An Efficient Agrobacterium-Mediated Transient Transformation System Using In Vitro Embryo-Derived Seedlings for Gene Function Elucidation in Paeonia ostii
Abstract
1. Introduction
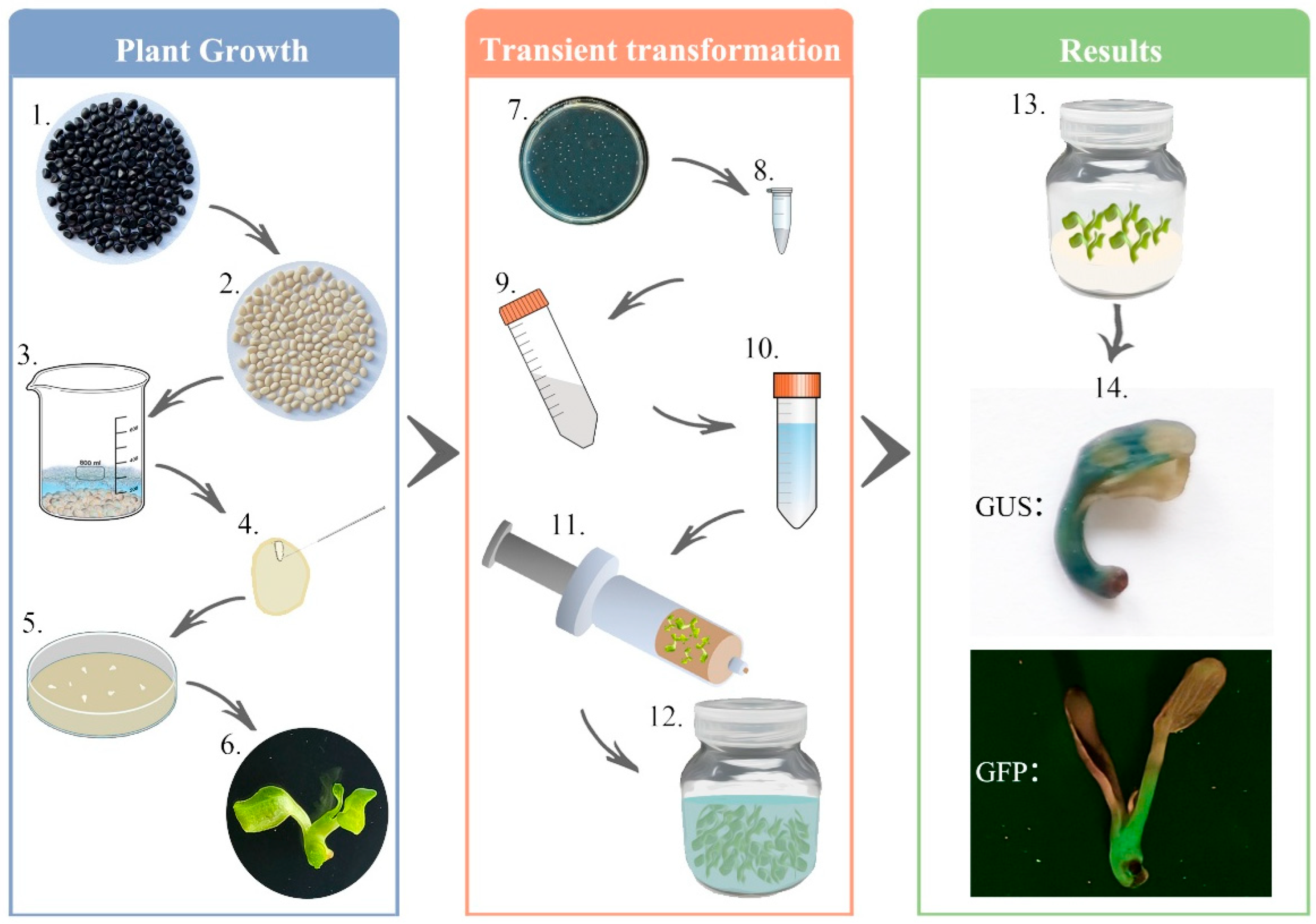
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. RNA Extraction and Gene Expression Profiling
2.3. Construction of Recombinant Plasmids
2.4. Agrobacterium Transformation, Cultivation, and Preparation of Infiltration Suspension
2.5. Infiltration of the P. ostii Seedlings
2.6. Orthogonal Experimental Design for Agrobacterium-Mediated Transient Genetic Transformation
2.7. GUS Histochemical Analysis and Enzyme Activity Assay
2.8. GFP Fluorescence Observation
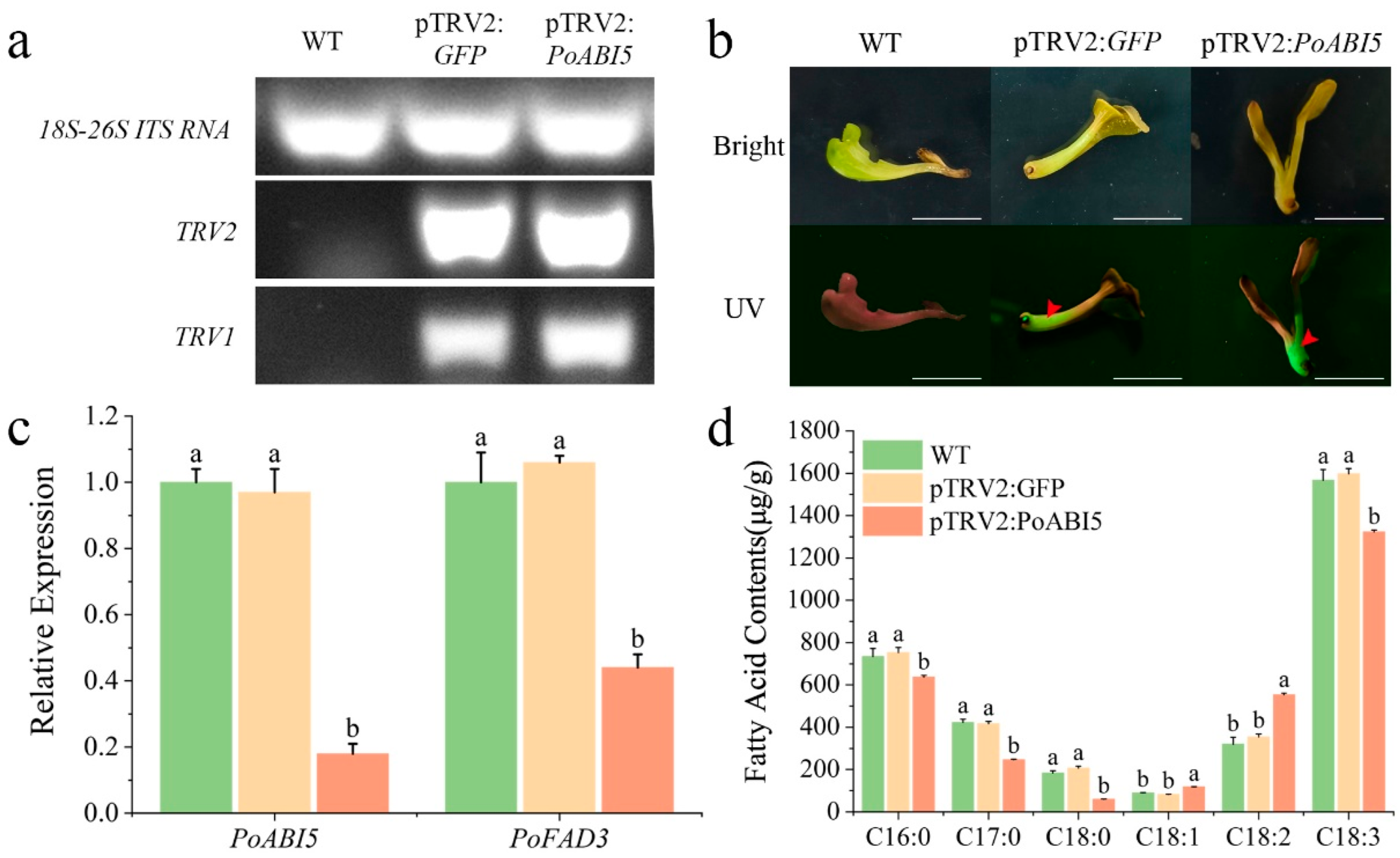
2.9. Virus-Induced Gene Silencing of PoABI5
2.10. FA Extraction and Analysis
3. Results
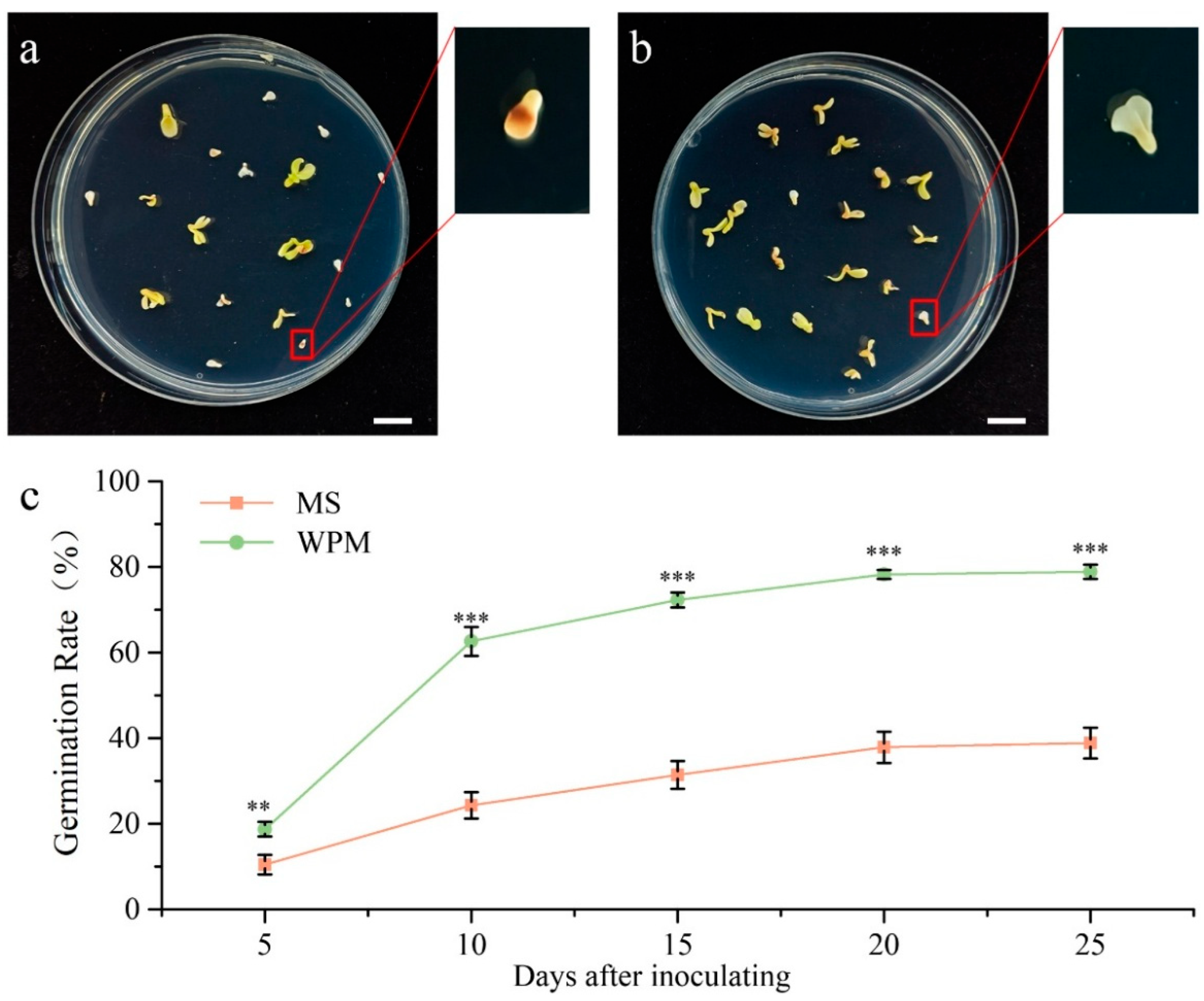
3.1. Optimal Germination Medium Screening for P. ostii Embryos
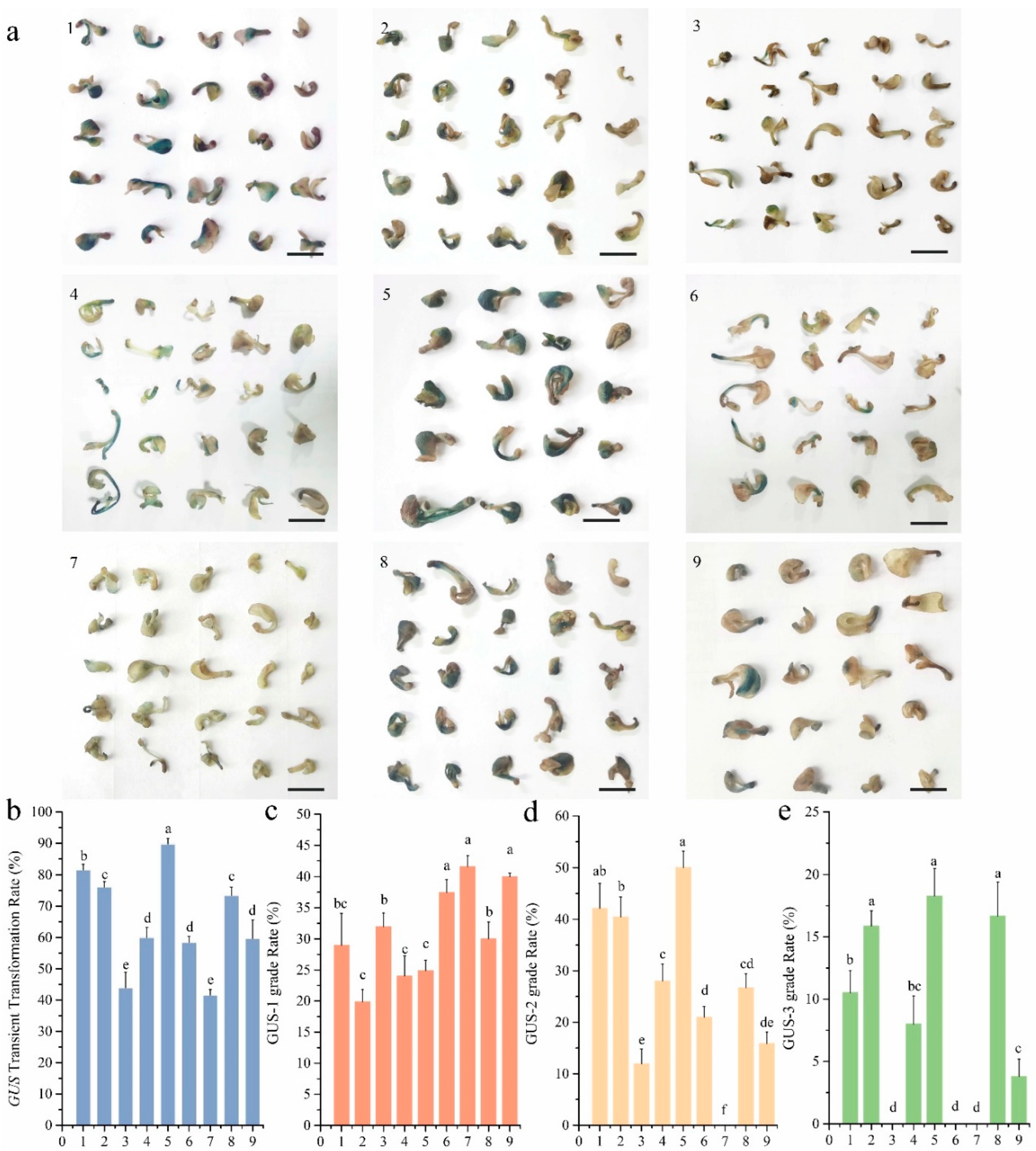
3.2. Orthogonal Optimization of Agrobacterium-Mediated Transient Transformation
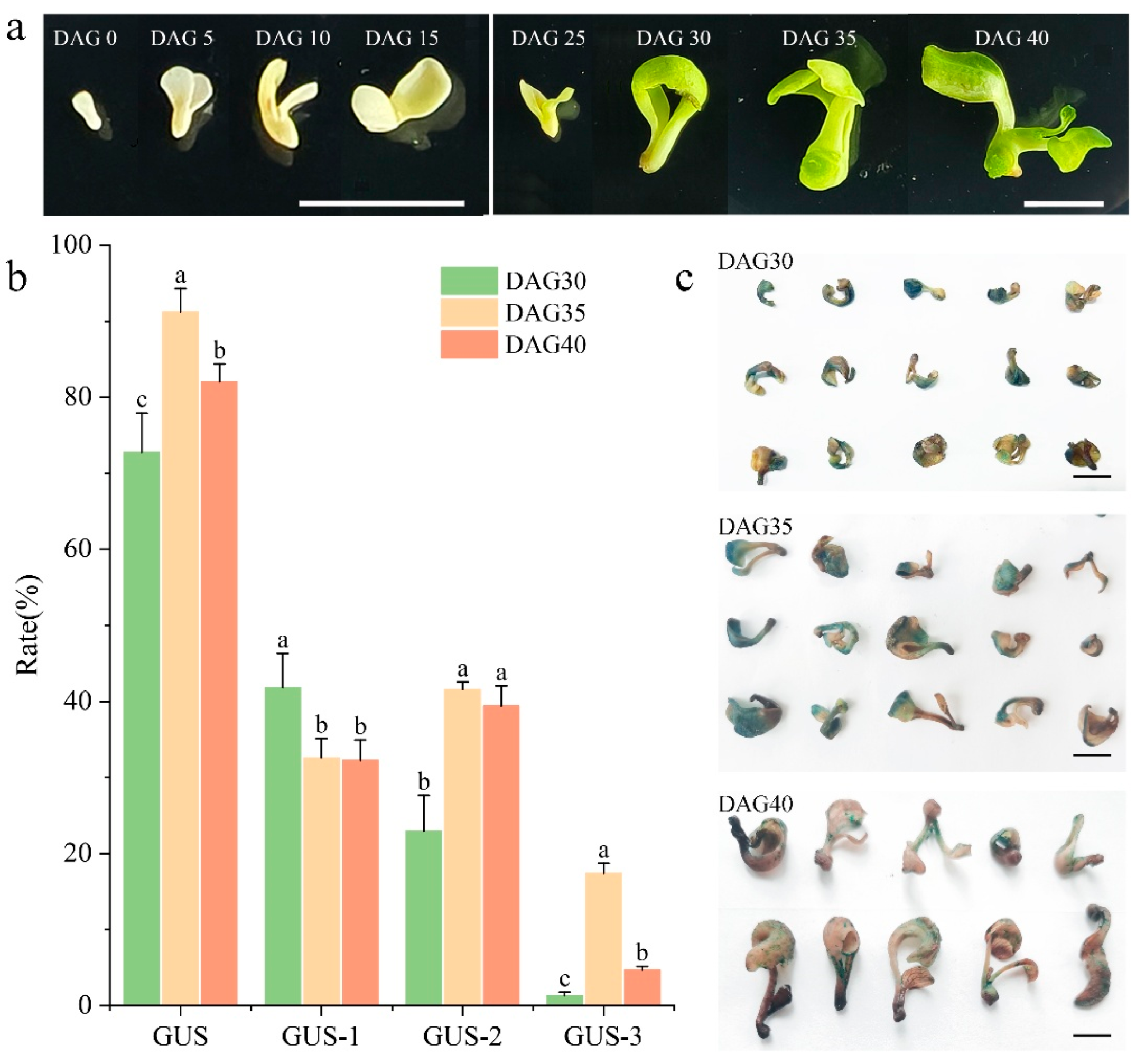
3.3. Impact of Growth Status of In Vitro Embryo-Derived Seedlings in P. ostii on Agrobacterium Infection Efficiency
3.4. Elucidation of TTAES for Assessing Transcription Factor-Mediated Target Gene Promoter Activation
3.5. Elucidation of TTAES as a GFP-Marked System for Gene Function Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Niu, L.; Zhang, M.; Zhang, H.; Liu, H.; Zhao, M.; Zhang, X.; Zhang, Q.; Zhang, Y. Application of Carbon-Based Nutrient Fertilizer Improved Soil Fertility and Seed Yield of Paeonia ostii ‘Feng Dan’. Ind. Crops Prod. 2024, 212, 118348. [Google Scholar] [CrossRef]
- Wang, X.; Liang, H.; Guo, D.; Guo, L.; Duan, X.; Jia, Q.; Hou, X. Integrated Analysis of Transcriptomic and Proteomic Data from Tree Peony (P. ostii) Seeds Reveals Key Developmental Stages and Candidate Genes Related to Oil Biosynthesis and Fatty Acid Metabolism. Hortic. Res. 2019, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Zuo, J.-Q.; Wang, Y.-T.; Duan, H.-Y.; Zhou, M.-H.; Li, H.-J.; Hu, Y.-H.; Yuan, J.-H. PoDPBT, a BAHD Acyltransferase, Catalyses the Benzoylation in Paeoniflorin Biosynthesis in Paeonia ostii. Plant Biotechnol. J. 2023, 21, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Shang, S.; Fan, L.; Zhou, S.; Gao, S.; Shaaban, M.; Fan, B.; Shi, G.; Wang, Z. The Role of IpSAG12 in Regulating Florescence and Senescence of Cut Itoh Peony Flowers. Postharvest Biol. Technol. 2025, 222, 113352. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wu, S.; Mei, X.; Song, W.; Shu, Q. Metabolic Pathway for Evaluation of Polyunsaturated Fatty Acids Content Variation and Regulation of Its Biosynthesis in Paeonia ostii through Abscisic Acid and ABSCISIC ACID-INSENSITIVE 5 (PoABI5). Ind. Crops Prod. 2025, 227, 120758. [Google Scholar] [CrossRef]
- Sun, J.; Guo, H.; Liu, M.; Chen, M.; Zhu, M.; Liu, D.; Tao, J. Histology and Transcriptomic Profiling Reveal the Dynamics of Seed Coat and Endosperm Formation in Tree Peony (Paeonia ostii). Hortic. Res. 2022, 9, uhac106. [Google Scholar] [CrossRef]
- Liu, R.; Ci, H.; Ren, X.; Gao, J.; Wang, S.; Zhang, X. Optimization of Callus Induction from Immature Embryo and Establishment Regeneration System of Paeonia ostii ‘Fengdan’. Acta Hortic. Sin. 2022, 49, 166. [Google Scholar] [CrossRef]
- Duan, S.; Xin, R.; Guan, S.; Li, X.; Fei, R.; Cheng, W.; Pan, Q.; Sun, X. Optimization of Callus Induction and Proliferation of Paeonia lactiflora Pall. and Agrobacterium-Mediated Genetic Transformation. Front. Plant Sci. 2022, 13, 996690. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, L.; Wang, X.; Gao, J.; Yi, J.; Deng, R. Characterization of Paeonia ostii Seed and Oil Sourced from Different Cultivation Areas in China. Ind. Crops Prod. 2019, 133, 63–71. [Google Scholar] [CrossRef]
- Luan, Y.; Chen, Z.; Fang, Z.; Meng, J.; Tao, J.; Zhao, D. PoWRKY69-PoVQ11 Module Positively Regulates Drought Tolerance by Accumulating Fructose in Paeonia ostii. Plant J. 2024, 119, 1782–1799. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Z.; Li, Y.; Wang, D.; Xiu, Y.; Wang, H. Characterization of Genes Encoding ω-6 Desaturase PoFAD2 and PoFAD6, and ω-3 Desaturase PoFAD3 for ALA Accumulation in Developing Seeds of Oil Crop Paeonia ostii Var. Lishizhenii. Plant Sci. 2021, 312, 111029. [Google Scholar] [CrossRef]
- Yuan, J.; Jiang, S.; Jian, J.; Liu, M.; Yue, Z.; Xu, J.; Li, J.; Xu, C.; Lin, L.; Jing, Y.; et al. Genomic Basis of the Giga-Chromosomes and Giga-Genome of Tree Peony Paeonia ostii. Nat. Commun. 2022, 13, 7328. [Google Scholar] [CrossRef]
- Gao, J.; Xue, J.; Xue, Y.; Liu, R.; Ren, X.; Wang, S.; Zhang, X. Transcriptome Sequencing and Identification of Key Callus Browning-Related Genes from Petiole Callus of Tree Peony (Paeonia suffruticosa Cv. Kao) Cultured on Media with Three Browning Inhibitors. Plant Physiol. Biochem. 2020, 149, 36–49. [Google Scholar] [CrossRef]
- Liu, R.; Xue, Y.; Ci, H.; Gao, J.; Wang, S.; Zhang, X. Establishment of Highly Efficient Plant Regeneration of Paeonia ostii ‘Fengdan’ through Optimization of Callus, Adventitious Shoot, and Rooting Induction. Hortic. Plant J. 2022, 8, 777–786. [Google Scholar] [CrossRef]
- Demei, N.; Fang, L.; Linqiang, G.; Huailong, Z.; Naibin, L.; Lu, Z.; Yanchao, Y.; Chunying, L.; Shupeng, G.; Yuxi, Z. MADS-Domain Transcription Factor AGAMOUS LIKE-9 Participates in the Gibberellin Pathway to Promote Bud Dormancy Release of Tree Peony. Hortic. Res. 2025, 12, uhaf043. [Google Scholar] [CrossRef] [PubMed]
- Xin, R.; Song, Y.; Zhang, X.; Kang, X.; Zhou, X.; Song, W.; Guan, S.; Sun, X. Optimization Strategy for Establishing an Efficient Mature Embryo Reproduction System of Paeonia lactiflora Pall. Sci. Hortic. 2025, 340, 113952. [Google Scholar] [CrossRef]
- Liu, S.; Ma, J.; Liu, H.; Guo, Y.; Li, W.; Niu, S. An Efficient System for Agrobacterium-Mediated Transient Transformation in Pinus tabuliformis. Plant Methods 2020, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yang, J.; Chen, Y.; Ding, L.; Wei, J.; Wang, H. An Improved and Efficient Method of Agrobacterium Syringe Infiltration for Transient Transformation and Its Application in the Elucidation of Gene Function in Poplar. BMC Plant Biol. 2021, 21, 54. [Google Scholar] [CrossRef]
- Liu, K.; Yang, Q.; Yang, T.; Wu, Y.; Wang, G.; Yang, F.; Wang, R.; Lin, X.; Li, G. Development of Agrobacterium-Mediated Transient Expression System in Caragana Intermedia and Characterization of CiDREB1C in Stress Response. BMC Plant Biol. 2019, 19, 237. [Google Scholar] [CrossRef] [PubMed]
- Xian, B.; Xi, Z.; Ren, C.; Yan, J.; Chen, J.; Pei, J. The Establishment of Transient Expression Systems and Their Application for Gene Function Analysis of Flavonoid Biosynthesis in Carthamus tinctorius L. BMC Plant Biol. 2023, 23, 186. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Gu, L.; Yang, Y.; He, D.; Luo, J.; Zhang, Y. The PlMYB73-PlMYB70-PlMYB108 Complex Regulates PlTPS1 to Promote Geraniol Biosynthesis in Paeonia lactiflora. Hortic. Res. 2025, 12, uhaf141. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Zhang, X.; Liu, Z.; Peng, L.; Hao, Q.; Liu, Z.; Men, S.; Tong, N.; Shu, Q. ABSCISIC ACID-INSENSITIVE 5-ω3 FATTY ACID DESATURASE3 Module Regulates Unsaturated Fatty Acids Biosynthesis in Paeonia ostii. Plant Sci. 2022, 317, 111189. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, Q.; Sun, D.; Yang, W.; Hu, J.; Niu, L.; Zhang, Y. Virus-Induced Gene Silencing in the Perennial Woody Paeonia ostii. PeerJ 2019, 7, e7001. [Google Scholar] [CrossRef]
- Yuan, Y.; Zeng, L.; Kong, D.; Mao, Y.; Xu, Y.; Wang, M.; Zhao, Y.; Jiang, C.-Z.; Zhang, Y.; Sun, D. Abscisic Acid–Induced Transcription Factor PsMYB306 Negatively Regulates Tree Peony Bud Dormancy Release. Plant Physiol. 2024, 194, 2449–2471. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xin, Z.; Hu, J.; Zhang, Y.; Zhang, Q.; Niu, L. The Tree Peony Nuclear Factor Y Transcription Factor PrNF-YC2 Promotes Seed Oil Accumulation. Plant J. 2023, 115, 546–562. [Google Scholar] [CrossRef] [PubMed]
- Ren, L. A Primary Study on the Selection in Field Forbreeding New Oil-Used Paeonia ostii “Fendan” Varieties. Master’s Thesis, Northwest A&F University, Yangling, China, 2016. [Google Scholar]
- Ren, H. Study on the Germination of Oil Tree Peony Seeds and Container Nursery. Master’s Thesis, Northwest A&F University, Yangling, China, 2016. [Google Scholar]
- Cheng, F.; Du, X. Effects of Chilling and Gibberellic Acid on the Seed Germination and Seedling Growth in Paeonia ostii ‘Feng Dan’. Acta Hortic. Sin. 2008, 35, 553–558. [Google Scholar]
- Li, X.U.; Fang-Yun, C.; Yuan, Z. Study on Rapid Seedling-Raising Technology of Tree Peony Embryo Culture. Bull. Bot. Res. 2017, 37, 690. [Google Scholar] [CrossRef]
- Guan, S.; Kang, X.; Ge, J.; Fei, R.; Duan, S.; Sun, X. An Efficient Agrobacterium-Mediated Transient Transformation System and Its Application in Gene Function Elucidation in Paeonia lactiflora Pall. Front. Plant Sci. 2022, 13, 999433. [Google Scholar] [CrossRef]
- Pinski, A.; Betekhtin, A. Efficient Agrobacterium-Mediated Transformation and Genome Editing of Fagopyrum tataricum. Front. Plant Sci. 2023, 14, 1270150. [Google Scholar] [CrossRef]
- Shen, M.; Wang, Q.; Yu, X.; Teixeira da Silva, J.A. Micropropagation of Herbaceous Peony (Paeonia lactiflora Pall.). Sci. Hortic. 2012, 148, 30–38. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Shen, M.; Yu, X. Tissue Culture and Micropropagation of Tree Peony (Paeonia suffruticosa Andr.). J. Crop Sci. Biotechnol. 2012, 15, 159–168. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, M.; Wang, X.; Zeng, Y.; Liao, Y.; Wang, Y.; Zeng, Z. Discrimination of Moutan Cortex from Different Sources and Geographical Origins for Quality Evaluation Using Microsatellite Markers Coupled with Chemical Analysis. Biochem. Syst. Ecol. 2020, 89, 104005. [Google Scholar] [CrossRef]
- Zhai, C.; Meng, X.; Fu, J.; Qin, Z.; Meng, Y. Research Progress of Peony Bark in Modern Pharmacy. Inf. Tradit. Chin. Med. 2020, 37, 109–114. [Google Scholar]
- Chen, M.; Yang, L.; Yang, Z.; Cai, Y.; Yin, L.; Zhang, Y. Callus Induction, Proliferation and Histological Observation of Paeoniaostii Cv. ‘Phoenix White’ Mature Embryo. Non-Wood For. Res. 2021, 39, 9–16. [Google Scholar]
- Liu, Z.; Zhou, H.; Cai, X.-f.; Li, J.; Hu, Y.-h. Paeonia ostii ‘Feng Dan’ Embryo Culture and Adventitious Buds Induction. In Advances in Chinese Ornamental Horticulture; China Forestry Press: Beijing, China, 2011; pp. 371–374. [Google Scholar]
- Ioannidis, K.; Koropouli, P. Effects of Different Media and Their Strengths in in Vitro Culture of Three Different Cistus creticus L. Populations and Their Genetic Assessment Using Simple Sequence Repeat Molecular Markers. Horticulturae 2024, 10, 104. [Google Scholar] [CrossRef]
- Berumen-Varela, G.; Ochoa-Jiménez, V.-A.; Burgara-Estrella, A.; Trillo-Hernández, E.-A.; Ojeda-Contreras, Á.-J.; Orozco-Avitia, A.; Rivera-Domínguez, M.; Troncoso-Rojas, R.; Báez-Sañudo, R.; Datsenka, T.; et al. Functional Analysis of a Tomato (Solanum lycopersicum L.) Rhamnogalacturonan Lyase Promoter. J. Plant Physiol. 2018, 229, 175–184. [Google Scholar] [CrossRef]
- Ma, S.; Du, C.; Ohlrogge, J.; Zhang, M. Accelerating Gene Function Discovery by Rapid Phenotyping of Fatty Acid Composition and Oil Content of Single Transgenic T 1 Arabidopsis and Camelina Seeds. Plant Direct. 2020, 4, e00253. [Google Scholar] [CrossRef]
- Dedow, L.K.; Oren, E.; Braybrook, S.A. Fake News Blues: A GUS Staining Protocol to Reduce False-Negative Data. Plant Direct. 2022, 6, e367. [Google Scholar] [CrossRef]
- Xue, J.; Wang, S.; Zhang, P.; Zhu, F.; Ren, X.; Liu, C.; Zhang, X. On the Role of Physiological Substances, Abscisic Acid and Its Biosynthetic Genes in Seed Maturation and Dormancy of Tree Peony (Paeonia ostii ‘Feng Dan’). Sci. Hortic. 2015, 182, 92–101. [Google Scholar] [CrossRef]
- Li, X. Study on in Vitro Regeneration via Meristematic Nodule Culture and Embryoculture in Tree Peony. Doctoral Dissertation, Beijing Forestry University, Beijing, China, 2022. [Google Scholar]
- An, B. Studies on the Establishment of in Vitro Regeneration System of Peonies Suffruticosa ANDR. Master’s Thesis, Northeast Forestry University, Harbin, China, 2005. [Google Scholar]
- Singh, P.; Khan, S.; Kumar, S.; ur Rahman, L. Establishment of an Efficient Agrobacterium-Mediated Genetic Transformation System in Pelargonium Graveolens: An Important Aromatic Plant. Plant Cell Tissue Organ Cult. 2017, 129, 35–44. [Google Scholar] [CrossRef]
- Yao, W.; Kong, L.; Lei, D.; Zhao, B.; Tang, H.; Zhou, X.; Lin, Y.; Zhang, Y.; Wang, Y.; He, W.; et al. An Effective Method for Establishing a Regeneration and Genetic Transformation System for Actinidia Arguta. Front. Plant Sci. 2023, 14, 1204267. [Google Scholar] [CrossRef]
- Gu, X.F.; Meng, H.; Qi, G.; Zhang, J.R. Agrobacterium-Mediated Transformation of the Winter Jujube (Zizyphus Jujuba Mill.). Plant Cell Tissue Organ Cult. 2008, 94, 23–32. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Wu, S.-H.; Chen, J.; Yi, X.-G.; Wang, X.-R.; Li, M. Efficient Plant Regeneration and Transient Genetic Transformation System of Prunus Xueluoensis via an Agrobacterium-Mediated Method. Int. J. Mol. Sci. 2025, 26, 3588. [Google Scholar] [CrossRef] [PubMed]
- Faizal, A.; Geelen, D. Agroinfiltration of Intact Leaves as a Method for the Transient and Stable Transformation of Saponin Producing Maesa Lanceolata. Plant Cell Rep. 2012, 31, 1517–1526. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Guo, J.; Qiao, Q.; Guo, X.; Ma, Y. The WRKY Transcription Factor PlWRKY65 Enhances the Resistance of Paeonia Lactiflora (Herbaceous peony) to Alternaria Tenuissima. Hort. Res. 2020, 7, 57. [Google Scholar] [CrossRef]
- Baros, C.J.; Beerkens, J.; Ludwig, M. Agrobacterium-Mediated Transient Transformation of Flaveria bidentis Leaves: A Novel Method to Examine the Evolution of C4 Photosynthesis. Plant Methods 2024, 20, 193. [Google Scholar] [CrossRef]
- Gao, L.; Niu, D.; Chi, T.; Yuan, Y.; Liu, C.; Gai, S.; Zhang, Y. PsRGL1 Negatively Regulates Chilling- and Gibberellin-Induced Dormancy Release by PsF-Box1-Mediated Targeting for Proteolytic Degradation in Tree Peony. Hortic. Res. 2023, 10, uhad044. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; He, C.; Li, Z.; Li, M.; He, S.; Qian, J.; Tan, B.; Zheng, X.; Cheng, J.; Wang, W.; et al. A Rapid and Efficient Agrobacterium-Mediated Transient Transformation System in Grape Berries. Protoplasma 2024, 261, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Acanda, Y.; Welker, S.; Orbović, V.; Levy, A. A Simple and Efficient Agroinfiltration Method for Transient Gene Expression in Citrus. Plant Cell Rep. 2021, 40, 1171–1179. [Google Scholar] [CrossRef]
- Pi, M.; Gao, Q.; Kang, C. Transient Expression Assay in Strawberry Fruits. Bio Protoc. 2019, 9, e3249. [Google Scholar] [CrossRef]
- Qi, Q.; Li, Y.; Xing, G.; Guo, J.; Guo, X. Fertility Variation among Paeonia lactiflora Genotypes and Fatty Acid Composition of Seed Oil. Ind. Crops Prod. 2020, 152, 112540. [Google Scholar] [CrossRef]
- Mao, Y.; Ji, X.; Meng, Q.; Xu, Z.; Yuan, Y.; Li, M.; Niu, L.; Zhang, Y.; Sun, D. Contribution of Anthocyanin and Polyunsaturated Fatty Acid Biosynthesis to Cold Tolerance during Bud Sprouting in Tree Peony. Ind. Crops Prod. 2022, 188, 115563. [Google Scholar] [CrossRef]
- Yeap, W.-C.; Lee, F.-C.; Shabari Shan, D.K.; Musa, H.; Appleton, D.R.; Kulaveerasingam, H. WRI1-1, ABI5, NF-YA3 and NF-YC2 Increase Oil Biosynthesis in Coordination with Hormonal Signaling during Fruit Development in Oil Palm. Plant J. 2017, 91, 97–113. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Primer Sequence (5′-3′) | Annotation |
|---|---|---|
| ProPoFAD3-GUS-F | ACCATGATTACGCCAAGCTTTGTTCGTCTCGTCGTCCTCTTC | Vector Construction |
| ProPoFAD3-GUS-R | GACTGACCACCCGGGGATCCTTGGCCTTCGGTTCACAGAT | |
| 35S:PoABI5-F | ACGGGGGACGAGCTCGGTACCATGGTTGTTCCTGAGTCCGAAA | |
| 35S:PoABI5-R | GCTCTGCAGGTCGACTCTAGATCAAAAAGGGGAACTCAAAGTCC | |
| PoABI5-pTRV2-F | GAAGGCCTCCATGGGGATCCTAAACCAGCGCGGTTATCCA | |
| PoABI5-pTRV2-R | GGACATGCCCGGGCCTCGAGGGATTGTCTTCCCAATGATGCA | |
| PoABI5-GFP-F | ACGGGGGACGAGCTCGGTACCATGGTTGTTCCTGAGTCCGAAA | |
| PoABI5-GFP-R | CTTGCTCACCATGGTGTCGACAAAAGGGGAACTCAAAGTCCTCC | |
| PoABI5-RT-F | TGGAAGCAGAGCTGAACCAG | RT-PCR /qRT-PCR |
| PoABI5-RT-R | CGAACACCTCAAAAAGGGGA | |
| PoFAD3-RT-F | GGCAGCCACATTTCCGTCTT | |
| PoFAD3-RT-R | AGTAGAGTGGCCAAACAGCC | |
| TRV1-RT-F | CAGTCTATACACAGAAACAGA | |
| TRV1-RT-R | GACGTGTGTACTCAAGGGTT | |
| TRV2-RT-F | GGCTAACAGTGCTCTTGGTG | |
| TRV2-RT-R | GTATCGGACCTCCACTCGC | |
| 18S-26S ITS RNA-F | ACCGTTGATTCGCACAATTGGTCATCG | |
| 18S-26S ITS RNA-R | TACTGCGGGTCGGCAATCGGACG |
| Test Number | OD600 | AS Concentration (μmol·L−1) | Number of Negative Pressure | Infection Time (h) |
|---|---|---|---|---|
| 1 | 0.8 | 150 | 2 | 2 |
| 2 | 0.8 | 200 | 4 | 4 |
| 3 | 0.8 | 250 | 6 | 8 |
| 4 | 1.0 | 150 | 4 | 8 |
| 5 | 1.0 | 200 | 6 | 2 |
| 6 | 1.0 | 250 | 2 | 4 |
| 7 | 1.2 | 150 | 6 | 4 |
| 8 | 1.2 | 200 | 2 | 8 |
| 9 | 1.2 | 250 | 4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; Xie, X.; Zhang, L.; Wang, X.; Zhang, Z.; Niu, L.; Zhang, Y. An Efficient Agrobacterium-Mediated Transient Transformation System Using In Vitro Embryo-Derived Seedlings for Gene Function Elucidation in Paeonia ostii. Plants 2025, 14, 2498. https://doi.org/10.3390/plants14162498
Zhai Y, Xie X, Zhang L, Wang X, Zhang Z, Niu L, Zhang Y. An Efficient Agrobacterium-Mediated Transient Transformation System Using In Vitro Embryo-Derived Seedlings for Gene Function Elucidation in Paeonia ostii. Plants. 2025; 14(16):2498. https://doi.org/10.3390/plants14162498
Chicago/Turabian StyleZhai, Yuhui, Xinrong Xie, Liping Zhang, Xuefei Wang, Zixuan Zhang, Lixin Niu, and Yanlong Zhang. 2025. "An Efficient Agrobacterium-Mediated Transient Transformation System Using In Vitro Embryo-Derived Seedlings for Gene Function Elucidation in Paeonia ostii" Plants 14, no. 16: 2498. https://doi.org/10.3390/plants14162498
APA StyleZhai, Y., Xie, X., Zhang, L., Wang, X., Zhang, Z., Niu, L., & Zhang, Y. (2025). An Efficient Agrobacterium-Mediated Transient Transformation System Using In Vitro Embryo-Derived Seedlings for Gene Function Elucidation in Paeonia ostii. Plants, 14(16), 2498. https://doi.org/10.3390/plants14162498







