https://doi.org/10.3390/plants14162505
Liu G, Ge J, Fan J, Zhao Y, Lu X, Liu C, Zhang B, Yang Q, Li M, Yang Y, et al. The Effect of Foliar Spraying of Different Selenium Fertilizers on the Growth, Yield, and Quality of Garlic (Allium sativum L.). Plants. 2025; 14(16):2505. https://doi.org/10.3390/plants14162505
Chicago/Turabian StyleLiu, Guangyang, Jie Ge, Jide Fan, Yongqiang Zhao, Xinjuan Lu, Canyu Liu, Biwei Zhang, Qingqing Yang, Mengqian Li, Yan Yang, and et al. 2025. "The Effect of Foliar Spraying of Different Selenium Fertilizers on the Growth, Yield, and Quality of Garlic (Allium sativum L.)" Plants 14, no. 16: 2505. https://doi.org/10.3390/plants14162505
APA StyleLiu, G., Ge, J., Fan, J., Zhao, Y., Lu, X., Liu, C., Zhang, B., Yang, Q., Li, M., Yang, Y., Feng, Y., & Yang, F. (2025). The Effect of Foliar Spraying of Different Selenium Fertilizers on the Growth, Yield, and Quality of Garlic (Allium sativum L.). Plants, 14(16), 2505. https://doi.org/10.3390/plants14162505






