Systematic Review of the State of Knowledge About Açaí-Do-Amazonas (Euterpe precatoria Mart., Arecaceae)
Abstract
1. Introduction
2. Results
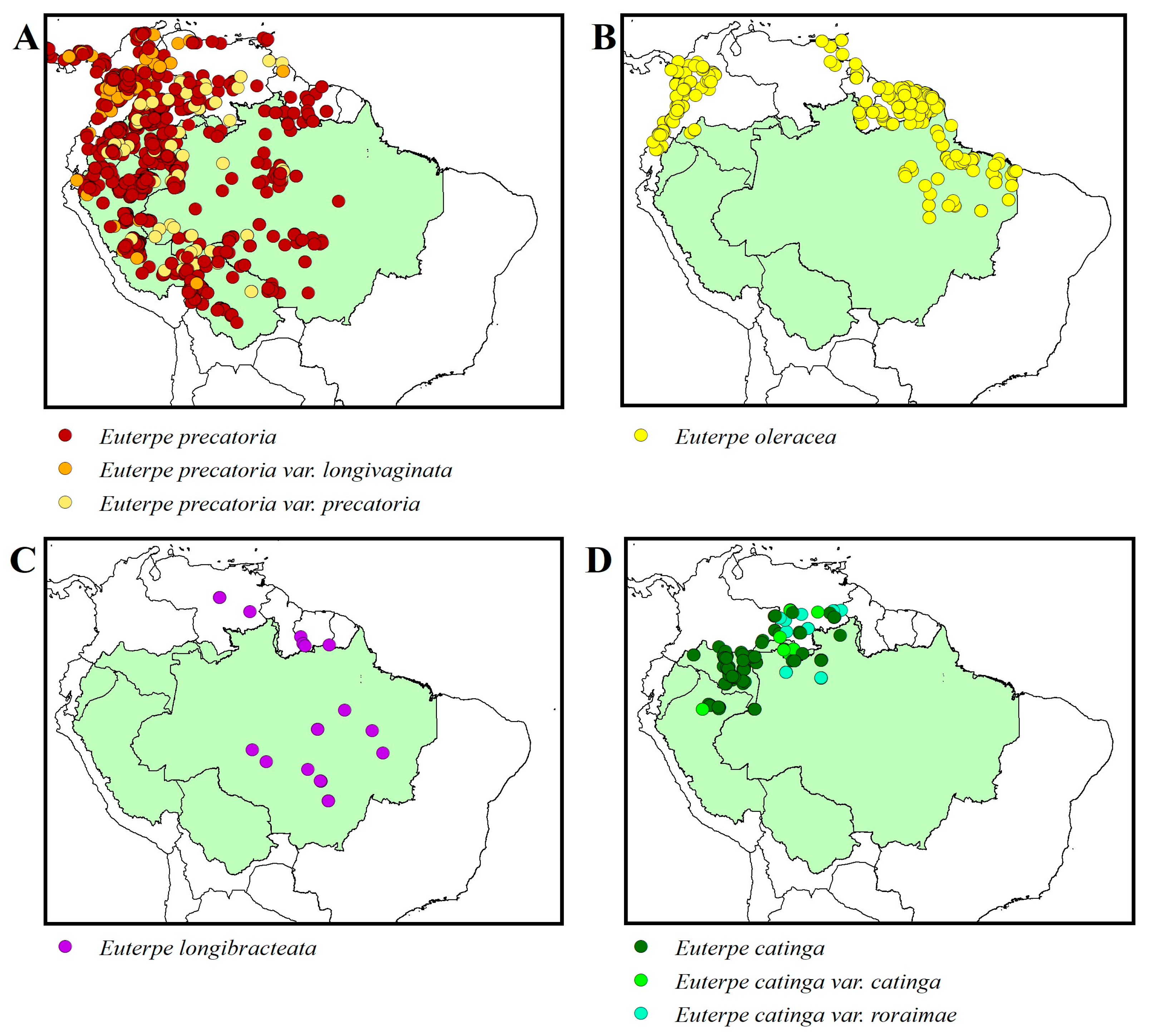
2.1. Botanical Classification
2.2. Botanical Descriptions
2.3. Phenology
| Location or River 3 | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Manaus, AM 1 | * | * | * | x | x | x | x | x | x | |||
| Manaus, AM 2 | x | x | *x | * | * | * | * | |||||
| Amazon River 4 | x | x | x | x | x | x | x | |||||
| Negro–Solimões Rivers 4 | x | x | x | x | x | x | x | x | x | x | ||
| Madeira River 4 | x | x | x | x | x | x | x | x | ||||
| Upper Negro River 4 | x | x | x | x | x | x | ||||||
| Jutaí–Juruá–Solimões Rivers 4 | x | x | x | x | x | x | ||||||
| Purus River 4 | x | x | x | x | x | x | x | |||||
| Upper Solimões River 4 | x | x | x | x | x | x |
2.4. Reproductive Biology
2.5. Interspecific Hybridization
2.6. Ecological Dynamics of Euterpe precatoria in Amazonian Ecosystems
| Environment | Abundance (Individuals/ha) | Number of Bunches (Plant/Year) | Fruit Weight (kg/Bunch) | Productivity (Tons of Fruits/ha/Year) |
|---|---|---|---|---|
| Terra firme 1−10 | 282 ± 229 | - | - | - |
| (69–517) | - | - | - | |
| Baixio 11−20 | 106 ± 33 | 2.25 ± 0.42 | 5.5 ± 2.3 | 3 |
| (60–129) | (2–3) | (3–7.5) | ||
| Várzea 21−33 | 202 ± 33 | 2.60 ± 0.52 | 8.0 ± 2.4 | 2.0 ± 0.3 |
| (170–248) | (2–3) | (5.9–11.9) | (1.8–2.2) |
2.7. Productivity
2.8. Extractive Production
2.9. Fruit Composition
2.10. Uses of Euterpe Precatoria
2.11. Management of Native Populations
- (i)
- (ii)
- (iii)
- (iv)
- (v)
- (vi)
- Select the most productive palms to use in enrichment and to have control over production estimates [41].
2.12. Cultivation
2.13. Production of Seedlings
2.14. Genetic Resources
3. Materials and Methods
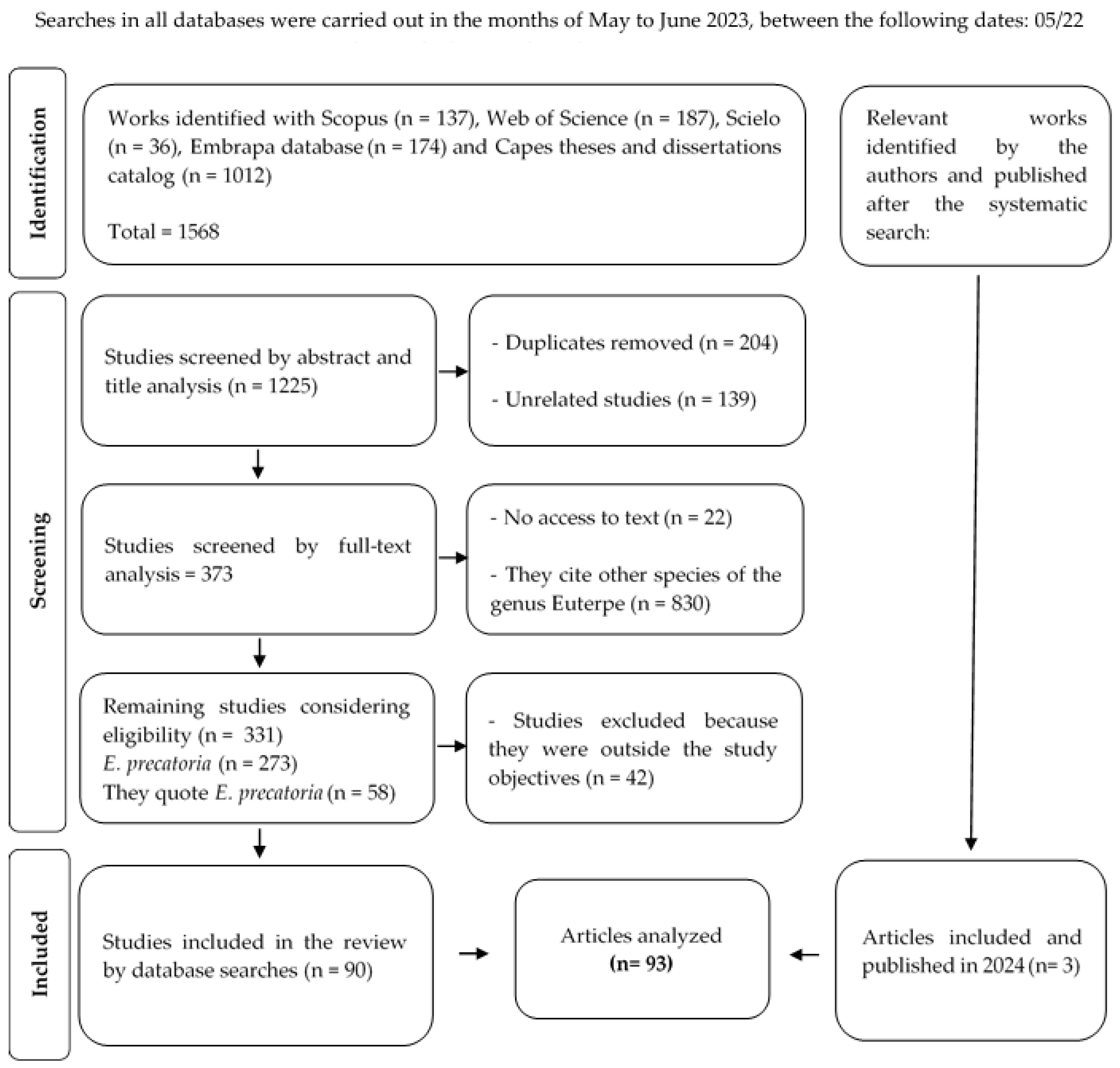
3.1. Literature Survey
3.2. Quantitative Data Extraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Henderson, A.; Galeano, G. Euterpe, Prestoea, and Neonicholsonia (Palmae). Flora Neotrop. 1996, 72, 1–99. [Google Scholar]
- Yamaguchi, K.K.L.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A Review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, L.K.O.; Aguiar, J.P.L.; Silva Filho, D.F.; Yuyama, K.; Jesus Varejão, M.D.; Fávaro, D.I.T.; Vasconcellos, M.B.A.; Pimentel, S.A.; Caruso, M.S.F. Caracterização físico-química do suco de açaí de Euterpe Precatoria Mart. oriundo de diferentes ecossistemas amazônicos. Acta Amaz. 2011, 41, 545–552. [Google Scholar] [CrossRef]
- Clement, C.R. 1492 and the Loss of Amazonian crop genetic resources. I. The Relation between domestication and human population decline. Econ. Bot. 1999, 53, 188–202. [Google Scholar] [CrossRef]
- Sosnowska, J.; Balslev, H. American palm ethnomedicine: A Meta-Analysis. J. Ethnobiol. Ethnomed. 2009, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.P.; Mochiutti, S.; de Farias Neto, J.T. Domesticação e melhoramento do açaizeiro. In Domesticação e melhoramento: Espécies amazônicas; Borém, A., Lopes, M.T.G., Clement, C.R., Eds.; Universidade Federal de Viçosa: Viçosa, MG, Brazil, 2009; pp. 206–235. [Google Scholar]
- Oliveira, M.S.P.; Mattietto, R.A.; Domingues, A.F.N.; Carvalho, A.V.; Oliveira, N.P.; Farias Neto, J.T. Euterpe oleracea e E. precatoria: Açaí. In Espécies Nativas da Flora Brasileira de Valor Econômico Atual ou Potencial: Plantas Para o Futuro: Região Norte; Coradin, L., Camillo, J., Vieira, I.C.G., Eds.; Série Biodiversidade; MMA: Brasília, DF, Brazil, 2022; pp. 303–323. [Google Scholar]
- Conab Mercado e Comercialização—Cenário Comparativo Da Região Norte e Nordeste: Brasília: Companhia Nacional de Abastecimento. 2019. Available online: https://www.conab.gov.br/info-agro/analises-do-mercado-agropecuario-e-extrativista/analises-do-mercado/historico-mensal-de-acai/item/download/27774_d8d651f3ab50fd8641c381639fd43c63. (accessed on 25 June 2024).
- IBGE Produção da Extração Vegetal e da Silvicultura|IBGE. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9105-producao-da-extracao-vegetal-e-da-silvicultura.html (accessed on 12 September 2024).
- Damasco, G.; Baraloto, C.; Vicentini, A.; Daly, D.C.; Baldwin, B.G.; Fine, P.V.A. Revisiting the hyperdominance of neotropical tree species under a taxonomic, functional and evolutionary perspective. Sci. Rep. 2021, 11, 9585. [Google Scholar] [CrossRef]
- Pichardo-Marcano, F.J.; Nieto-Blázquez, M.E.; MacDonald, A.N.; Galeano, G.; Roncal, J. Phylogeny, historical biogeography and diversification rates in an economically important group of neotropical palms: Tribe Euterpeae. Mol. Phylogenet. Evol. 2019, 133, 67–81. [Google Scholar] [CrossRef]
- Ter Steege, H.; Pitman, N.C.A.; Sabatier, D.; Baraloto, C.; Salomão, R.P.; Guevara, J.E.; Phillips, O.L.; Castilho, C.V.; Magnusson, W.E.; Molino, J.-F.; et al. Hyperdominance in the Amazonian tree flora. Science 2013, 342, 1243092. [Google Scholar] [CrossRef]
- Marinho, T.A.S.; Lopes, A.; Assis, R.L.; Ramos, S.L.F.; Gomes, L.R.P.; Wittmann, F.; Schöngart, J. Distribuição e crescimento de Garcinia brasiliensis Mart. e Hevea spruceana (Benth.) Müll. Arg. em uma floresta inundável em Manaus, Amazonas. Ciênc. Florest. 2013, 23, 223–232. [Google Scholar] [CrossRef]
- Rocha, E. Potencial ecológico para o manejo de frutos de açaizeiro (Euterpe precatoria Mart.) em áreas extrativistas no Acre, Brasil. Acta Amaz. 2004, 34, 237–250. [Google Scholar] [CrossRef]
- Rocha, E.; Viana, V.M. Manejo de Euterpe precatoria Mart. (Açaí) no Seringal Caquetá, Acre, Brasil. Sci. For. 2004, 65, 59–69. [Google Scholar]
- Rogez, H. Açaí: Preparo, Composição e Melhoramento da Consevação; Universidade Federal Do Pará. Ufpa: Belém, PA, Brazil, 2000; ISBN 978-85-247-0202-0. [Google Scholar]
- Aguiar, J. Tabela de composição de alimentos da Amazônia. Acta Amaz. 2018, 26, 121–126. [Google Scholar] [CrossRef]
- Abramovay, R.; Ferreira, J.; Assis Costa, F.; Ehrlich, M.; Castro Euler, A.M.; Young, C.E.F.; Kaimowitz, D.; Moutinho, P.; Nobre, I.; Rogez, H.; et al. Chapter 30: Opportunities and challenges for a healthy standing forest and flowing rivers bioeconomy in the Amazon. In Amazon Assessment Report 2021; Nobre, C., Encalada, A., Anderson, E., Roca Alcazar, F.H., Bustamante, M., Mena, C., Peña-Claros, M., Poveda, G., Rodriguez, J.P., Saleska, S., et al., Eds.; UN Sustainable Development Solutions Network (SDSN): New York, NY, USA, 2021; ISBN 978-1-7348080-0-1. [Google Scholar]
- Nobre, C.A.; Feltran-Barbieri, R.; De Assis Costa, F.; Haddad, E.A.; Schaeffer, R.; Domingues, E.P.; Rocha Frasson, C.M.; Camuri, P.; Genin, C.; Szklo, A.; et al. Nova Economia da Amazônia; World Resources Institute: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Nogueira, O.L.; Figueiredo, F.J.C.; Muller, A.A. Açaí; (Embrapa Amazônia Oriental. Sistemas de Produção, 4); Embrapa Amazônia Oriental: Belém, Brazil, 2005. [Google Scholar]
- Clement, C.R.; Santos Pereira, H.; Vieira, I.C.G.; Homma, A.K.O. Challenges for a Brazilian Amazonian bioeconomy based on forest foods. Trees For. People 2024, 16, 100583. [Google Scholar] [CrossRef]
- Costa Ayres, M.I.; Almeida Souza, É.I.; Uguen, K.; Sena Alfaia, S.; Santos Pereira, H. Caracterização dos agroecossistemas de açaí-do-amazonas em Codajás, Amazonas-Brasil. Rev. Bras. De Agroecol. 2024, 19, 167–190. [Google Scholar] [CrossRef]
- Dransfield, J.; Uhl, N.; Lange, C.; Baker, W.; Harley, M.; Lewis, C. Genera Palmarum. The Evolution and Classification of Palms; Royal Botanical Gardens; Kew Publishing: Kew, Australia, 2008. [Google Scholar]
- Flora e fungo do Brasil Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 10 May 2023).
- Silva, A.J.B.; Sevalho, E.S.; Miranda, I.P.A. Potencial das palmeiras nativas da Amazônia Brasileira para a bioeconomia: Análise em rede da produção científica e tecnológica. Ciênc. Florest. 2021, 31, 1020–1046. [Google Scholar] [CrossRef]
- Henderson, A. The genus Euterpe in Brazil. Sellowia 2000, 49–52, 1–22. [Google Scholar]
- Ramos, S.L.F.; Dequigiovanni, G.; Lopes, M.T.G.; Aguiar, A.V.; Lopes, R.; Veasey, E.A.; Macêdo, J.L.V.; Alves-Pereira, A.; Fraxe, T.D.J.P.; Wrege, M.S.; et al. Genetic structure in populations of Euterpe precatoria Mart. in the Brazilian Amazon. Front. Ecol. Evol. 2021, 8, 603448. [Google Scholar] [CrossRef]
- Barreiro, J.M.S. Estructura genética de Euterpe precatoria (Mart.) en los Andes Tropicales. Master’s Thesis, Pontifícia Universidad Católica del Ecuador, Quito, Ecuador, 2013. [Google Scholar]
- Isaza, C.; Galeano, G.; Bernal, R. Manejo actual de Mauritia flexuosa 13. para la producción de frutos en el sur de la Amazonia colombiana. In Morichales y Cananguchales de la Orinoquia y Amazonia: Colombia-Venezuela; Lasso, C.A., Rial, A., González, R.B., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2014; pp. 247–276. [Google Scholar]
- Aguiar, M.O.; Mendonça, M.S. Morfo-anatomia da semente de Euterpe precatoria Mart. (Palmae). Rev. Bras. De Sementes 2003, 25, 37–42. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Oliveira, M.S.P.; Davide, L.C.; Augusta Torres, G. Karyotype and genome size in Euterpe Mart. (Arecaceae) species. Comp. Cytogenet. 2016, 10, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.; Cunha, R.N.V.; Tavares, M.S.; Raizer, M.D.M.; Santos, C.A.; Silva, E.J.D.; Lopes, M.T.G. Seasonality of fruit production of Euterpe oleracea and E. precatoria açaí palm trees cultivated in the metropolitan region of Manaus (AM). Rev. Agro@mbiente On-line 2022, 16, 1–14. [Google Scholar] [CrossRef]
- Henderson, A. The Palms of the Amazon; Oxford University Press: Oxford, UK, 1995; ISBN 978-0-19-508311-8. [Google Scholar]
- Oliveira, M.S.P. Biologia floral do açaizeiro em Belém, PA. Embrapa Amaz. Oriental. Bol. de Pesqui. e Desenvolv. 2002, 8, 26. [Google Scholar]
- Câmara, G.M.S. Fenologia é ferramenta auxiliar de técnicas de produção. Visão Agrícola 2006, 3, 63–66. [Google Scholar]
- Rodríguez, S.Y.C.; García, J.A.B.; Bautista, M.P.C.; Gómez, M.S.H. Asaí (Euterpe precatoria): Cadena de Valor En El Sur de La Región Amazónica; Instituto Amazónico de Investigaciones Científicas-Sinchi: Bogotá, Colombia, 2015. [Google Scholar]
- Peres, C.A. Composition, density, and fruiting phenology of arborescent palms in an Amazonian terra firme forest. Biotropica 1994, 26, 285. [Google Scholar] [CrossRef]
- Gama, M.A.M. Estudo comparativo da biologia reprodutiva de Euterpe oleracea Martius e Euterpe precatoria Martius (Arecaceae), na região de Manaus-AM. Master’s Thesis, Instituto Nacional de Pesquisas da Amazônia/UFAM, Manaus, Brazil, 2004. [Google Scholar]
- Melo, G.S.; Costa, F.S.; Silva, L.C. O cenário da produção do açaí (Euterpe spp.) no Estado do Amazonas. Braz. J. Dev. 2021, 7, 71536–71549. [Google Scholar] [CrossRef]
- Cartaxo, C.B.C.; Vasconcelos, M.A.M.; Papa, D.A.; Gonzaga, D.S.O.M.; Álvares, V.S. Euterpe precatoria Mart.: Boas Práticas de Produção na Coleta e Pós-Coleta de Açaí-Solteiro; (Documentos/Embrapa Acre, 166); Embrapa Acre: Rio Branco, AC, Brazil, 2020. [Google Scholar]
- Wadt, L.H.O.; Rigamonte-Azevedo, O.C.; Ferreira, E.J.L.; Cartaxo, C.B.C. Manejo de Açaí Solteiro (Euterpe Precatoria Mart.) Para Produção de Frutos; (Secretaria de Extensão Agroflorestal e Produção Familiar. Documento técnico, 2); Embrapa Acre: Rio Branco, AC, Brazil, 2004. [Google Scholar]
- Gomes, J.P.; Condé, T.M.; de Lima Santos, R.; Dionisio, L.F.S.; Duarte, O.R.; de Miranda, D.L.; Da Silva, F. Seplanct—Secretaria de Estado de Planejamento, Desenvolvimento, Ciência, Tecnologia e Inovação. In Amazonas Em Mapas, 1st ed.; Amazonas Portal do Planejamento: Manaus, AM, Brazil, 2016. [Google Scholar]
- Evangelista-Vale, J.C.; Weihs, M.; José-Silva, L.; Arruda, R.; Sander, N.L.; Gomides, S.C.; Machado, T.M.; Pires-Oliveira, J.C.; Barros-Rosa, L.; Castuera-Oliveira, L.; et al. Climate change may affect the future of extractivism in the Brazilian Amazon. Biol. Conserv. 2021, 257, 109093. [Google Scholar] [CrossRef]
- Vieira, M.F.; Fonseca, R. Biologia Reprodutiva Em Angiospermas: Síndromes Florais, Polinização e Sistemas Reprodutivos Sexuados; Série Conhecimento; UFV: Viçosa, MG, Brazil, 2014. [Google Scholar]
- Hamrick, J.L. Response of forest trees to global environmental changes. For. Ecol. Manag. 2004, 197, 323–335. [Google Scholar] [CrossRef]
- Degen, B.; Sebbenn, A.M. Genetics and tropical forests. In Tropical Forestry Handbook; Köhl, M., Pancel, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–30. ISBN 978-3-642-41554-8. [Google Scholar]
- Ramos, S.L.F.; Dequigiovanni, G.; Sebbenn, A.M.; Lopes, M.T.G.; Macêdo, J.L.V.D.; Veasey, E.A.; Alves-Pereira, A.; Silva, P.P.; Garcia, J.N.; Kageyama, P.Y. Paternity analysis, pollen flow, and spatial genetic structure of a natural population of Euterpe Precatoria in the Brazilian Amazon. Ecol. Evol. 2018, 8, 11143–11157. [Google Scholar] [CrossRef]
- Henderson, A. Pollination systems of palms (Arecaceae). J. Poll. Ecol. 2024, 35, 144–248. [Google Scholar] [CrossRef]
- Küchmeister, H.; Silberbauer-Gottsberger, I.; Gottsberger, G. Flowering, pollination, nectar standing crop, and nectaries of Euterpe precatoria (Arecaceae), an Amazonian rain forest palm. Plant Syst. Evol. 1997, 206, 71–97. [Google Scholar] [CrossRef]
- Goodwillie, C.; Kalisz, S.; Eckert, C. The evolutionary enigma of mixed mating systems in plants: Occurrence, theoretical explanations, and empirical evidence. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 47–79. [Google Scholar] [CrossRef]
- Zona, S.; Henderson, A. A Review of animal-mediated seed dispersal of palms. Selbyana 1989, 11, 6–21. [Google Scholar]
- Smith, N. Euterpe precatoria. In Palms and People in the Amazon; Springer International Publishing: Cham, Switzerland, 2015; pp. 259–273. ISBN 978-3-319-05509-1. [Google Scholar]
- Ramos, S.L.F.; Lopes, M.T.G.; Lopes, R.; Dequigiovanni, G.; Macêdo, J.L.V.; Sebbenn, A.M.; Silva, E.B.D.; Garcia, J.N. Mating system analysis of açaí-do-Amazonas (Euterpe precatoria Mart.) using molecular markers. Crop Breed. Appl. Biotechnol. 2019, 19, 126–130. [Google Scholar] [CrossRef]
- Perrut-Lima, P.; Sebbenn, A.M.; Francisconi, A.F.; Picanço-Rodrigues, D.; Clement, C.R. Genetic diversity and mating system of Euterpe precatoria in three localities along the lower Solimões river in Central Amazonia. Silvae Genet. 2023, 72, 81–91. [Google Scholar] [CrossRef]
- Rosa, L.Z.; Almeida, C.; Brasil, A.; Laindorf, B.; Cogo, M.R.D.; Kuhn, S.; Bacega, A.; Santos, N.; Silveira, D.; Vestena Cassol, A.; et al. A importância da hibridização para a preservação da variabilidade genética da família Arecaceae (Palmeiras) frente a fatores antropogênicos: Uma revisão sobre o caso da palmeira x Butyagrus nabonnandii (Prosch.) Vorste. Res. Soc. Dev. 2021, 10, e347101422104. [Google Scholar] [CrossRef]
- Bovi, M.L.A.; Godoy Júnior, G.; Sáes, L.A. Híbridos interespecíficos de palmiteiro (Euterpe oleracea x Euterpe edulis). Bragantia 1987, 46, 343–363. [Google Scholar] [CrossRef]
- Chaves, S.F.d.S.; Alves, R.M.; dos Santos Dias, L.A. Contribution of breeding to agriculture in the Brazilian Amazon. I. Açaí palm and oil palm. Crop Breed. Appl. Biotechnol. 2021, 21, e386221S8. [Google Scholar] [CrossRef]
- Santos, J.F.; Mariguele, K.H.; Pereira, A.; Zambonim, F.M.; Venturieri, G.A. Parâmetros genéticos e teste de paternidade em progênies de Euterpe oleracea produto da hibridação espontânea com Euterpe edulis em clima subtropical. Rev. De La Fac. De Agron. 2020, 119, 036. [Google Scholar] [CrossRef]
- Lima, L.C.S.; Oliveira, M.S.P. Fases de floração e viabilidade polínica em acessos híbridos interespecíficos de açaizeiro. Res. Soc. Dev. 2023, 12, e5812842879. [Google Scholar] [CrossRef]
- Delgado, J.P.M. Características vegetativas na fase jovem de híbridos interespecíficos entre açaizeiros Amazônicos. In Proceedings of the Caminhos da Produção Agroflorestal na Amazônia: II Workshop Cadeias de Produção Agroflorestal Prioritárias da Amazônia, Rondônia, Brasil, 5 May 2023; Sociedade Brasileira de Ciências do solo- Núcleo Regional Noroeste: Porto Velho, Brazil, 2024; pp. 63–77. [Google Scholar]
- Dransfield, J. Growth forms of rain forest palms. In Tropical Trees as Living Systems; Tomilinson, P.B., Zimmermman, M., Eds.; Cambridge University Press: Cambridge, UK, 1978; pp. 247–268. [Google Scholar]
- Kahn, F. The distribution of palms as a function of local topography in Amazonian terra-firme forests. Experientia 1987, 43, 251–259. [Google Scholar] [CrossRef]
- Anderson, A.B.; Gely, A.; Strudwick, J.; Sobel, G.L.; Pinto, M.G.C. Um sistema agroflorestal na várzea do estuário amazônico (Ilha das Onças, município de Barcarena, Estado do Pará). Acta Amaz. 1985, 15, 195–224. [Google Scholar] [CrossRef]
- FAO. Especies Forestales Productoras de Frutas y Otros Alimentos, 3. Ejemplos de América Latina; FAO Forestry Paper 44/3; FAO: Roma, Italy, 1987. [Google Scholar]
- Kageyama, P.; Sebbenn, A.; Arruda Ribas, L.; Gandara, F.; Castellen, M.; Perecim, M.; Vencovsky, R. Genetic diversity in tropical tree species from different successional stages determined with genetic markers. Sci. For. 2003, 64, 93–107. [Google Scholar][Green Version]
- Prance, G.T.; Rodrigues, W.A.; Silva, M.F. Inventário florestal de um hectare de mata de terra firme km 30 da estrada Manaus-Itacoatiara. Acta Amaz. 1976, 6, 9–35. [Google Scholar] [CrossRef]
- Kahn, F.; Granville, J.-J. Palms in Forest Ecosystems of Amazonia; Springer: Berlin/Heidelberg, Germany, 1992; ISBN 978-3-642-76852-1. [Google Scholar]
- Homma, A.K.O.; Müller, A.A.; Müller, C.H.; Ferreira, C.A.P.; Figueirêdo, F.J.C.; Viégas, I.J.M.; Farias Neto, J.T.; Carvalho, J.E.U.; Cohen, K.O.; Souza, L.A.; et al. Açaí. In Sistema de Produção: Açaí; (Embrapa Amazônia Oriental. Sistemas de Produção, 4); Embrapa Amazônia Oriental: Belém, PA, Brazil, 2005; pp. 15–19. [Google Scholar]
- Falesi, Í.C. O Estado Atual de Conhecimento de Solos da Amazônia Brasileira. Boletim Técnico, 54. IPEAN: Belém, Pará, 1972, 17–61. Available online: http://www.alice.cnptia.embrapa.br/alice/handle/doc/394287 (accessed on 31 July 2024).
- Lamotte, S. Fluvial dynamics and succession in the lower Ucayali river basin, Peruvian Amazonia. For. Ecol. Manag. 1990, 33, 141–156. [Google Scholar] [CrossRef]
- Castro, A. O Extrativismo do Acaí no Amazonas; INPA-CNPq/ORSTOM: Manaus, AM, Brazil, 1992; pp. 779–782. [Google Scholar]
- Brum, H.D.; Souza, A.F. Flood disturbance and shade stress shape the population structure of açaí palm Euterpe precatoria, the most abundant amazon species. Botany 2020, 98, 147–160. [Google Scholar] [CrossRef]
- Bovi, M.L.A.; Castro, A. Assaí. In Selected Species and Strategies to Enhance Income Generation from Amazonian Forests; Clay, J.W., Clement, C.R., Eds.; FAO: Rome, Italy, 1993; p. 260. [Google Scholar]
- Martinot, J.F.; Pereira, H.S.; Silva, S.C.P. Coletar ou cultivar: As escolhas dos produtores de açaí-da-mata (Euterpe precatoria) do Amazonas. Rev. Econ. Sociol. Rural 2017, 55, 751–766. [Google Scholar] [CrossRef]
- Nogueira, O.L.; Conceição, H.E.O. Análise de Crescimento de Açaizeiros Em Áreas de Várzea Do Estuário Amazônico. Pesqui. Agropecuária Bras. 2000, 35, 2167–2173. Available online: https://www.google.com/search?q=Nogueira (accessed on 31 July 2024). [CrossRef]
- Junk, W.; Wittmann, F.; Schöngart, J.; Piedade, M.T. A classification of the major habitats of Amazonian black-water river floodplains and a comparison with their white-water counterparts. Wetl. Ecol. Manag. 2015, 23, 677–693. [Google Scholar] [CrossRef]
- Wittmann, F.; Householder, J.E.; Piedade, M.T.F.; Schöngart, J.; Demarchi, L.O.; Quaresma, A.C.; Junk, W.J. A review of the ecological and biogeographic differences of Amazonian floodplain forests. Water 2022, 14, 3360. [Google Scholar] [CrossRef]
- Alarcón, J.G.S.; Peixoto, A.L. Florística e fitossociologia de um trecho de um hectare de floresta de terra firme, em Caracaraí, Roraima, Brasil. Bol. Mus. Para. Emílio Goeldi. Ciências Nat. 2007, 2, 33–60. [Google Scholar] [CrossRef]
- Silva Carvalho, C.; Ribeiro, M.C.; Côrtes, M.C.; Galetti, M.; Collevatti, R.G. Contemporary and historic factors influence differently genetic differentiation and diversity in a tropical Palm. Heredity 2015, 115, 216–224. [Google Scholar] [CrossRef]
- Alvarez Montalván, C.; Manrique-León, S.; Fonseca, M.; Cardozo-Soarez, J.; Callo-Ccorcca, J.; Bravo-Camara, P.; Castañeda-Tinco, I.; Alvarez-Orellana, J. Floristic composition, structure and tree diversity of an Amazon forest in Peru. Sci. Agropecu. 2021, 12, 73–82. [Google Scholar] [CrossRef]
- Campos, T.d.; Azêvedo, H.S.F.d.S.; Azevedo, J.M.A.d.; Rufino, P.B.; Silva, S.M.M.; Oliveira, J.C.d.; Silva, L.M.d. Rendimento de polpa de frutos de açaizeiro em áreas de baixio e terra firme em Feijó, AC. In Proceedings of the Inovação: Sustentabilidade e Desenvolvimento Regional; Edufac: Rio Branco, AC, Brazil, 2016. [Google Scholar]
- Melo, G.D.S. Produtividade e sustentabilidade do cultivo do açaizeiro (Euterpe precatoria Mart.) no município de Humaitá-Amazonas. Master’s Thesis, Universidade Federal do Estado do Amazonas, Humaitá, AM, Brazil, 2022. [Google Scholar]
- Raupp, S.V. Distribuição, abundância e fenologia produtiva de palmeiras em uma floresta de bterra firme da Amazônia Central. Ph.D. Thesis, Instituto Nacional de Pesquisas da Amazônia, Manaus, AM, Brazil, 2010. [Google Scholar]
- Pardo-Molina, G.; Pereira, L.; Feldpausch, T.; Vos, V.A.; Aramayo-Parada, R.; Arancibia-Rocabado, I.; Mamio, R.; Enríquez, S.; Mamani-Loza, M.A.; Suarez-Tabo, N.; et al. Composición florística del bosque amazónico de tierra firme del sector Alto Madera, Bolivia. Ecol. En Boliv. Rev. Del Inst. De Ecol. 2020, 55, 111–126. [Google Scholar]
- Cochev, J.S.; Rossi, A.A.B.; Neves, S.M.A.S.; Zortéa, K.É.M.; Rodrigues, A.D.S. Dinâmica espaço-temporal da paisagem e estrutura populacional de Euterpe precatoria Mart. em fragmento florestal no município mato-grossense de Alta Floresta, Brasil. Ciênc. Florest. 2019, 29, 1398–1414. [Google Scholar] [CrossRef]
- Gomes, J.P.; Condé, T.M.; Santos, R.L.; Dionisio, L.F.S.; Duarte, O.R.; Miranda, D.L.C.; Silva, F. Efeitos de gradientes ambientais na fitossociologia de assembleias de palmeiras no sudeste de Roraima, Brasil. Nativa 2016, 4, 317–327. [Google Scholar] [CrossRef]
- Martinot, J.F. Majejo Agro-Extrativista do Açaí-Da Mata na Amazônia Central. Master’s Thesis, Universidade Federal do Estado do Amazonas, Manaus, AM, Brazil, 2013. [Google Scholar]
- Dias, F.O. Diagnóstico Para o Reconhecimento do Açaí de Codajás-Amazonas como Indicação Geográfica. Master’s Thesis, Universidade Federal do Estado do Amazonas, Manaus, Brazil, 2021. [Google Scholar]
- Bayma, M.M.A.; Wadt, L.H.O.; Sa, C.P.; Balzon, T.A.; Sousa, M.M.M. Custo e Rentabilidade da Atividade de Extração de Açaí em Áreas de Baixio na Reserva Extrativista Chico Mendes, Seringais Porvir, Filipinas, Etelvi, no Acre; (Embrapa Acre. Comunicado técnico, 170); Embrapa Acre: Rio Branco, Brazil, 2008. [Google Scholar]
- Pinto, F.R. Análise produtiva de sistemas agroextrativistas de açaí-da-mata (Euterpe precatoria) na Amazônia Central. Ph.D. Thesis, Universidade Federal do Estado do Amazonas, Manaus, AM, Brazil, 2018. [Google Scholar]
- de Albuquerque, T.C.S.; Queiroz, N.B. Floristíca em áreas de ocorrências de açaizeiros (Euterpe precatoria Mart.) No Município de Rorainópolis, RR. In Avanços Científicos Tecnológicos e de Inovação na Botânica 2; Silva-Matos, R.R.S., Moraes, L.F., Sousa, L.A.M., Eds.; Atena: Ponta Grossa, PR, Brazil, 2022; pp. 26–38. [Google Scholar]
- Cabrera Amaya, D.M.; Rivera Diaz, O. Composición florística y estructura de los bosques ribereños de la cuenca baja del río Pauto, Casanare, Colombia. Caldasia 2016, 38, 53–85. [Google Scholar] [CrossRef]
- Isaza, C.; Bernal, R.; Galeano, G.; Martorell, C. Demography of Euterpe precatoria and Mauritia flexuosa in the Amazon: Application of integral projection models for their harvest. Biotropica 2017, 49, 653–664. [Google Scholar] [CrossRef]
- Andrade Miranda, I.P.; Marques Barbosa, E.; Rabelo, A.; Ferreira Santiago, F. Palmas de comunidades ribereñas como recurso sustentable en la Amazonía brasileña. Rev. Peru. Biol. 2008, 15, 115–120. [Google Scholar] [CrossRef]
- Lima, C.B.A. Padrões Distributivos das Assembléias de Palmeiras ao Longo de Gradientes Ripário na Estação Ecológica do Cuniã, Interflúvio Purus-Madeira, Rondônia. Master’s Thesis, Universidade Federal de Rondônia, Rondonia, Brazil, 2012. [Google Scholar]
- Rubiano, L.J.; Ortiz, R.; Dueñas, H. Caracterización fisionómica, estructural y florística de un área selvática en la Sierra Nevada de Santa Marta, Colombia. Rev. Biol. Trop. 1994, 42, 89–105. [Google Scholar][Green Version]
- Lopes, E.; Soares-Filho, B.; Souza, F.; Rajão, R.; Merry, F.; Carvalho Ribeiro, S. Mapping the socio-ecology of non timber forest products (NTFP) extraction in the brazilian Amazon: The case of açaí (Euterpe precatoria Mart) in Acre. Landsc. Urban Plan. 2019, 188, 110–117. [Google Scholar] [CrossRef]
- Velarde, M.J.; Moraes, M. Density of adults and fruit production of asaí (Euterpe precatoria, Arecaceae) in Riberalta, Bolivia. Ecol. En Boliv. 2008, 43, 99–110. [Google Scholar]
- Zanatta, G.V. O extrativismo de açaí (Euterpe precatoria Mart.) e os sistemas produtivos tradicionais na terra indígena Kwatá-Laranjal, Amazonas. In Mestrado em Ciências de Florestas Tropicais; Instituto Nacional de Pesquisas da Amazônia: Manaus, AM, Brazil, 2012. [Google Scholar]
- Zárate-Gómez, R.; Del-Águila-Cachiqe, H.K.J.; Ramos-Rodríguez, M.C.; Palacios-Vega, J.J.; Pérez Macedo, C.P.; Valles-Pérez, L.A. Diversidade de flora y vegetación del interfluvio Napo-Putumayo-Amazonas, Perú. Folia Amaz. 2020, 29, 189–266. [Google Scholar] [CrossRef]
- Kahn, F.; Mejia, K. Palm communities in wetland forest ecosystems of peruvian Amazonia. For. Ecol. Manag. 1990, 33, 169–179. [Google Scholar] [CrossRef]
- Phillips, O. The Potential for harvesting fruits in tropical rainforests: New data from Amazonian Peru. Biodivers. Conserv. 1993, 2, 18–38. [Google Scholar] [CrossRef]
- Sousa, L.A.S.; Jardim, M.A.G. Produção foliar de mudas de açaizeiro (Euterpe oleracea Mart.) em área de vegetação secundária no nordeste paraense. Rev. Bras. De Biociências 2007, 5, 225–227. [Google Scholar]
- Matos, C.B.E.; Sampaio, P.; Rivas, A.A.A.; Matos, J.C.S.; Hodges, D.G. Economic profile of two species of genus der Euterpe, producers of açaí fruits, from the Pará and Amazonas States-Brazil. IJEAB J. 2017, 2, 1822–1828. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Paniagua Zambrana, N.Y. Facing Global Markets—Usage changes in western Amazonian plants: The example of Euterpe precatoria Mart. and E. oleracea Mart. Acta Soc. Bot. Pol. 2012, 81, 257–261. [Google Scholar] [CrossRef]
- Conab Açaí—Análise Mensal—Dezembro 2020. Available online: http://www.conab.gov.br/info-agro/analises-do-mercado-agropecuario-e-extrativista/analises-do-mercado/historico-mensal-de-sociobiodiversidade/item/15517-acai-analise-mensal-dezembro-2020 (accessed on 28 August 2024).
- Suframa Açaí de Codajás (AM) Passa a Ser Mais um Produto da Amazônia Ocidental Com Registro de Indicação Geográfica. Available online: https://www.gov.br/suframa/pt-br/publicacoes/noticias/acai-de-codajas-passa-a-ser-mais-um-produto-da-amazonia-ocidental-com-registro-de-indicacao-geografica (accessed on 30 August 2024).
- Sebrae Açaí de Feijó (AC) Conquista a Primeira Indicação Geográfica Para o Produto no País. Available online: https://agenciasebrae.com.br/inovacao-e-tecnologia/acai-de-feijo-ac-conquista-a-primeira-indicacao-geografica-para-o-produto-no-pais/ (accessed on 30 August 2024).
- Silveira, J.T.; Rosa, A.P.C.; Morais, M.G.; Victoria, F.N.; Costa, J.A.V. An Integrative Review of Açaí (Euterpe oleracea and Euterpe precatoria): Traditional Uses, Phytochemical Composition, Market Trends, and Emerging Applications. Food Res. Int. 2023, 173, 113304. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Teixeira, G.; Darnet, E.; Schaller, H.; Rogez, H.; Darnet, S. A review of the genus Euterpe: Botanical and genetic aspects of açai, the purple gold of the Amazon. Bot. J. Linn. Soc. 2024, 208, 1–13. [Google Scholar] [CrossRef]
- Kang, J.; Thakali, K.M.; Xie, C.; Kondo, M.; Tong, Y.; Ou, B.; Jensen, G.; Medina, M.B.; Schauss, A.G.; Wu, X. Bioactivities of açaí (Euterpe precatoria Mart.) Fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleracea Mart. Food Chem. 2012, 133, 671–677. [Google Scholar] [CrossRef]
- Fernandes, E.T.M.B.; Maciel, V.T.; Souza, M.L.; Furtado, C.M.; Wadt, L.H.O.; Cunha, C.R. Physicochemical Composition, Color and Sensory Acceptance of Low-Fat Cupuaçu and Açaí Nectar: Characterization and Changes during Storage. Food Sci. Technol 2016, 36, 413–420. [Google Scholar] [CrossRef]
- Neves, L.C.; Tosin, J.M.; Benedette, R.M.; Cisneros-Zevallos, L. Post-harvest nutraceutical behaviour during ripening and senescence of 8 highly perishable fruit species from the northern Brazilian Amazon Region. Food Chem. 2015, 174, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.V.C.; Costa, C.M.; Rebelo, K.S.; Yamaguchi, K.K.D.L. Análise da composição centesimal dos açaís amazônicos: Euterpe precatoria Mart. e Euterpe oleracea Mart. Rev. Ifes Ciência 2023, 9, 1–7. [Google Scholar] [CrossRef]
- Brasil Instrução Normativa—Secretaria de Defesa a Agropecuária No 37 de 01/10/2018—Federal-LegisWeb. Available online: https://www.legisweb.com.br/legislacao/?id=368178 (accessed on 28 August 2024).
- Macía, M.J.; Armesilla, P.J.; Cámara-Leret, R.; Paniagua-Zambrana, N.; Villalba, S.; Balslev, H.; Pardo-de-Santayana, M. Palm Uses in Northwestern South America: A Quantitative Review. Bot. Rev. 2011, 77, 462–570. [Google Scholar] [CrossRef]
- Prance, G.T.; Balee, W.; Boom, B.; Carneiro, R. Quantitative ethnobotany and the case for conservation in Amazonia. Conserv. Biol. 1987, 1, 296–310. [Google Scholar] [CrossRef]
- Almeida, S.S.; Silva, P.J. As palmeiras: Aspectos botânicos, ecológicos e econômicos. In Cauxianã; Lisboa, P.L.B., Ed.; Museu Paraense Emílio Goeldi: Belém, PA, Brazil, 1997; pp. 235–251. [Google Scholar]
- Nowak, D.; Gośliński, M.; Przygoński, K.; Wojtowicz, E. The antioxidant properties of exotic fruit juices from acai, maqui berry and coni berries. Eur. Food Res. Technol. 2018, 244, 1897–1905. [Google Scholar] [CrossRef]
- Gama, M.M.B.; Ribeiro, G.D.; Fernandes, C.F.; Medeiros, I.M. de Açaí (Euterpe spp.): Características, formação de mudas e plantio para a produção de frutos. Circular Técnica 2005, 80, 6. [Google Scholar]
- Díaz, R.O.; Cardona, J.E.C.; Carrillo, M.; Hernández, M.S.; Fernández-Trujillo, J.P.; Gutiérrez, R.H.; Lares, M. Postharvest Handling and uses of asaí (Euterpe precatoria) fruit. Acta Hortic. 2014, 1047, 269–274. [Google Scholar] [CrossRef]
- Siviero, A.; Ming, L.; Silveira, M.; Daly, D.; Wallace, R. Etnobotânica e Botânica Econômica Do Acre; Edufac: Rio Branco, AC, Brazil, 2016; ISBN 978-85-8236-027-9. [Google Scholar]
- Reis, B.d.O.; Silva, I.T.d.; Silva, I.M.O.d.; Rocha, B.R.P.d. Produção de briquetes energéticos a partir de caroços de açaí. In Proceedings of the 4th Encontro de Energia no Meio Rural, Campinas, SP, Brazil, 29–31 October 2002. [Google Scholar]
- Tinoco, A.C. Açaí Amazônico: Novas perspectivas de negócio. In Workshop Regional do Açaizeiro: Pesquisa, Produção e Comercialização; Embrapa Amazônia Oriental: Belém, PA, Brzail, 2005. [Google Scholar]
- Shanley, P.; Medina, G. Frutíferas e Plantas Uteis Na Vida Amazonica; CIFOR e Imazon: Belém, Brazil, 2005. [Google Scholar]
- Lorenzi, H. Arvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas do Brasil, 8th ed.; Plantarum: Nova Odessa, SP, Brazil, 2021; Volume 1, ISBN 978-65-87655-01-7. [Google Scholar]
- Jensen, J.F.; Kvist, L.P.; Christensen, S.B. An antiplasmodial lignan from Euterpe precatoria. J. Nat. Prod. 2002, 65, 1915–1917. [Google Scholar] [CrossRef]
- Alves, V.M.; Asquieri, E.R.; Araújo, E.D.S.; Martins, G.A.S.; Melo, A.A.M.; Freitas, B.C.B.; Damiani, C. Provenient residues from industrial processing of açaí berries (Euterpe precatoria Mart): Nutritional and antinutritional contents, phenolic profile, and pigments. Food Sci. Technol. 2022, 42, e77521. [Google Scholar] [CrossRef]
- Rufino, J.P.F.; Cruz, F.G.G.; Filho, P.A.O.; Brasil, R.J.M.; Melo, L.D.; Andrade, P.G.C. Análise de viabilidade econômica do farelo do resíduo de açaí na alimentação de poedeiras comerciais leves. Rev. Em Agronegócio E Meio Ambiente 2020, 13, 867–882. [Google Scholar] [CrossRef]
- Boeira, L.S.; Cád, S.V.; Bezerra, J.A.; Benavente, C.T.; Neta, M.T.S.L.; Sandes, R.D.D.; Narain, N. Development of alcohol vinegars macerated with açai (Euterpe precatoria Mart.) berries and their quality evaluations with emphasis on color, antioxidant capacity, and volatiles profile. J. Food Sci. 2023, 88, 666–680. [Google Scholar] [CrossRef]
- EMBRAPA Manejo e Uso Dos Recursos Naturais—Portal Embrapa. Available online: https://www.embrapa.br/agencia-de-informacao-tecnologica/tematicas/bioma-cerrado/ecologia/manejo-e-uso-dos-recursos-naturais (accessed on 28 August 2024).
- MacDicken, K.G.; Sola, P.; Hall, J.E.; Sabogal, C.; Tadoum, M.; Wasseige, C. Global Progress toward Sustainable Forest Management. For. Ecol. Manag. 2015, 352, 47–56. [Google Scholar] [CrossRef]
- National Geographic Açaí Certificado do Amapá Mostra que Soluções Tradicionais são Futuro Para a Amazônia. Available online: https://www.nationalgeographicbrasil.com/meio-ambiente/2022/12/acai-certificado-do-amapa-mostra-que-solucoes-tradicionais-sao-futuro-para-a-amazonia (accessed on 17 September 2024).
- Bernal, R.; Torres, C.; García, N.; Isaza, C.; Navarro López, J.; Vallejo, M.; Galeano, G.; Balslev, H. Palm Management in South America. Bot. Rev. 2011, 77, 607–646. [Google Scholar] [CrossRef]
- Vieira, A.H.; Ramalho, A.R.; Rosa, C.N.; Cararo, D.C.; Costa, J.N.M.; Vieira, J.R.J.; Wadt, P.G.S.; Souza, V.F. Cultivo do Açaizeiro (Euterpe oleracea Martius) no Noroeste do Brasil; (Embrapa Rondônia. Sistemas de produção, 36); Embrapa Rondônia: Porto Velho, Brazil, 2018. [Google Scholar]
- Delgado, J.P.M.; Cunha, R.N.V.; Morais, R.R.; Lopes, M.T.G.; Ramos, S.L.F.; Rodrigues, M.D.R.L.; Medeiros, N.M.C.; Meneses, C.H.S.G.; Barcelos, E.; Lopes, R. Morphology and allometry of juvenile açaí palms under cultivation conditions in Central Amazonia. Horticulturae 2024, 10, 1119. [Google Scholar] [CrossRef]
- Viana, L.F.; Homma, A.K.O.; de Menezes, A.J.E.A.; dos Santos, J.C.; Neto, J.T.F.; Pena, H.W.A. Análise econômica do cultivo de açaizeiro (Euterpe oleracea Mart.) irrigado no nordeste paraense. Rev. Terceira Margem Amaz. 2021, 7, 155–169. [Google Scholar] [CrossRef]
- Sousa, D.; Fernandes, T.; Tavares, L.; Farias, V.; Lima, M.; Nunes, H.; Costa, D.; Ortega-Farias, S.; Souza, P. Estimation of evapotranspiration and single and dual crop coefficients of acai palm in the Eastern Amazon (Brazil) using the bowen ratio system. Irrig. Sci. 2021, 39, 5–22. [Google Scholar] [CrossRef]
- Conforto, E.C.; Contin, D.R. Desenvolvimento do açaizeiro de terra firme, cultivar pará, sob atenuação da radiação solar em fase de viveiro. Bragantia 2009, 68, 979–983. [Google Scholar] [CrossRef]
- Uzzo, R.P. Resposta Fisiológica e Anatômica do Açaizeiro e da Palmeira Real Australiana ao Sombreamento. Ph.D. Thesis, Universidade de São Paulo, Piracicaba, Brazil, 2008. [Google Scholar]
- Carvalho, N.O.S.; Pelacani, C.R.; Rodrigues, M.O.S.; Crepaldi, I.C. Crescimento inicial de plantas de licuri (Syagrus coronata (Mart.) Becc.) em diferentes níveis de luminosidade. Rev. Árvore 2006, 30, 351–357. [Google Scholar] [CrossRef]
- Almeida, U.O.; Andrade Neto, R.C.; Lunz, A.M.P.; Nogueira, S.R.; Costa, D.A.; Araújo, J.M. Environment and Sslow-release fertilizer in the production of Euterpe precatoria seedlings. Pesqui. Agropecu. Trop. 2018, 48, 382–389. [Google Scholar] [CrossRef]
- Carvalho, J.E.U.; Nascimento, W.M.O. Technological innovations in the propagation of açaí palm and bacuri. Rev. Bras. Frutic. 2018, 40, e-679. [Google Scholar] [CrossRef]
- Ferreira, J.; Araújo Silva-Cardoso, I.; Meira, R.; Schewinski-Pereira, J. Somatic Embryogenesis and plant regeneration from zygotic embryos of the palm tree Euterpe precatoria Mart. Plant Cell Tissue Organ. Cult. 2022, 148, 667–686. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Silva-Cardoso, I.M.A.; Ferreira, J.C.B.; Costa, F.H.D.S.; Scherwinski-Pereira, J.E. Morphostructural and histochemical dynamics of Euterpe precatoria (Arecaceae) germination. J. Plant Res. 2020, 133, 693–713. [Google Scholar] [CrossRef]
- Pinheiro, C.U.B. Germination strategies of palms: The case of Schippia concolor Burret in Belize. Brittonia 2001, 53, 519–527. [Google Scholar] [CrossRef]
- Bentes-Gama, M.M.; Rocha, R.B.; Capelasso, P.H.S.; Pereira, N.S. Desenvolvimento Inicial de Espécies Nativas Utilizadas na Recuperação de Paisagem Alterada em Rondônia; (Embrapa Rondônia. Circular Técnica, 108); Embrapa Rondônia: Porto Velho, Brazil, 2009; p. 9. [Google Scholar]
- Flores Romayna, M.A.; Ortega Chávez, W.; Ortega Mallqui, A. Evaluación de Tratamientos Pregerminativos En Semillas de Euterpe precatoria Mart. (Huasaí) en la ciudad de Pucallpa-Perú. Rev. Cuba. De Cienc. For. 2020, 8, 88–103. [Google Scholar]
- Santos, J.A.; Oliveira, I.V. Diferentes recipientes na produção de mudas de açaizeiro. Res. Soc. Dev. 2021, 10, e33810414174. [Google Scholar] [CrossRef]
- Gude Butzke, A.; Brito, R.S.; Carvalho Andrade Neto, R.; Pereira Lunz, A.M.; Silva Fiuza, S. Produção de mudas de açaizeiro solteiro submetidas a doses de nitrogênio e potássio. Rev. Agric. Neotrop. 2023, 10, e7316. [Google Scholar] [CrossRef]
- Araújo, C.S.; Lunz, A.M.P.; Santos, V.B.; Andrade Neto, R.C.; Nogueira, S.R.; Santos, R.S. Use of agro-industry residues as substrate for the production of Euterpe precatoria seedlings. Pesqui. Agropecu. Trop. 2020, 50, e58709. [Google Scholar] [CrossRef]
- Viégas, I.J.M.; Frazão, D.A.C.; Thomaz, M.A.A.; Conceição, H.E.O.; Pinheiro, E. Limitações nutricionais para o cultivo de açaizeiro em latossolo amarelo textura média, Estado do Pará. Rev. Bras. Frutic. 2004, 26, 382–384. [Google Scholar] [CrossRef]
- da Costa, K.K.; Rufino, C.P.B.; de Macedo, P.E.F.; Nogueira, S.R. Antagonismo de Trichoderma spp. sobre Colletotrichum gloeosporioides, agente causal da antracnose de Euterpe precatoria. S. Am. J. Basic Educ. Tech. Technol. 2019, 6, 391–397. [Google Scholar]
- Rufino, C.P.B.; Nogueira, S.R.; Araújo, C.S.; Rossi, A.J.D.; Neto, R.C.A.; Lunz, A.M.P.; Macedo, P.E.F. Chemical of Colletotrichum cloeosporioides on seedlings of single assai palm. Rev. De Agric. Neotrop. 2023, 10, e7264. [Google Scholar] [CrossRef]
- Nogueira, S.R.; Silva, I.M.; Macedo, P.E.; Lunz, A.M.P.; Andrade Neto, R.C. Controle de antracnose em açaí-solteiro (Euterpe precatoria) no Acre; (Embrapa Acre. Comunicado técnico, 197); Embrapa Rondônia: Porto Velho, Brazil, 2017; pp. 1–15. [Google Scholar]
- Nogueira, S.R.; Andrade Neto, R.C.; Lunz, A.M.P. Sombreamento Para Controle da Antracnose na Produção de Mudas de Açaí-solteiro. 2016. (Embrapa Acre. Folder/folhetos/Cartilha) Embrapa Acre: Rio Branco, 2016. Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/661589 (accessed on 17 September 2024).
- Valois, A.C.C. Reprodução em plantas de espécies tropicais e implicações na seleção de matrizes. Rev. RG News 2016, 2, 57–65. [Google Scholar]
- Nass, L.L. Recursos Genéticos Vegetais; Embrapa Recursos Genéticos e Biotecnologia: Brasília, DF, Brazil, 2007. [Google Scholar]
- Oliveira, M.S.P.; Pinheiro, T.M.S.; Fiala, M.A. Práticas Para a Renovação do Banco Ativo de Germoplasma de Espécies do Gênero Euterpe (Açaizeiros); (Embrapa Amazônia Oriental. Comunicado Técnico, 315); Embrapa Amazônia Oriental: Belém, Brazil, 2019; p. 10. [Google Scholar]
- Alelo Recursos Genéticos AleloVegetal Dados Abertos. Available online: https://av.cenargen.embrapa.br/avconsulta/Passaporte/busca.do (accessed on 17 September 2024).
- O’Dea, R.E.; Lagisz, M.; Jennions, M.D.; Koricheva, J.; Noble, D.W.A.; Parker, T.H.; Gurevitch, J.; Page, M.J.; Stewart, G.; Moher, D.; et al. Preferred reporting items for systematic reviews and meta-analyses in ecology and evolutionary biology: A PRISMA extension. Biol. Rev. 2021, 96, 1695–1722. [Google Scholar] [CrossRef] [PubMed]
- Lanza, T.; Ming, L.; Haverroth, M.; Ferreira, A. Etnobotânica no Acre: Três décadas de pesquisas científicas realizadas no Estado (1990–2020). Ethnoscientia 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Hidayati, S.; Franco, F.M.; Bussmann, R.W. Ready for Phase 5—Current status of ethnobiology in Southeast Asia. J. Ethnobiol. Ethnomed. 2015, 11, 17. [Google Scholar] [CrossRef] [PubMed]

| Characters | E. precatoria var. longivaginata | E. precatoria var. precatoria | E. longibracteata | E. oleracea | E. catinga var. catinga | E. catinga var. roraimae |
|---|---|---|---|---|---|---|
| Stem | Solitary, rarely cespitose | Solitary | Solitary, occasionally cespitose | Cespitose | Cespitose with few stems, or solitary | Solitary or cespitose |
| Trunk diameter | 4–23 cm | 4–23 cm | 5–8 cm | 7–18 cm | 3.5–9 cm | 7–15 cm |
| Leaf | Broad leaflets, generally pendulous | Narrow leaflets, occasionally pendulous | Broad leaflets, generally pendulous | Broad leaflets, pendulous | Medium leaflets spread horizontally | Medium leaflets, pendulous |
| Seed | Homogeneous endosperm | Homogeneous endosperm | Homogeneous endosperm | Ruminated endosperm | Homogeneous endosperm | Homogeneous endosperm |
| Fruit diameter | 0.9–1.0 cm | 1.0–1.3 cm | 1.0–1.2 cm | 1.0–2.0 cm | 0.8–1.0 cm | 0.8–1.3 |
| Fruit maturation | - | 7–8 months | - | 6 months | - | - |
| Number of bunches | 2–4 | 2–4 | - | 2–3 per stem | - | - |
| Germination (time) | - | 30 to 40 days | - | 15 to 25 days | - | - |
| Eophil | Palmate | Palmate | Palmate | Bifid | Bifid | Bifid |
| Time to first fruiting | - | 6 to 7 years | - | 4 to 5 years | - | - |
| Centesimal Composition | Mean ± Standard Deviation | Minimum–Maximum |
|---|---|---|
| Humidity (%) | 87.94 ± 3.73 | 84.4–94.1 |
| Total lipids (%) | 4.8 ± 2.33 | 1.83–9.74 |
| Total proteins (%) | 0.82 ± 0.13 | 0.76–1.03 |
| Ash (%) | 0.30 ± 0.08 | 0.2–0.46 |
| Total fibers (%) | 7.39 ± 0.32 | 7.1–7.15 |
| Carbohydrates (%) | 3.78 | |
| Total titratable acidity (%) | 0.29 ± 0.01 | 0.28–0.29 |
| pH | 4.34 | - |
| Energy (kcal/100 g) | 48.64 ± 20.05 | 22–91 |
| Soluble solids (°Brix) | 4.92± 2.24 | 3.33–6.5 |
| Glycides (%) | 0.80 ± 0.54 | 0.13–1.95 |
| Concentration of macro- and microelements | ||
| Na (mg) | 2.44 ± 4.22 | 0.26–13.92 |
| Ca (mg) | 27.14 ± 12.05 | 15.99–57.85 |
| K (mg) | 122.03 ± 90.85 | 73.78–376.69 |
| Fe (mg) | 0.75 ± 0.25 | 0.46–1.16 |
| Zn (mg) | 283 ± 116.80 | 163.43–585.37 |
| Bo (µg) | 32.38 ± 29.11 | 5.17–62.24 |
| Co (µg) | 0.76 ± 0.26 | 0.42–1.07 |
| Cr (µg) | 58.12 ± 37.66 | 22.9–148.53 |
| Functional features | ||
| Phenolic compounds (mg GAE/100 g) | 4607.4 | - |
| Vitamin C (mg/100 g) | 68.5 | - |
| Palmitic acid (g/100 g) | 1.4 ± 1.84 | 0.1–4.3 |
| Oleic acid (g/100 g) | 68.2 ± 5.79 | 58.7–74.6 |
| Linoleic acid (g/100 g) | 7.5 ± 3.64 | 2–11.6 |
| Linolenic acid (g/100 g) | 1.0 ± 0.40 | 1–1.7 |
| Centesimal Composition | Mean ± Standard Deviation | Minimum–Maximum |
|---|---|---|
| Humidity (%) | 30.89 | - |
| Total lipids (%) | 11.78 | - |
| Total proteins (%) | 3.31 | - |
| Ash (%) | 1.34 | - |
| Total fibers (%) | - | - |
| Carbohydrates (%) | 52.69 | - |
| Functional features | ||
| Anthocyanins (mg/100 g) | 498.6 ± 226.22 | 128–868.9 |
| Phenolic compounds (mg GAE/100 g) | 320.7 | - |
| Antioxidant capacity (µmol TE/g) | 423.15 ± 145.45 | 320.3–526.0 |
| Total carotenoids (µg/g) | 963.7 | - |
| Institution | Species | Accessions |
|---|---|---|
| Embrapa Eastern Amazon | E. oleracea, E. precatoria and E. edulis | 307 |
| Embrapa Amapá | E. oleracea and E. precatória | 77 |
| Embrapa Acre | E. oleracea and E. precatoria | 25 |
| Federal University of Amazonas | E. oleracea and E. precatoria | 4 |
| Federal University of Tocantins | E. oleracea | 10 |
| Federal University of Acre | E. oleracea and E. precatoria | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, S.Y.N.d.; Ferreira, M.J.; Clement, C.R.; Lopes, R. Systematic Review of the State of Knowledge About Açaí-Do-Amazonas (Euterpe precatoria Mart., Arecaceae). Plants 2025, 14, 2439. https://doi.org/10.3390/plants14152439
Rocha SYNd, Ferreira MJ, Clement CR, Lopes R. Systematic Review of the State of Knowledge About Açaí-Do-Amazonas (Euterpe precatoria Mart., Arecaceae). Plants. 2025; 14(15):2439. https://doi.org/10.3390/plants14152439
Chicago/Turabian StyleRocha, Sabrina Yasmin Nunes da, Maria Julia Ferreira, Charles R. Clement, and Ricardo Lopes. 2025. "Systematic Review of the State of Knowledge About Açaí-Do-Amazonas (Euterpe precatoria Mart., Arecaceae)" Plants 14, no. 15: 2439. https://doi.org/10.3390/plants14152439
APA StyleRocha, S. Y. N. d., Ferreira, M. J., Clement, C. R., & Lopes, R. (2025). Systematic Review of the State of Knowledge About Açaí-Do-Amazonas (Euterpe precatoria Mart., Arecaceae). Plants, 14(15), 2439. https://doi.org/10.3390/plants14152439







