Full-Length Transcriptome Sequencing of Pinus massoniana Under Simulated Monochamus alternatus Feeding Highlights bHLH Transcription Factor Involved in Defense Response
Abstract
1. Introduction
2. Results
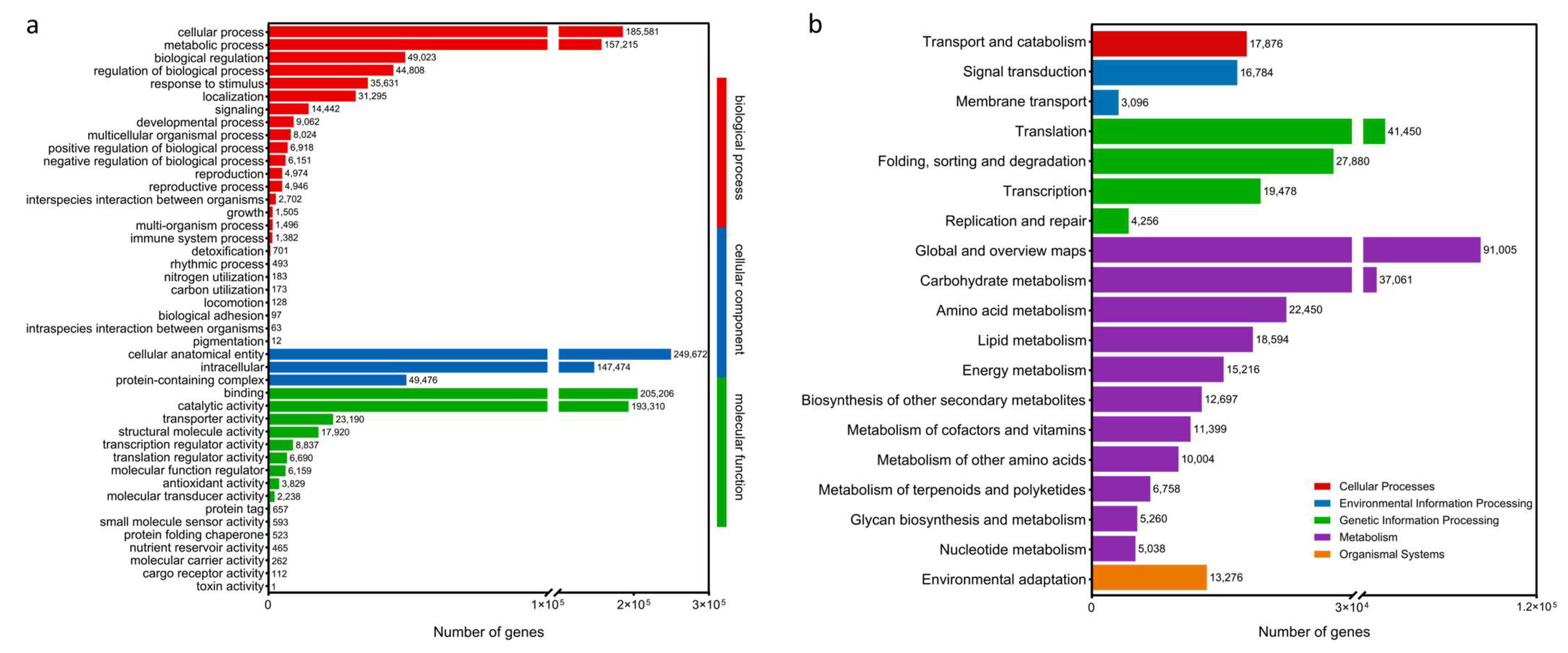
2.1. mRNA Sequencing and Assembly
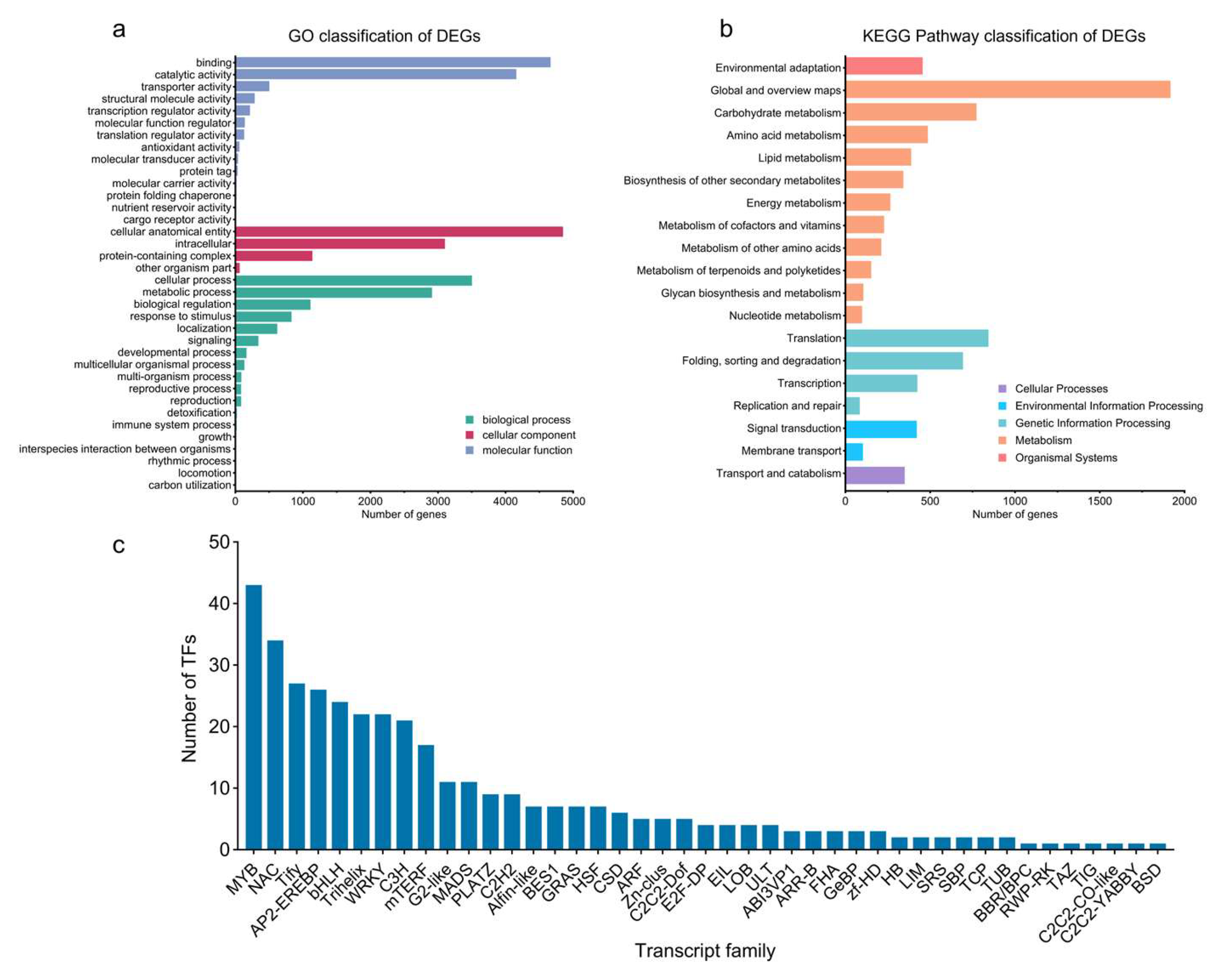
2.2. DEG Identification
2.3. Transcription Factor Analysis of DEGs
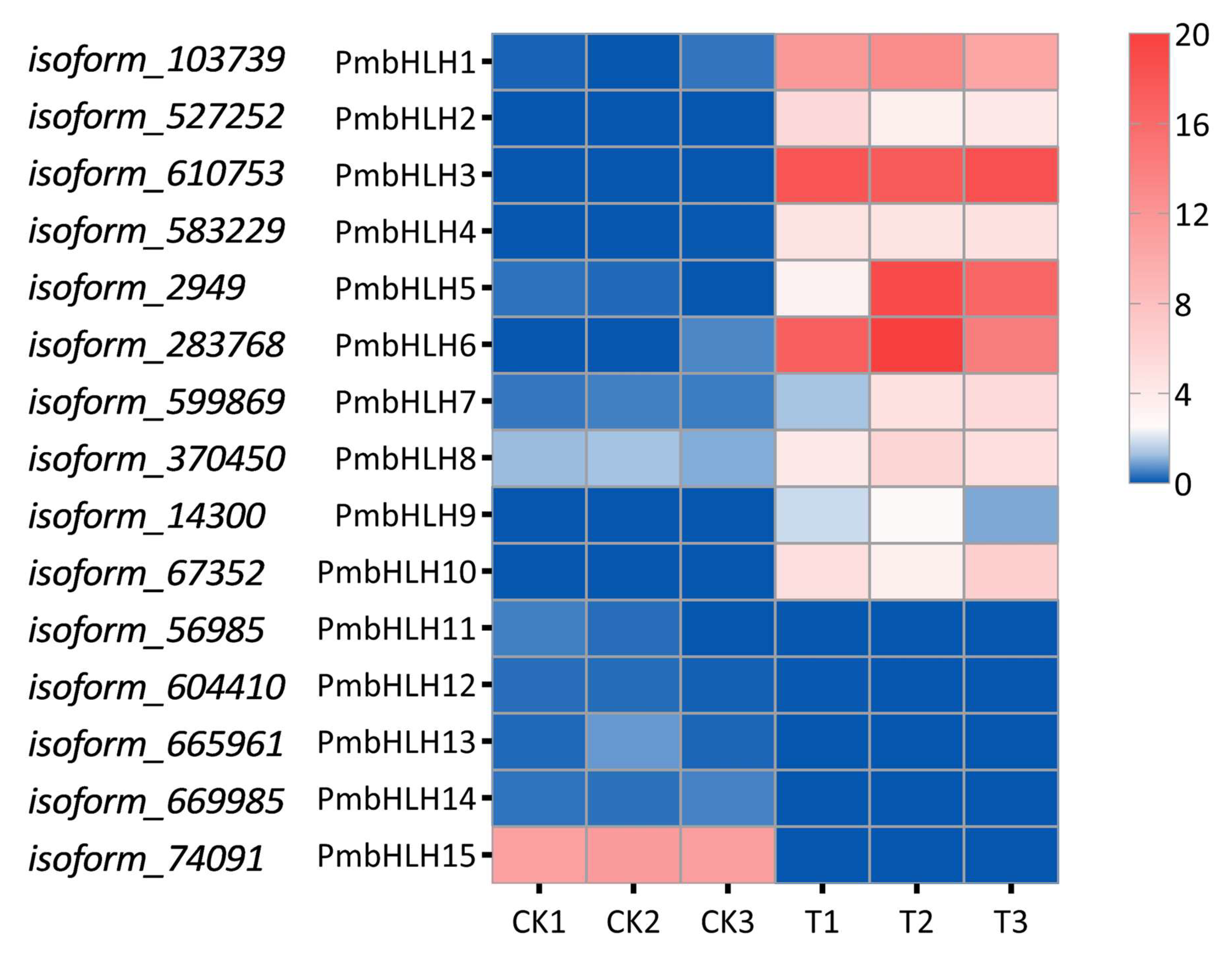
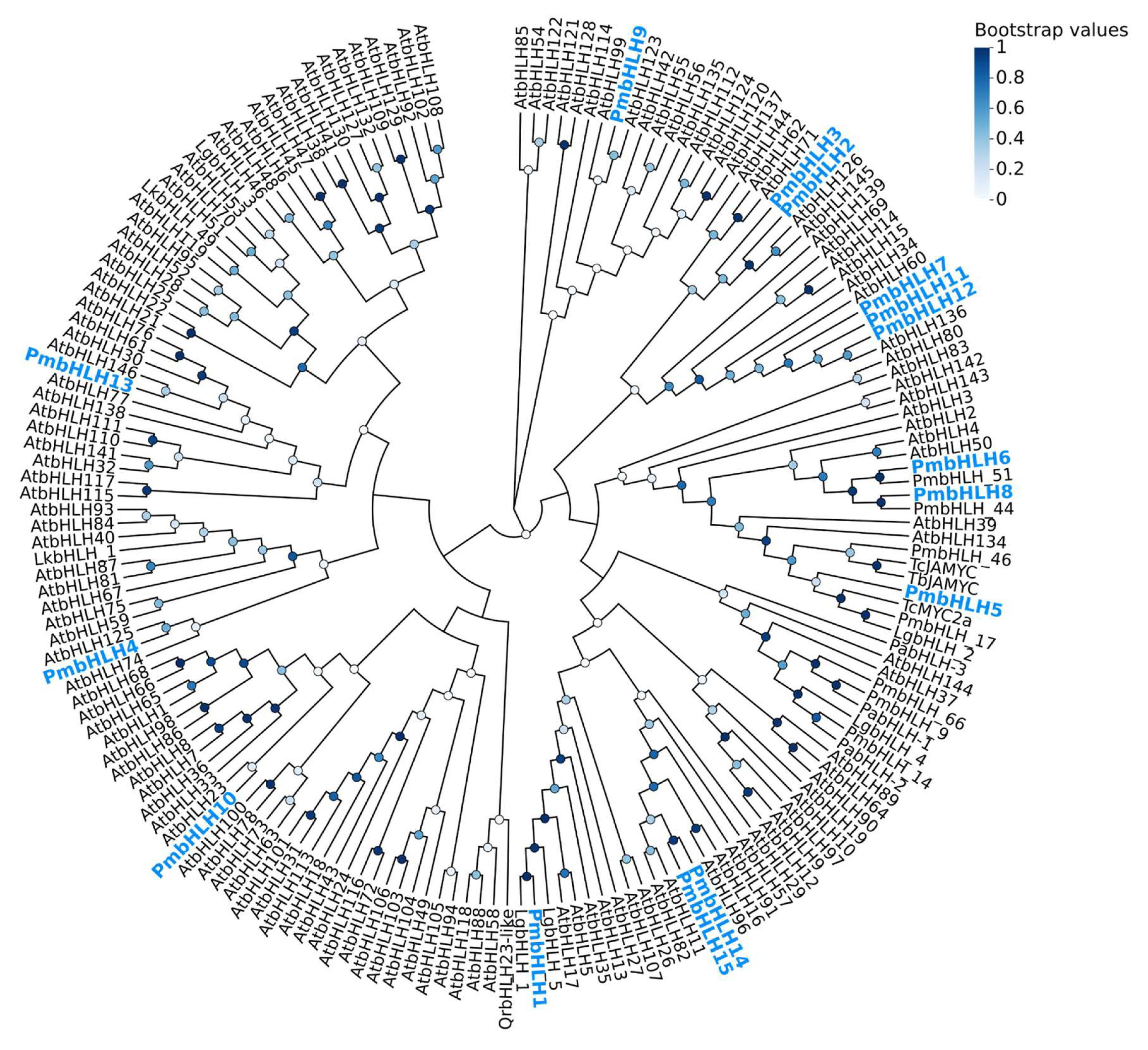
2.4. Identification of bHLH Transcription Factors of DEGs in P. massoniana
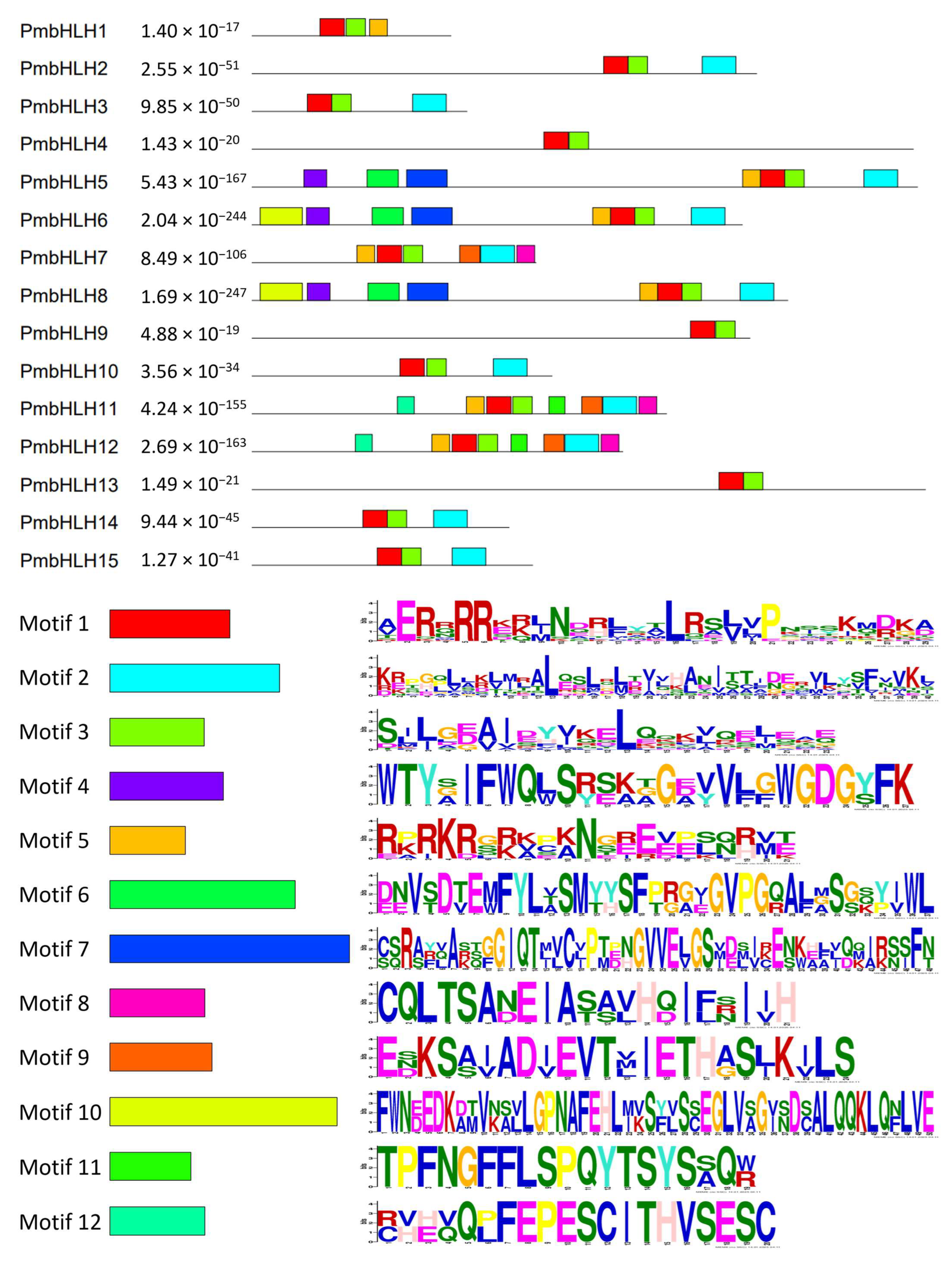
2.5. Protein Motif Analysis
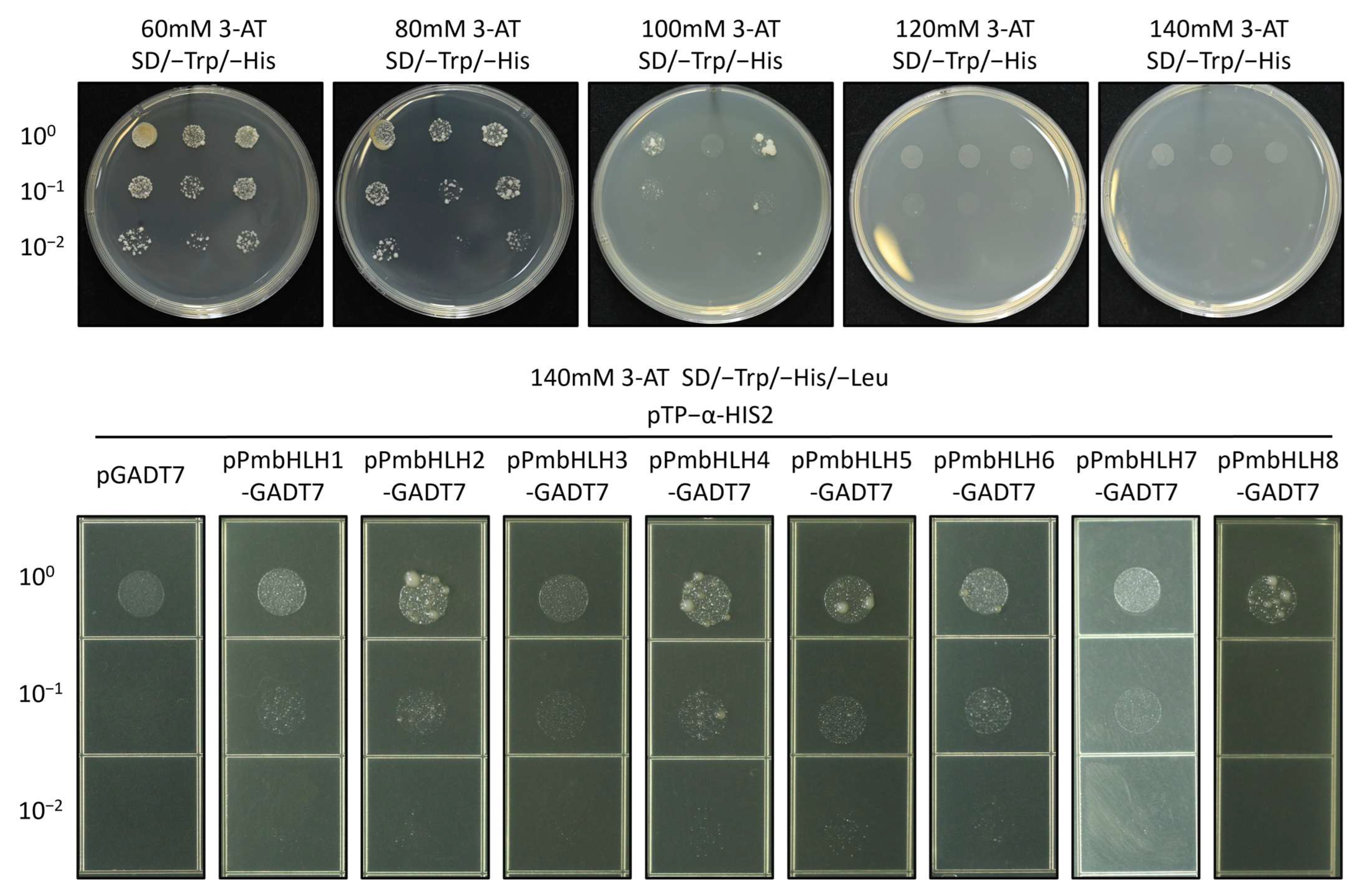
2.6. Yeast One-Hybrid (Y1H) Assay of the Interaction of PmbHLHs with Pm TPS (−)-α-Pinene Promoter
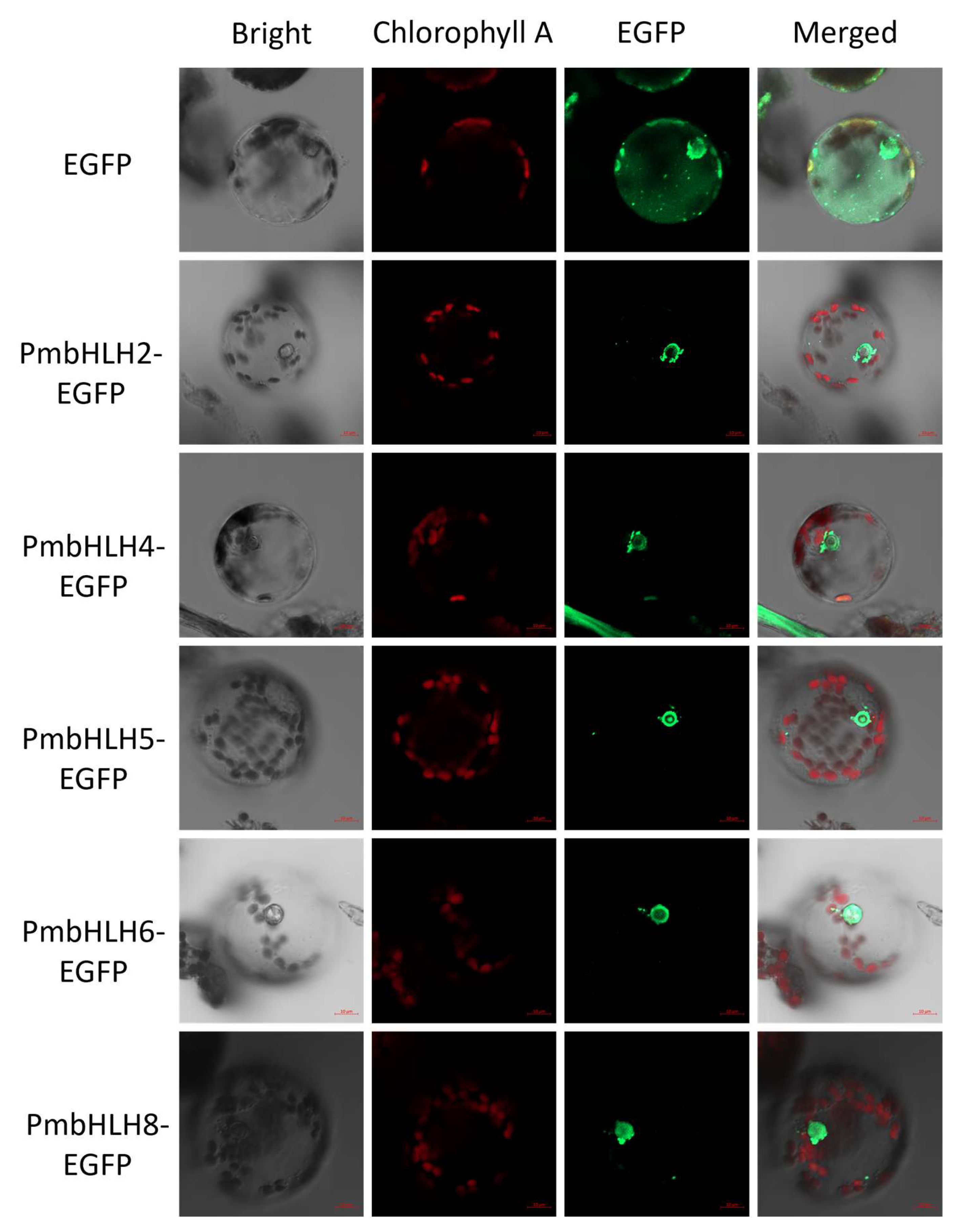
2.7. Subcellular Localization Analysis
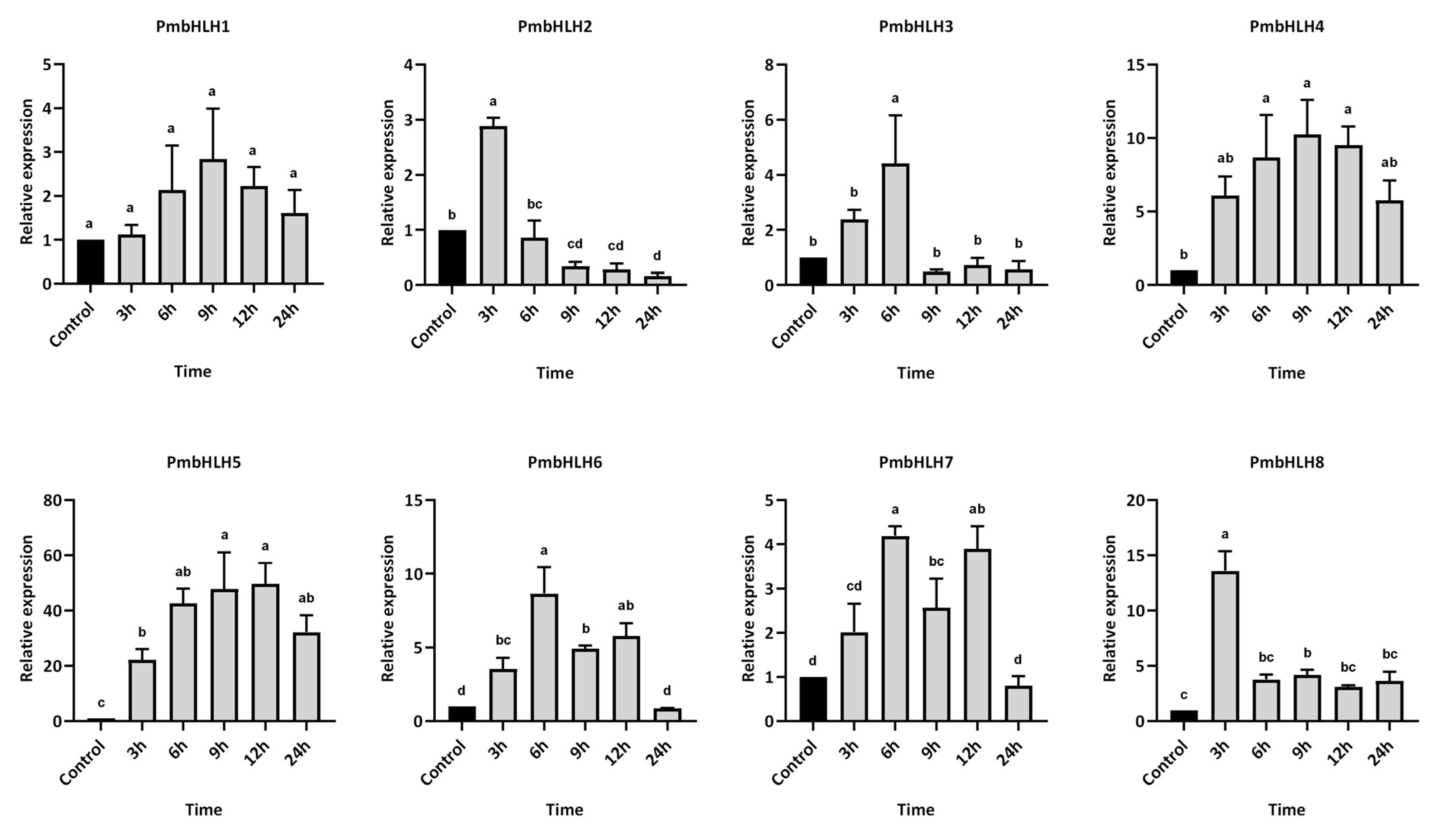
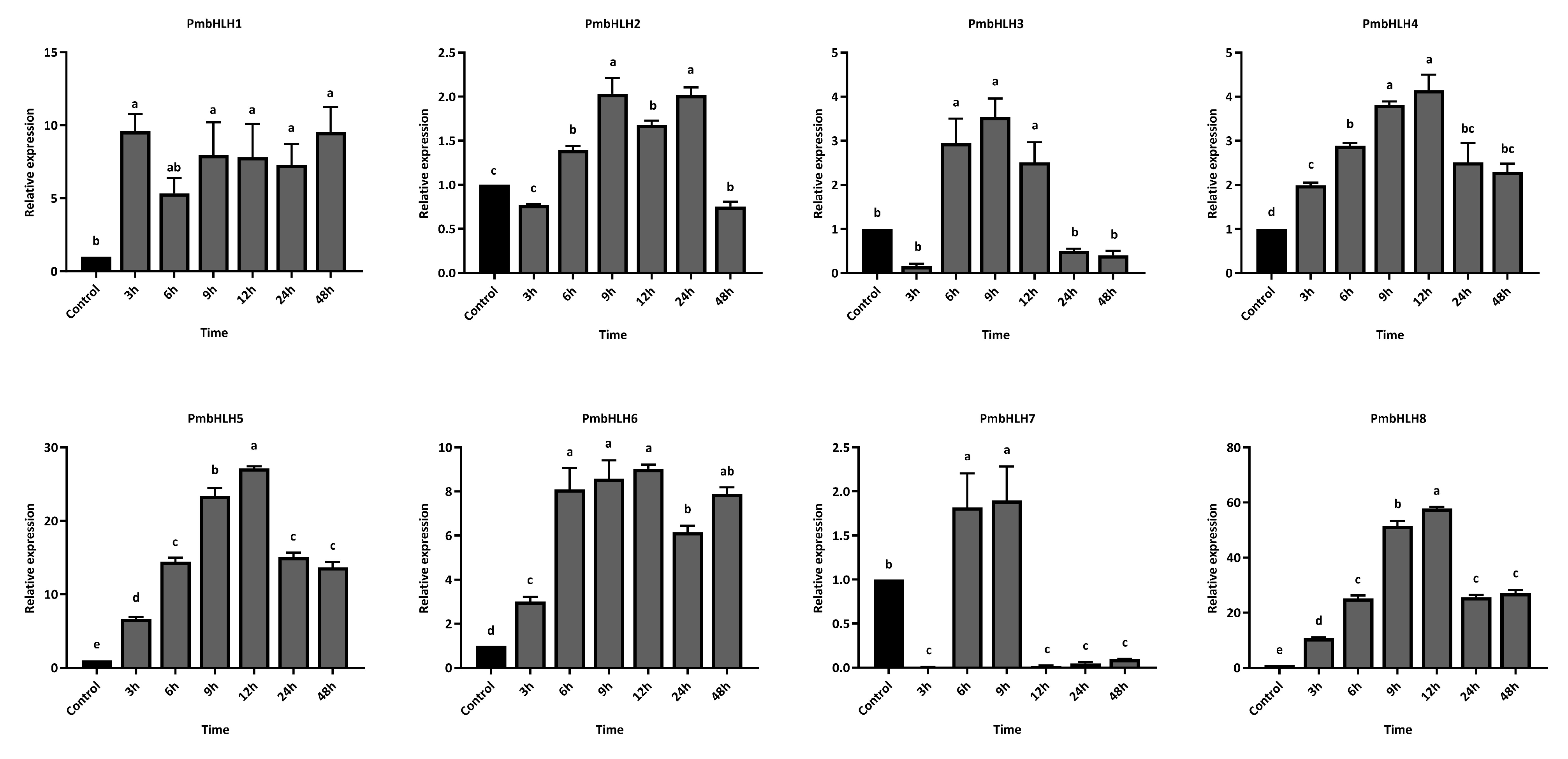
2.8. Gene Expression Analysis Under MeJA and M. alternatus Adult Feeding Treatment
3. Discussion
4. Materials and Methods
4.1. Plant and Insect Materials
4.2. MeJA Treatment and Feeding Treatment
4.3. RNA Isolation and Assessment
4.4. NGS Library Construction and Sequencing
4.5. Full-Length Transcriptome Library (PacBio) Preparation and Sequencing
4.6. Transcriptome Data Processing
4.7. Bioinformatics Analysis
4.8. Y1H Assay
4.9. Protoplast Transfection
4.10. qRT-PCR
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Pm | Pinus massoniana |
| MeJA | Methyl jasmonate |
| bHLH | basic helix–loop–helix |
| NGS | Next-generation sequencing |
| DEG | Differentially expressed gene |
| TF | Transcription factor |
| Y1H | Yeast one-hybrid |
| TPS | Terpene synthase |
| 3-AT | 3-aminotriazole |
| EGFP | Enhanced green fluorescent protein |
References
- Trapp, S.; Croteau, R. Defensive resin biosynthesis in conifers. Annu. Rev. Plant Biol. 2001, 52, 689–724. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, J. Pine terpenoid defences in the mountain pine beetle epidemic and in other conifer pest interactions: Specialized enemies are eating holes into a diverse, dynamic and durable defence system. Tree Physiol. 2012, 32, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Madilao, L.L.; Ralph, S.; Bohlmann, J. Insect-induced conifer defense. White pine weevil and methyl jasmonate induce traumatic resinosis, de novo formed volatile emissions, and accumulation of terpenoid synthase and putative octadecanoid pathway transcripts in Sitka spruce. Plant Physiol. 2005, 137, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; He, X.; Chen, J.; Gu, T.; Liu, P.; Xu, T.; Teale, S.A.; Hao, D. Traumatic Resin Duct Development, Terpenoid Formation, and Related Synthase Gene Expression in Pinus massoniana Under Feeding Pressure of Monochamus alternatus. J. Plant Growth Regul. 2018, 38, 897–908. [Google Scholar] [CrossRef]
- Hamberger, B.; Ohnishi, T.; Hamberger, B.; Séguin, A.; Bohlmann, J. Evolution of diterpene metabolism: Sitka spruce CYP720B4 catalyzes multiple oxidations in resin acid biosynthesis of conifer defense against insects. Plant Physiol. 2011, 157, 1677–1695. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Conrath, U. Priming of Induced Plant Defense Responses. Adv. Bot. Res. 2009, 51, 361–395. [Google Scholar]
- Phillips, M.A.; Croteau, R.B. Resin-based defenses in conifers. Trends Plant Sci. 1999, 4, 184–190. [Google Scholar] [CrossRef]
- Sheard, L.B.; Tan, X.; Mao, H.; Withers, J.; Ben-Nissan, G.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.-F.; Sharon, M.; Browse, J. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 2010, 468, 400–405. [Google Scholar] [CrossRef]
- Santner, A.; Estelle, M. Recent advances and emerging trends in plant hormone signalling. Nature 2009, 459, 1071–1078. [Google Scholar] [CrossRef]
- Vanstraelen, M.; Benková, E. Hormonal Interactions in the Regulation of Plant Development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.; Chico, J.M.; Solano, R. The jasmonate pathway: The ligand, the receptor and the core signalling module. Curr. Opin. Plant Biol. 2009, 12, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Monte, I. Jasmonates and salicylic acid: Evolution of defense hormones in land plants. Curr. Opin. Plant Biol. 2023, 76, 102470. [Google Scholar] [CrossRef] [PubMed]
- Mageroy, M.H.; Wilkinson, S.W.; Tengs, T.; Cross, H.; Almvik, M.; Pétriacq, P.; Vivian-Smith, A.; Zhao, T.; Fossdal, C.G.; Krokene, P. Molecular underpinnings of methyl jasmonate-induced resistance in Norway spruce. Plant Cell Environ. 2020, 43, 1827–1843. [Google Scholar] [CrossRef]
- Anurag, A. Induced resistance for plant defense: A sustainable approach to crop protection. Q. Rev. Biol. 2008, 83, 221. [Google Scholar]
- Tianzi, G.; Congcong, Z.; Changyu, C.; Hui, L.; Kairu, H.; Shuo, T.; Xudong, Z.; Dejun, H. Effects of exogenous methyl jasmonate-induced resistance in Populus× euramericana ‘Nanlin895’on the performance and metabolic enzyme activities of Clostera anachoreta. Arthropod-Plant Interact. 2018, 12, 247–255. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. Effect of methyl jasmonate (MeJA)-induced defenses in rice against the rice leaffolder Cnaphalocrocis medinalis (Guenèe)(Lepidoptera: Pyralidae). Pest. Manag. Sci. 2019, 75, 460–465. [Google Scholar] [CrossRef]
- Fang, J.; Gan, W.; Wang, Z.; Zhang, R.; Zhang, S.; Liu, F.; Zhao, X.; Kong, X. Induction of antiherbivore defense responses in poplars using a methyl jasmonate and mesoporous silica nanoparticle complex. Pest. Manag. Sci. 2024, 80, 6310–6321. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Wang, J.; Wu, D.; Wang, Y.; Xie, D. Jasmonate action in plant defense against insects. J. Exp. Bot. 2019, 70, 3391–3400. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Melotto, M.; Yao, J.; He, S.Y. Jasmonate signaling and manipulation by pathogens and insects. J. Exp. Bot. 2017, 68, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calvo, P.; Chini, A.; Fernández-Barbero, G.; Chico, J.-M.; Gimenez-Ibanez, S.; Geerinck, J.; Eeckhout, D.; Schweizer, F.; Godoy, M.; Franco-Zorrilla, J.M.; et al. The Arabidopsis bHLH Transcription Factors MYC3 and MYC4 Are Targets of JAZ Repressors and Act Additively with MYC2 in the Activation of Jasmonate Responses. Plant Cell 2011, 23, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Stolz, S.; Chetelat, A.; Reymond, P.; Pagni, M.; Dubugnon, L.; Farmer, E.E. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 2007, 19, 2470–2483. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Y.; Liu, L.; Ma, K.S.; Wang, W.J.; Lv, H.M.; Gao, M.; Wang, X.M.; Zhang, X.C.; Ren, S.X.; Zhang, N.; et al. The jasmonate-induced bHLH gene functions in terpene biosynthesis and resistance to insects and fungus. J. Integr. Plant Biol. 2022, 64, 1102–1115. [Google Scholar] [CrossRef]
- Tan, Z.; Lu, D.; Yu, Y.; Li, L.; Dong, W.; Xu, L.; Yang, Q.; Wan, X.; Liang, H. Genome-Wide Identification and Characterization of the bHLH Gene Family and Its Response to Abiotic Stresses in Carthamus tinctorius. Plants 2023, 12, 3764. [Google Scholar] [CrossRef]
- Gao, F.; Dubos, C. The arabidopsis bHLH transcription factor family. Trends Plant Sci. 2024, 29, 668–680. [Google Scholar] [CrossRef]
- Xu, F.; Tang, J.; Wang, S.; Cheng, X.; Wang, H.; Ou, S.; Gao, S.; Li, B.; Qian, Y.; Gao, C. Antagonistic control of seed dormancy in rice by two bHLH transcription factors. Nat. Genet. 2022, 54, 1972–1982. [Google Scholar] [CrossRef]
- Zumajo-Cardona, C.; Gabrieli, F.; Anire, J.; Albertini, E.; Ezquer, I.; Colombo, L. Evolutionary studies of the bHLH transcription factors belonging to MBW complex: Their role in seed development. Ann. Bot. 2023, 132, 383–400. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, J.; Wu, Y.; Wang, Y.; Zhang, J.; Zheng, Y.; Li, Y.; Li, X. bHLH transcription factors LP1 and LP2 regulate longitudinal cell elongation. Plant Physiol. 2021, 187, 2577–2591. [Google Scholar] [CrossRef]
- Zuch, D.T.; Herrmann, A.; Kim, E.-D.; Torii, K.U. Cell Cycle Dynamics during Stomatal Development: Window of MUTE Action and Ramification of Its Loss-of-Function on an Uncommitted Precursor. Plant Cell Physiol. 2023, 64, 325–335. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Dong, J. Establishing asymmetry: Stomatal division and differentiation in plants. New Phytol. 2021, 232, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, X.; Li, K.; Klejnot, J.; Yang, H.; Lisiero, D.; Lin, C. Photoexcited CRY2 Interacts with CIB1 to Regulate Transcription and Floral Initiation in Arabidopsis. Science 2008, 322, 1535–1539. [Google Scholar] [CrossRef]
- Hao, Y.; Oh, E.; Choi, G.; Liang, Z.; Wang, Z.-Y. Interactions between HLH and bHLH Factors Modulate Light-Regulated Plant Development. Mol. Plant 2012, 5, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Mitsuda, N.; Herde, M.; Koo, A.J.; Moreno, J.E.; Suzuki, K.; Howe, G.A.; Ohme-Takagi, M. A bHLH-Type Transcription Factor, ABA-INDUCIBLE BHLH-TYPE TRANSCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, Acts as a Repressor to Negatively Regulate Jasmonate Signaling in Arabidopsis. Plant Cell 2013, 25, 1641–1656. [Google Scholar] [CrossRef]
- Song, S.; Qi, T.; Fan, M.; Zhang, X.; Gao, H.; Huang, H.; Wu, D.; Guo, H.; Xie, D. The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development. PLos Genet. 2013, 9, e1003653. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Pan, J.; Lou, D.; Hu, Y.; Yu, D. The bHLH transcription factors MYC2, MYC3, and MYC4 are required for jasmonate-mediated inhibition of flowering in Arabidopsis. Mol. Plant 2017, 10, 1461–1464. [Google Scholar] [CrossRef]
- Sánchez-Pérez, R.; Pavan, S.; Mazzeo, R.; Moldovan, C.; Aiese Cigliano, R.; Del Cueto, J.; Ricciardi, F.; Lotti, C.; Ricciardi, L.; Dicenta, F. Mutation of a bHLH transcription factor allowed almond domestication. Science 2019, 364, 1095–1098. [Google Scholar] [CrossRef]
- Chen, C.; Liu, F.; Zhang, K.X.; Niu, X.L.; Zhao, H.; Liu, Q.X.; Georgiev, M.; Xu, X.H.; Zhang, X.Q.; Zhou, M.L. MeJA-responsive bHLH transcription factor LjbHLH7 regulates cyanogenic glucoside biosynthesis in Lotus japonicus. J. Exp. Bot. 2022, 73, 2650–2665. [Google Scholar] [CrossRef]
- Li, X.; Cao, L.; Jiao, B.; Yang, H.; Ma, C.; Liang, Y. The bHLH transcription factor AcB2 regulates anthocyanin biosynthesis in onion (Allium cepa L.). Hortic. Res. 2022, 9, uhac128. [Google Scholar] [CrossRef]
- Broucke, E.; Dang, T.T.V.; Li, Y.; Hulsmans, S.; Van Leene, J.; De Jaeger, G.; Hwang, I.; Wim, V.d.E.; Rolland, F. SnRK1 inhibits anthocyanin biosynthesis through both transcriptional regulation and direct phosphorylation and dissociation of the MYB/bHLH/TTG1 MBW complex. Plant J. 2023, 115, 1193–1213. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, W.; Li, J.; Wang, D.; Bai, H.; Li, H.; Shi, L. The transcription factor LaMYC4 from lavender regulates volatile Terpenoid biosynthesis. BMC Plant Biol. 2022, 22, 289. [Google Scholar] [CrossRef] [PubMed]
- Koini, M.A.; Alvey, L.; Allen, T.; Tilley, C.A.; Harberd, N.P.; Whitelam, G.C.; Franklin, K.A. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr. Biol. 2009, 19, 408–413. [Google Scholar] [CrossRef]
- Liu, W.; Tai, H.; Li, S.; Gao, W.; Zhao, M.; Xie, C.; Li, W.X. bHLH 122 is important for drought and osmotic stress resistance in A rabidopsis and in the repression of ABA catabolism. New Phytol. 2014, 201, 1192–1204. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Vishal, B.; Khoo, K.; Rajappa, S.; Loh, C.-S.; Kumar, P.P. Expression of AoNHX1 increases salt tolerance of rice and Arabidopsis, and bHLH transcription factors regulate AtNHX1 and AtNHX6 in Arabidopsis. Plant Cell Rep. 2019, 38, 1299–1315. [Google Scholar] [CrossRef]
- Long, T.A.; Tsukagoshi, H.; Busch, W.; Lahner, B.; Salt, D.E.; Benfey, P.N. The bHLH Transcription Factor POPEYE Regulates Response to Iron Deficiency in Arabidopsis Roots. Plant Cell 2010, 22, 2219–2236. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Li, M.; Feng, D.; Jin, H.; Wang, P.; Liu, J.; Xiong, F.; Wang, J.; Wang, H.-B. The bHLH transcription factor bHLH104 interacts with IAA-LEUCINE RESISTANT3 and modulates iron homeostasis in Arabidopsis. Plant Cell 2015, 27, 787–805. [Google Scholar] [CrossRef]
- Aparicio, F.; Pallás, V. The coat protein of Alfalfa mosaic virus interacts and interferes with the transcriptional activity of the bHLH transcription factor ILR3 promoting salicylic acid-dependent defence signalling response. Mol. Plant Pathol. 2017, 18, 173–186. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, X.; Nie, X.; Qu, M.; Zheng, L.; Tan, Z.; Zhao, H.; Huo, L.; Liu, S.; Zhang, B. Arabidopsis Atb HLH 112 regulates the expression of genes involved in abiotic stress tolerance by binding to their E-box and GCG-box motifs. New Phytol. 2015, 207, 692–709. [Google Scholar] [CrossRef]
- Guo, P.; Cheng, X.; Wang, Y.; Chen, G.; Chen, X.; Yang, Y.; Zhang, X.; Hu, Z. SlUPA-like, a bHLH Transcription Factor in Tomato (Solanum lycopersicum), Serves as the Crosstalk of GA, JA and BR. Int. J. Mol. Sci. 2024, 25, 13419. [Google Scholar] [CrossRef]
- Feller, A.; Machemer, K.; Braun, E.L.; Grotewold, E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011, 66, 94–116. [Google Scholar] [CrossRef]
- Lu, Y.; Ran, J.; Guo, D.; Yang, Z.; Wang, X. Phylogeny and divergence times of gymnosperms inferred from single-copy nuclear genes. PLoS ONE 2014, 9, e107679. [Google Scholar] [CrossRef] [PubMed]
- Nemesio-Gorriz, M.; Blair, P.B.; Dalman, K.; Hammerbacher, A.; Arnerup, J.; Stenlid, J.; Mukhtar, S.M.; Elfstrand, M. Identification of Norway spruce MYB-bHLH-WDR transcription factor complex members linked to regulation of the flavonoid pathway. Front. Plant Sci. 2017, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Brueggemann, J.; Weisshaar, B.; Sagasser, M. A WD40-repeat gene from Malus × domestica is a functional homologue of Arabidopsis thaliana TRANSPARENT TESTA GLABRA1. Plant Cell Rep. 2010, 29, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, P.; Uimari, A.; Mehto, M.; Albert, V.A.; Laitinen, R.A.E.; Teeri, T.H. Activation of Anthocyanin Biosynthesis in Gerbera hybrida (Asteraceae) Suggests Conserved Protein-Protein and Protein-Promoter Interactions between the Anciently Diverged Monocots and Eudicots. Plant Physiol. 2003, 133, 1831–1842. [Google Scholar] [CrossRef]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Haruta, K.S.; Pitaksutheepong, C.; Abe, Y.; Kakizaki, Y.; Yamamoto, K.; Shimada, N.; Yamamura, S.; Nishihara, M. Identification and characterization of R2R3-MYB and bHLH transcription factors regulating anthocyanin biosynthesis in gentian flowers. Plant Cell Physiol. 2008, 49, 1818–1829. [Google Scholar] [CrossRef]
- Ralph, S.G.; Yueh, H.; Friedmann, M.; Aeschliman, D.; Zeznik, J.A.; Nelson, C.C.; Butterfield, Y.S.; Kirkpatrick, R.; Liu, J.; Jones, S.J. Conifer defence against insects: Microarray gene expression profiling of Sitka spruce (Picea sitchensis) induced by mechanical wounding or feeding by spruce budworms (Choristoneura occidentalis) or white pine weevils (Pissodes strobi) reveals large-scale changes of the host transcriptome. Plant Cell Environ. 2006, 29, 1545–1570. [Google Scholar]
- Delhomme, N.; García-Gil, M.R.; Ranade, S.S. Transcriptome analysis of shade avoidance and shade tolerance in conifers. Planta 2019, 250, 299–318. [Google Scholar]
- Liu, H.; Zhou, C.; Nisa, Z.U.; El-Kassaby, Y.A.; Li, W. Exogenous 6-BA inhibited hypocotyl elongation under darkness in Picea crassifolia Kom revealed by transcriptome profiling. Front. Plant Sci. 2023, 14, 1086879. [Google Scholar] [CrossRef]
- Guo, Y.; Deng, C.; Feng, G.; Liu, D. Genome-wide analysis of phytochrome-interacting factor (PIF) families and their potential roles in light and gibberellin signaling in Chinese pine. BMC Genom. 2024, 25, 1017. [Google Scholar] [CrossRef]
- Ranade, S.S.; Delhomme, N.; Garcia-Gil, M.R. Global gene expression analysis in etiolated and de-etiolated seedlings in conifers. PLoS ONE 2019, 14, e0219272. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, R.; Yang, Y.; Luo, Y.; Wei, G.; Bian, L.; Xu, J. Integrative analysis of the transcriptome, targeted metabolome, and anatomical observation provides insights into the brassinosteroids-mediated seasonal variation of cambial activity in Chinese fir. Ind. Crops Prod. 2024, 222, 119977. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, M.; Cai, K.; Liu, L.; Han, R.; Pei, X.; Zhang, L.; Zhao, X. Phytohormone biosynthesis and transcriptional analyses provide insight into the main growth stage of male and female cones Pinus koraiensis. Front. Plant Sci. 2023, 14, 1273409. [Google Scholar] [CrossRef]
- Housset, J.M.; Nadeau, S.; Isabel, N.; Depardieu, C.; Duchesne, I.; Lenz, P.; Girardin, M.P. Tree rings provide a new class of phenotypes for genetic associations that foster insights into adaptation of conifers to climate change. New Phytol. 2018, 218, 630–645. [Google Scholar] [CrossRef]
- Lenka, S.K.; Nims, N.E.; Vongpaseuth, K.; Boshar, R.A.; Roberts, S.C.; Walker, E.L. Jasmonate-responsive expression of paclitaxel biosynthesis genes in Taxus cuspidata cultured cells is negatively regulated by the bHLH transcription factors TcJAMYC1, TcJAMYC2, and TcJAMYC4. Front. Plant Sci. 2015, 6, 115. [Google Scholar] [CrossRef]
- Bai, Q.; He, B.; Cai, Y.; Lian, H.; Zhang, Q. Transcriptomic and metabolomic analyses reveal several critical metabolic pathways and candidate genes involved in resin biosynthesis in Pinus massoniana. Mol. Genet. Genom. 2020, 295, 327–341. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Quail, P.H. The Arabidopsis Basic/Helix-Loop-Helix Transcription Factor Family. Plant Cell 2003, 15, 1749–1770. [Google Scholar] [CrossRef]
- Pires, N.; Dolan, L. Origin and diversification of basic-helix-loop-helix proteins in plants. Mol. Biol. Evol. 2010, 27, 862–874. [Google Scholar] [CrossRef]
- Jianren, Y. Epidemic Status of Pine Wilt Disease in China and Its Prevention and Control Techniques and Counter Measures. Sci. Silvae Sin. 2019, 55, 1–10. (In Chinese) [Google Scholar]
- Tahir, S.; Hassan, S.S.; Yang, L.; Ma, M.; Li, C. Detection Methods for Pine Wilt Disease: A Comprehensive Review. Plants 2024, 13, 2876. [Google Scholar] [CrossRef]
- Jianguo, W.; Xu, J.; Qingshu, L.; Jian, F.; Jianjun, W.; Mu, L. Effects of Bursaphelenchus xylophilus Infestation on Physiological Indexes of Larix kaempferi. J. Southwest For. Univ. 2023, 43, 135–140. [Google Scholar]
- Wang, X.N.; Li, S.Z.; Huang, S.T.; Cui, Y.J.; Fu, H.J.; Li, T.; Zhao, W.H.; Yang, X.Y. Pinus massoniana population dynamics: Driving species diversity during the pioneer stage of ecological restoration. Global Ecol. Conserv. 2021, 27, e01593. [Google Scholar] [CrossRef]
- Liu, Q.H.; Zhou, Z.C.; Fan, H.H.; Liu, Y.R. Genetic variation and correlation among resin yield, growth, and morphologic traits of Pinus massoniana. Silvae Genet. 2013, 62, 38–44. [Google Scholar] [CrossRef]
- Zhao, L.L.; Wei, W.; Kang, L.; Sun, J.H. Chemotaxis of the pinewood nematode, Bursaphelenchus xylophilus, to volatiles associated with host pine, Pinus massoniana, and its vector Monochamus alternatus. J. Chem. Ecol. 2007, 33, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.M.; Chen, R.X.; Xu, T.; Hao, D.J. Functional Characterization of Terpene Synthases from Masson Pine (Pinus massoniana) under Feeding of Monochamus alternatus Adults. Forests 2024, 15, 244. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Xie, Y. Overexpression of geranyl diphosphate synthase (PmGPPS1) boosts monoterpene and diterpene production involved in the response to pine wood nematode invasion. Tree Physiol. 2022, 42, 411–424. [Google Scholar] [CrossRef]
- Meng, F.; Li, Y.; Liu, Z.; Feng, Y.; Wang, X.; Zhang, X. Expression of the thaumatin-like protein-1 gene (Bx-tlp-1) from pine wood nematode Bursaphelenchus xylophilus affects terpene metabolism in pine trees. Phytopathology 2022, 112, 888–897. [Google Scholar] [CrossRef]
- Xie, W.; Lai, X.; Wu, Y.; Li, Z.; Zhu, J.; Huang, Y.; Zhang, F. Transcription Factor and Protein Regulatory Network of PmACRE1 in Pinus massoniana Response to Pine Wilt Nematode Infection. Plants 2024, 13, 2672. [Google Scholar] [CrossRef]
- Chen, R.; Huang, K.; Pan, S.; Xu, T.; Tan, J.; Hao, D. Jasmonate induced terpene-based defense in Pinus massoniana depresses Monochamus alternatus adult feeding. Pest. Manag. Sci. 2021, 77, 731–740. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Yi, S.; Wei, Y.; Wang, M. Odorant-binding protein 19 in Monochamus alternatus involved in the recognition of a volatile strongly emitted from ovipositing host pines. Insect Sci. 2024, 31, 134–146. [Google Scholar] [CrossRef]
- Dong, Y.F.; Chen, D.P.; Zhou, S.Y.; Mao, Z.Y.; Fan, J.T. Identification of Attractants from Three Host Plants and How to Improve Attractiveness of Plant Volatiles for Monochamus saltuarius. Plants 2024, 13, 1732. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Xiao, Y.T.; Köllner, T.G.; Zhang, W.N.; Wu, J.X.; Wu, J.; Guo, Y.Y.; Zhang, Y.J. Identification and characterization of (E)-β-caryophyllene synthase and α/β-pinene synthase potentially involved in constitutive and herbivore-induced terpene formation in cotton. Plant Physiol. Biochem. 2013, 73, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Trindade, H.; Sena, I.; Figueiredo, A.C. Characterization of α-pinene synthase gene in Pinus pinaster and P. pinea in vitro cultures and differential gene expression following Bursaphelenchus xylophilus inoculation. Acta Physiol. Plant. 2016, 38, 143. [Google Scholar] [CrossRef]
- Diao, S.; Zhang, Y.; Luan, Q.; Ding, X.; Sun, J.; Jiang, J. Identification of TPS-d subfamily genes and functional characterization of three monoterpene synthases in slash pine. Ind. Crops Prod. 2022, 188, 115609. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Xie, Y.; Wei, Y. Two terpene synthases in resistant Pinus massoniana contribute to defence against Bursaphelenchus xylophilus. Plant Cell Environ. 2020, 44, 257–274. [Google Scholar] [CrossRef]
- Maleki, S.S.; Mohammadi, K.; Ji, K.S. Study on factors influencing transformation efficiency in Pinus massoniana using Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 133, 437–445. [Google Scholar] [CrossRef]
- Riley, P.; Anaon-Cartwight, L.; Cross, J.C. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat. Genet. 1998, 18, 271–275. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J.-R. Global identification, structural analysis and expression characterization of bHLH transcription factors in wheat. BMC Plant Biol. 2017, 17, 90. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, P.; Wu, F.; Wang, X.; Zhang, J.; Ji, K. Identification and characterization of the basic helix-loop-helix transcription factor family in Pinus massoniana. Forests 2020, 11, 1292. [Google Scholar] [CrossRef]
- Ke, Y.-Z.; Wu, Y.-W.; Zhou, H.-J.; Chen, P.; Wang, M.-M.; Liu, M.-M.; Li, P.-F.; Yang, J.; Li, J.-N.; Du, H. Genome-wide survey of the bHLH super gene family in Brassica napus. BMC Plant Biol. 2020, 20, 115. [Google Scholar] [CrossRef]
- Wei, K.; Chen, H. Comparative functional genomics analysis of bHLH gene family in rice, maize and wheat. BMC Plant Biol. 2018, 18, 309. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Li, J.; Bo, W.; Yang, W.; Zuccolo, A.; Giacomello, S.; Chen, X.; Han, F.; Yang, J.; Song, Y. The Chinese pine genome and methylome unveil key features of conifer evolution. Cell 2022, 185, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ren, X.; Zhang, J.; Yu, W.; Yu, Q.; Ji, K. Identification and Analysis of Stress-Associated Protein (SAP) Transcription Factor Family Members in Pinus massoniana. Plants 2025, 14, 1592. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Song, Y.H.; Josephson-Day, A.R.; Miller, R.J.; Breton, G.; Olmstead, R.G.; Imaizumi, T. FLOWERING BHLH transcriptional activators control expression of the photoperiodic flowering regulator CONSTANS in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 3582–3587. [Google Scholar] [CrossRef]
- He, X.; Zhang, W.; Sabir, I.A.; Jiao, C.; Li, G.; Wang, Y.; Zhu, F.; Dai, J.; Liu, L.; Chen, C. The spatiotemporal profile of Dendrobium huoshanense and functional identification of bHLH genes under exogenous MeJA using comparative transcriptomics and genomics. Front. Plant Sci. 2023, 14, 1169386. [Google Scholar] [CrossRef]
- Yin, J.; Li, X.; Zhan, Y.; Li, Y.; Qu, Z.; Sun, L.; Wang, S.; Yang, J.; Xiao, J. Cloning and expression of BpMYC4 and BpbHLH9 genes and the role of BpbHLH9 in triterpenoid synthesis in birch. BMC Plant Biol. 2017, 17, 214. [Google Scholar] [CrossRef]
- Liang, J.; Fang, Y.; An, C.; Yao, Y.; Wang, X.; Zhang, W.; Liu, R.; Wang, L.; Aslam, M.; Cheng, Y. Genome-wide identification and expression analysis of the bHLH gene family in passion fruit (Passiflora edulis) and its response to abiotic stress. Int. J. Biol. Macromol. 2023, 225, 389–403. [Google Scholar] [CrossRef]
- Ali, A.; Khan, N.M.; Jiang, Y.; Zhou, G.; Wan, Y. Comprehensive Genome-Wide Identification and Expression Profiling of bHLH Transcription Factors in Areca catechu Under Abiotic Stress. Int. J. Mol. Sci. 2024, 25, 12936. [Google Scholar] [CrossRef]
- Massari, M.E.; Murre, C. Helix-loop-helix proteins: Regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 2000, 20, 429–440. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Z.; Zhao, T.; Yang, Y.; Chen, T.; Yang, M.; Yu, W.; Zhang, B. Genome-wide analysis of bHLH transcription factor and involvement in the infection by yellow leaf curl virus in tomato (Solanum lycopersicum). BMC Genom. 2015, 16, 39. [Google Scholar] [CrossRef]
- López-Gómez, P.; De La Mora-Franco, D.; Herrera-Ubaldo, H.; Díaz-Quezada, C.; Brieba, L.G.; de Folter, S. Site-Directed Mutagenesis Mediated by Molecular Modeling and Docking and Its Effect on the Protein–Protein Interactions of the bHLH Transcription Factors SPATULA, HECATE1, and INDEHISCENT. Plants 2025, 14, 1756. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Dong, Q.; Li, C.; Liu, C.; Ma, F. Genome wide identification and characterization of apple bHLH transcription factors and expression analysis in response to drought and salt stress. Front. Plant Sci. 2017, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lv, W.; Zhang, H.; Ma, L.; Li, P.; Ge, L.; Li, G. Genome-wide analysis of the basic Helix-Loop-Helix (bHLH) transcription factor family in maize. BMC Plant Biol. 2018, 18, 235. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ohashi-Ito, K.; Bergmann, D.C. Orthologs of Arabidopsis thaliana stomatal bHLH genes and regulation of stomatal development in grasses. Development 2009, 136, 2265–2276. [Google Scholar] [CrossRef]
- Heim, M.A.; Jakoby, M.; Werber, M.; Martin, C.; Weisshaar, B.; Bailey, P.C. The basic helix-loop-helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 2003, 20, 735–747. [Google Scholar] [CrossRef]
- Atchley, W.R.; Terhalle, W.; Dress, A. Positional dependence, cliques, and predictive motifs in the bHLH protein domain. J. Mol. Evol. 1999, 48, 501–516. [Google Scholar] [CrossRef]
- Dönnes, P.; Höglund, A. Predicting Protein Subcellular Localization: Past, Present, and Future. Genom. Proteom. Bioinf. 2004, 2, 209–215. [Google Scholar] [CrossRef]
- Nebenführ, A. Identifying Subcellular Protein Localization with Fluorescent Protein Fusions After Transient Expression in Onion Epidermal Cells; Springer: Berlin/Heidelberg, Germany, 2014; pp. 77–85. [Google Scholar]
- Vogeli-Lange, R.; Wagner, G.J. Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves: Implication of a transport function for cadmium-binding peptides. Plant Physiol. 1990, 92, 1086–1093. [Google Scholar] [CrossRef]
- Trofimov, K.; Ivanov, R.; Eutebach, M.; Acaroglu, B.; Mohr, I.; Bauer, P.; Brumbarova, T. Mobility and localization of the iron deficiency-induced transcription factor bHLH039 change in the presence of FIT. Plant Direct 2019, 3, e00190. [Google Scholar] [CrossRef]
- Pendle, A.F.; Clark, G.P.; Boon, R.; Lewandowska, D.; Lam, Y.W.; Andersen, J.; Mann, M.; Lamond, A.I.; Brown, J.W.; Shaw, P.J. Proteomic analysis of the Arabidopsis nucleolus suggests novel nucleolar functions. Mol. Biol. Cell 2005, 16, 260–269. [Google Scholar] [CrossRef]
- Wang, R.; Wang, R.; Liu, M.; Yuan, W.; Zhao, Z.; Liu, X.; Peng, Y.; Yang, X.; Sun, Y.; Tang, W. Nucleocytoplasmic trafficking and turnover mechanisms of BRASSINAZOLE RESISTANT1 in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2021, 118, e2101838118. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Krylov, D.M.; Makarova, K.S.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; Rao, B.S. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 2004, 5, R7. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Boeckmann, B.; Bairoch, A.; Apweiler, R.; Blatter, M.-C.; Estreicher, A.; Gasteiger, E.; Martin, M.J.; Michoud, K.; O’Donovan, C.; Phan, I. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 2003, 31, 365–370. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Johnson, L.S.; Eddy, S.R.; Portugaly, E. Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinf. 2010, 11, 431. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Boyle, E.I.; Weng, S.A.; Gollub, J.; Jin, H.; Botstein, D.; Cherry, J.M.; Sherlock, G. GO::TermFinder—open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics 2004, 20, 3710–3715. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Thiel, T.; Michalek, W.; Varshney, R.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012, 40, 115. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.-Q.; Liu, X.-Q.; Zhao, S.-Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, 345–349. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, 166. [Google Scholar] [CrossRef]
- Xie, J.M.; Chen, Y.R.; Cai, G.J.; Cai, R.L.; Hu, Z.; Wang, H. Tree Visualization By One Table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, 587–592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Q.; Cui, Y.; Xu, T.; Deng, Y.; Hao, D.; Chen, R. Full-Length Transcriptome Sequencing of Pinus massoniana Under Simulated Monochamus alternatus Feeding Highlights bHLH Transcription Factor Involved in Defense Response. Plants 2025, 14, 2038. https://doi.org/10.3390/plants14132038
Wen Q, Cui Y, Xu T, Deng Y, Hao D, Chen R. Full-Length Transcriptome Sequencing of Pinus massoniana Under Simulated Monochamus alternatus Feeding Highlights bHLH Transcription Factor Involved in Defense Response. Plants. 2025; 14(13):2038. https://doi.org/10.3390/plants14132038
Chicago/Turabian StyleWen, Quanmin, Yajie Cui, Tian Xu, Yadi Deng, Dejun Hao, and Ruixu Chen. 2025. "Full-Length Transcriptome Sequencing of Pinus massoniana Under Simulated Monochamus alternatus Feeding Highlights bHLH Transcription Factor Involved in Defense Response" Plants 14, no. 13: 2038. https://doi.org/10.3390/plants14132038
APA StyleWen, Q., Cui, Y., Xu, T., Deng, Y., Hao, D., & Chen, R. (2025). Full-Length Transcriptome Sequencing of Pinus massoniana Under Simulated Monochamus alternatus Feeding Highlights bHLH Transcription Factor Involved in Defense Response. Plants, 14(13), 2038. https://doi.org/10.3390/plants14132038




