Beyond Pairwise Interactions: How Other Species Regulate Competition Between Two Plants?
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Species
4.2. Experimental Design
4.3. Harvest and Measurement
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ninkovic, V.; Rensing, M.; Dahlin, I.; Markovic, D. Who is my neighbor? Volatile cues in plant interactions. Plant Signal. Behav. 2019, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Bascompte, J. Disentangling the web of life. Science 2009, 325, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.J.; Anthelme, F.; et al. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef]
- Adler, P.B.; Smull, D.; Beard, K.H.; Choi, R.T.; Furniss, T.; Kulmatiski, A.; Meiners, J.M.; Tredennick, A.T.; Veblen, K.E. Competition and coexistence in plant communities: Intraspecific competition is stronger than interspecific competition. Ecol. Lett. 2018, 21, 1319–1329. [Google Scholar] [CrossRef]
- Fukano, Y.; Tachiki, Y.; Kasada, M.; Uchida, K. Evolution of competitive traits changes species diversity in a natural field. Proc. R. Soc. B-Biol. Sci. 2022, 289, 8. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Novoplansky, A. Picking battles wisely: Plant behaviour under competition. Plant Cell Environ. 2009, 32, 726–741. [Google Scholar] [CrossRef]
- Golivets, M.; Wallin, K.F. Neighbour tolerance, not suppression, provides competitive advantage to non-native plants. Ecol. Lett. 2018, 21, 745–759. [Google Scholar] [CrossRef]
- Laird, R.A.; Schamp, B.S. Does local competition increase the coexistence of species in intransitive networks? Ecology 2008, 89, 237–247. [Google Scholar] [CrossRef]
- Eigentler, L. Species coexistence in resource-limited patterned ecosystems is facilitated by the interplay of spatial self-organisation and intraspecific competition. Oikos 2021, 130, 609–623. [Google Scholar] [CrossRef]
- Trinder, C.J.; Brooker, R.W.; Robinson, D. Plant ecology’s guilty little secret: Understanding the dynamics of plant competition. Funct. Ecol. 2013, 27, 918–929. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Y.F.; Cheng, J.H.; Cui, G.L.; Huang, Y.; Yang, Z. Warming mitigates the enhancement effect of elevated air CO2 on anti-grazer morphological defense in Scenedesmus obliquus. Sci. Total Environ. 2021, 770, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, C.R.; Wang, Z.C.; Zhang, J.L.; Li, L.J.; Huang, S.; Li, D.H. The species-specific responses of freshwater diatoms to elevated temperatures are affected by interspecific interactions. Microorganisms 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Germain, S.J.; Lutz, J.A. Climate warming may weaken stabilizing mechanisms in old forests. Ecol. Monogr. 2022, 92, 21. [Google Scholar] [CrossRef]
- Bazzaz, F.A.; Jasienski, M.; Thomas, S.C.; Wayne, P. Microevolutionary responses in experimental populations of plants to CO2-enriched environments: Parallel results from two model systems. Proc. Natl. Acad. Sci. USA 1995, 92, 8161–8165. [Google Scholar] [CrossRef]
- Kaciene, G.; Miskelyte, D.; AbdElgawad, H.; Beemster, G.; Asard, H.; Diksaityte, A.; Zaltauskaite, J.; Sujetoviene, G.; Januskaitiene, I.; Juknys, R. O3 pollution in a future climate increases the competition between summer rape and wild mustard. Plant Physiol. Biochem. 2019, 135, 194–205. [Google Scholar] [CrossRef]
- Yu, K.; Saha, M.V.; D’Odorico, P. The effects of interannual rainfall variability on tree-grass composition along kalahari rainfall gradient. Ecosystems 2017, 20, 975–988. [Google Scholar] [CrossRef]
- Cournault, Q.; Colbach, N.; Busset, H.; Matejicek, A.; Souche-Suchovsky, P.; Prudent, M.; Moreau, D. Interspecies diversity in morphological responses to water stress: Study on a panel of weed and crop species. Environ. Exp. Bot. 2024, 224, 11. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.X.; Lin, X.; Gu, S.B.; Wang, D. The effects of intraspecific competition and light transmission within the canopy on wheat yield in a wide-precision planting pattern. J. Integr. Agric. 2020, 19, 1577–1585. [Google Scholar] [CrossRef]
- Liu, S.; Streich, J.; Borevitz, J.O.; Rice, K.J.; Li, T.; Li, B.; Bradford, K.J. Environmental resource deficit may drive the evolution of intraspecific trait variation in invasive plant populations. Oikos 2019, 128, 171–184. [Google Scholar] [CrossRef]
- Sheng, M.; Chen, X.D.; Yu, X.J.; Yan, J.; Zhang, X.L.; Hamel, C.; Sheng, Y.Y.; Tang, M. Neighborhood effects on soil properties, mycorrhizal attributes, tree growth, and nutrient status in afforested zones. Restor. Ecol. 2020, 28, 459–467. [Google Scholar] [CrossRef]
- Li, Y.; Duan, B.L.; Chen, J.; Korpelainen, H.; Niinemets, Ü.; Li, C.Y. Males exhibit competitive advantages over females of Populus deltoides under salinity stress. Tree Physiol. 2016, 36, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shi, H.J.; Kang, X.J.; Liu, C.Q.; Chen, L.C.; Liang, X.F.; Jin, L. Inter- and intra-specific competition of duckweed under multiple heavy metal contaminated water. Aquat. Toxicol. 2017, 192, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Qin, Y.Z.; Chai, Q.; Feng, F.X.; Zhao, C.; Yu, A.Z. Interspecies interactions in relation to root distribution across the rooting profile in wheat-maize intercropping under different plant densities. Front. Plant Sci. 2018, 9, 17. [Google Scholar] [CrossRef]
- Li, Z.W.; Khan, K.; Yang, L.; Pan, Y.Q.; Zhou, X.B. Continuous straw returning combined with nitrogen application improve soil properties and yield of double cropping maize in subtropical regions. Sustainability 2024, 16, 13. [Google Scholar] [CrossRef]
- Hejda, M.; Sádlo, J.; Kutlvasr, J.; Petrík, P.; Vítková, M.; Vojík, M.; Pysek, P.; Pergl, J. Impact of invasive and native dominants on species richness and diversity of plant communities. Preslia 2021, 93, 181–201. [Google Scholar] [CrossRef]
- Driscoll, D.A. Disturbance maintains native and exotic plant species richness in invaded grassy woodlands. J. Veg. Sci. 2017, 28, 573–584. [Google Scholar] [CrossRef]
- Sena, K.; Gauger, L.; Johnson, T.; Shirkey, F.; Caldbeck, W.; Hammock, J.; Kim, A.; Mazza, B.; Pethtel, I.; Leuenberger, W. Honeysuckle (Lonicera maackii) presence is associated with reduced diversity and richness of flowering spring flora in Central Kentucky. Nat. Areas J. 2021, 41, 249–257. [Google Scholar] [CrossRef]
- Gallien, L.; Zimmermann, N.E.; Levine, J.M.; Adler, P.B. The effects of intransitive competition on coexistence. Ecol. Lett. 2017, 20, 791–800. [Google Scholar] [CrossRef]
- Martin, G.D.; Coetzee, J.A.; Compton, S. Plant-herbivore-parasitoid interactions in an experimental freshwater tritrophic system: Higher trophic levels modify competitive interactions between invasive macrophytes. Ecol. Model. 2018, 817, 307–318. [Google Scholar] [CrossRef]
- Kim, T.N.; Underwood, N.; Inouye, B.D. Insect herbivores change the outcome of plant competition through both inter- and intraspecific processes. Ecology 2013, 94, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, A.; Jin, Z.C.; Long, X.L.; Jiang, S.J.; Zhang, Q.; Pan, J.B.; Liu, Y.J.; Feng, H.Y. Arbuscular mycorrhizal fungi alter plant interspecific interaction under nitrogen fertilization. Eur. J. Soil Biol. 2019, 93, 7. [Google Scholar] [CrossRef]
- Vázquez-de-Aldana, B.R.; Zabalgogeazcoa, I.; García-Ciudad, A.; García-Criado, B. An Epichloë endophyte affects the competitive ability of Festuca rubra against other grassland species. Plant Soil 2013, 362, 201–213. [Google Scholar] [CrossRef]
- Simberloff, D.; Von Holle, B. Positive interactions of nonindigenous species: Invasional meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Catford, J.A.; Bode, M.; Tilman, D. Introduced species that overcome life history tradeoffs can cause native extinctions. Nat. Commun. 2018, 9, 2131. [Google Scholar] [CrossRef]
- Peng, L.; Xue, W.; Yu, F.H. Live under strong power: A third plant species alters interspecific interactions between two plant species. Ecol. Indic. 2023, 146, 8. [Google Scholar] [CrossRef]
- Liu, J.N.; Wu, F.R.; Roiloa, S.R.; Xue, W.; Lei, N.F.; Yu, F.H. Emergent plant presence and richness alter competitive interactions between two floating plants. J. Plant Ecol. 2024, 17, 11. [Google Scholar] [CrossRef]
- Liu, J.N.; Wu, F.R.; Xue, W.; Wu, C.P.; Tang, M.; Yu, F.H. Genetic diversity of a dominant plant alters interspecific interactions between two subordinate species and facilitates their coexistence. Environ. Exp. Bot. 2024, 222, 8. [Google Scholar] [CrossRef]
- Arroyo, A.I.; Pueyo, Y.; Saiz, H.; Alados, C.L. Plant–plant interactions and local patterns of diversity from semi-arid to subalpine Mediterranean plant communities. Biodivers. Conserv. 2021, 30, 3481–3508. [Google Scholar] [CrossRef]
- Sotomayor, D.A.; Lortie, C.J. Indirect interactions in terrestrial plant communities: Emerging patterns and research gaps. Ecosphere 2015, 6, 23. [Google Scholar] [CrossRef]
- Chen, X.F.; Qian, L.S.; Shi, H.H.; Zhang, Y.Z.; Song, M.S.; Sun, H.; Chen, J.G. Allelopathic potentials of surrounding vegetation on seedling establishment of alpine cushion Arenaria polytrichoides Edgew. J. Plant Ecol. 2024, 17, 15. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Dong, M.; de Kroon, H. Plasticity in Morphology and Biomass Allocation in Cynodon dactylon, a Grass Species Forming Stolons and Rhizomes. Oikos 1994, 70, 99–106. [Google Scholar] [CrossRef]
- Mohammad, J.S.; Pouria, Z.; Fatemeh, K. Essential oil analysis and phytotoxic activity of catnip (Nepeta cataria L.). Am. J. Essent. Oil Nat. Prod. 2016, 4, 40–45. [Google Scholar]
- Wu, S.N.; Wen, L.; Dong, S.K.; Gao, X.X.; Xu, Y.D.; Li, S.; Dong, Q.M.; Wessell, K. The plant interspecific association in the revegetated alpine grasslands determines the productivity stability of plant community across restoration time on qinghai-tibetan plateau. Front. Plant Sci. 2022, 13, 9. [Google Scholar] [CrossRef]
- Queijeiro-Bolaños, M.E.; González, E.J.; Martorell, C.; Cano-Santana, Z. Competition and facilitation determine dwarf mistletoe infection dynamics. J. Ecol. 2017, 105, 775–785. [Google Scholar] [CrossRef]
- Sun, L.J.; Ataka, M.; Han, M.G.; Han, Y.F.; Gan, D.Y.; Xu, T.L.; Guo, Y.P.; Zhu, B. Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol. 2021, 229, 259–271. [Google Scholar] [CrossRef]
- Bennett, J.A.; Riibak, K.; Tamme, R.; Lewis, R.J.; Pärtel, M. The reciprocal relationship between competition and intraspecific trait variation. J. Ecol. 2016, 104, 1410–1420. [Google Scholar] [CrossRef]
- Guiz, J.; Ebeling, A.; Eisenhauer, N.; Hacker, N.; Hertzog, L.; Oelmann, Y.; Roscher, C.; Wagg, C.; Hillebrand, H. Interspecific competition alters leaf stoichiometry in 20 grassland species. Oikos 2018, 127, 903–914. [Google Scholar] [CrossRef]
- Semchenko, M.; Lepik, A.; Abakumova, M.; Zobel, K. Different sets of belowground traits predict the ability of plant species to suppress and tolerate their competitors. Plant Soil 2018, 424, 157–169. [Google Scholar] [CrossRef]
- Schofield, E.J.; Brooker, R.W.; Rowntree, J.K.; Price, E.A.C.; Brearley, F.Q.; Paterson, E. Plant-plant competition influences temporal dynamism of soil microbial enzyme activity. Soil Biol. Biochem. 2019, 139, 9. [Google Scholar] [CrossRef]
- Liu, Q.; Sterck, F.J.; Zhang, J.L.; Scheire, A.; Konings, E.; Cao, M.; Sha, L.Q.; Poorter, L. Traits, strategies, and niches of liana species in a tropical seasonal rainforest. Oecologia 2021, 196, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Baptist, F.; Aranjuelo, I.; Legay, N.; Lopez-Sangil, L.; Molero, G.; Rovira, P.; Nogués, S. Rhizodeposition of organic carbon by plants with contrasting traits for resource acquisition: Responses to different fertility regimes. Plant Soil 2015, 394, 391–406. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J. A review of processes behind diversity-productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef]
- Zhu, D.H.; Wang, P.; Zhang, W.Z.; Yuan, Y.; Li, B.; Wang, J. Sampling and complementarity effects of plant diversity on resource use increases the invasion resistance of communities. PLoS ONE 2015, 10, 14. [Google Scholar] [CrossRef]
- Freilich, M.A.; Wieters, E.; Broitman, B.R.; Marquet, P.A.; Navarrete, S.A. Species co-occurrence networks: Can they reveal trophic and non-trophic interactions in ecological communities? Ecology 2018, 99, 690–699. [Google Scholar] [CrossRef]
- Allesina, S.; Levine, J.M. A competitive network theory of species diversity. Proc. Natl. Acad. Sci. USA 2011, 108, 5638–5642. [Google Scholar] [CrossRef]
- Adler, P.B.; Seabloom, E.W.; Borer, E.T.; Hillebrand, H.; Hautier, Y.; Hector, A.; Harpole, W.S.; O’Halloran, L.R.; Grace, J.B.; Anderson, T.M.; et al. Productivity is a poor predictor of plant species richness. Science 2011, 333, 1750–1753. [Google Scholar] [CrossRef]
- Castagneri, D.; Vacchiano, G.; Hacket-Pain, A.; DeRose, R.J.; Klein, T.; Bottero, A. Meta-analysis reveals different competition effects on tree growth resistance and resilience to drought. Ecosystems 2022, 25, 30–43. [Google Scholar] [CrossRef]
- Inouye, B.D. Response surface experimental designs for investigating interspecific competition. Ecology 2001, 82, 2696–2706. [Google Scholar] [CrossRef]
- Gibson, D.J.; Connolly, J.; Hartnett, D.C.; Weidenhamer, J.D. Designs for greenhouse studies of interactions between plants. J. Ecol. 1999, 87, 1–16. [Google Scholar] [CrossRef]
- Zhang, X.M.; Jin, Y.; Xue, W.; Gao, J.Q.; Lei, N.F.; Chen, J.S.; Yu, F.H. Clonal transgenerational effects transmit for multiple generations in a floating plant. Phyton-Int. J. Exp. Bot. 2023, 92, 1589–1601. [Google Scholar] [CrossRef]
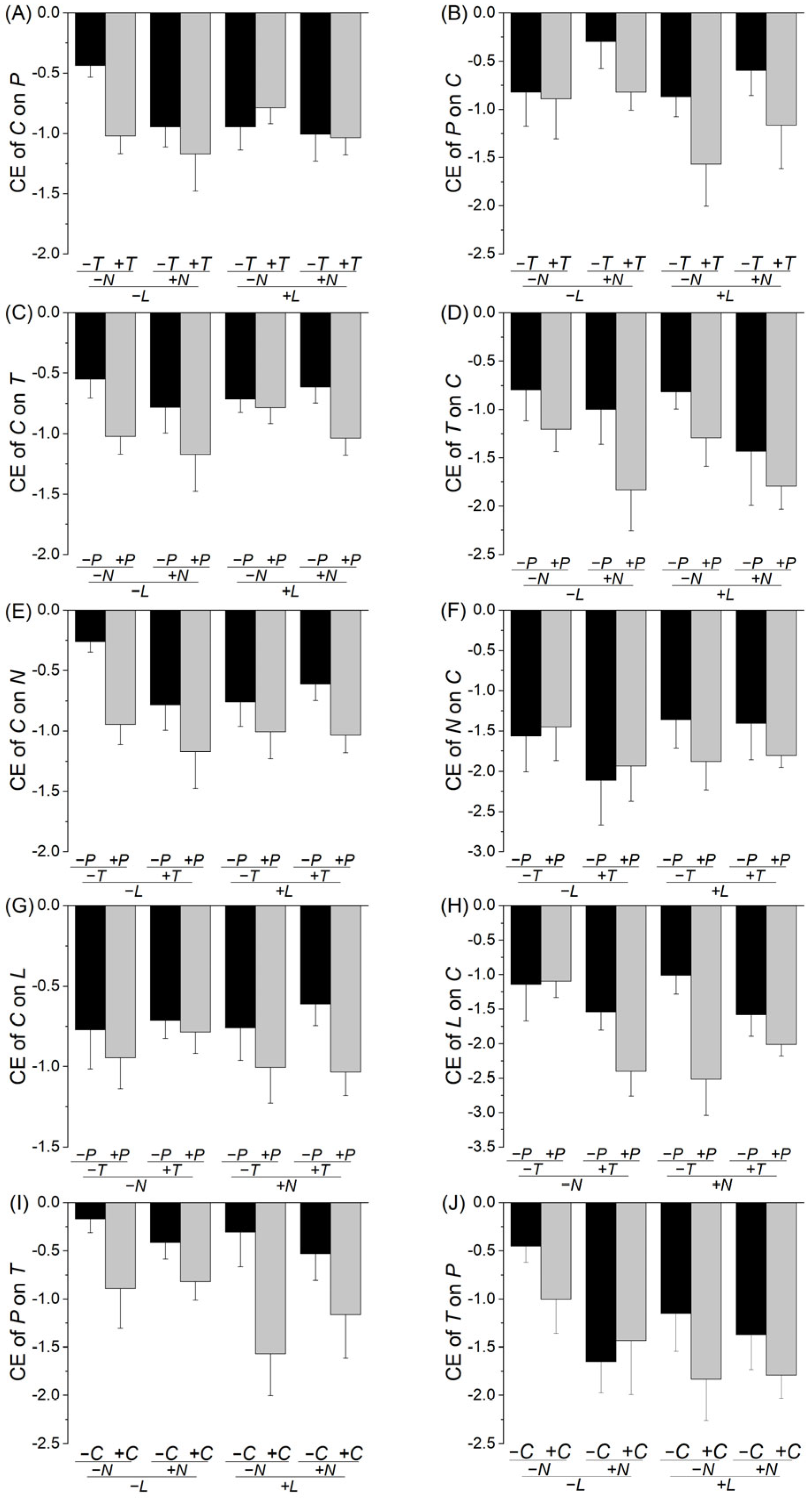
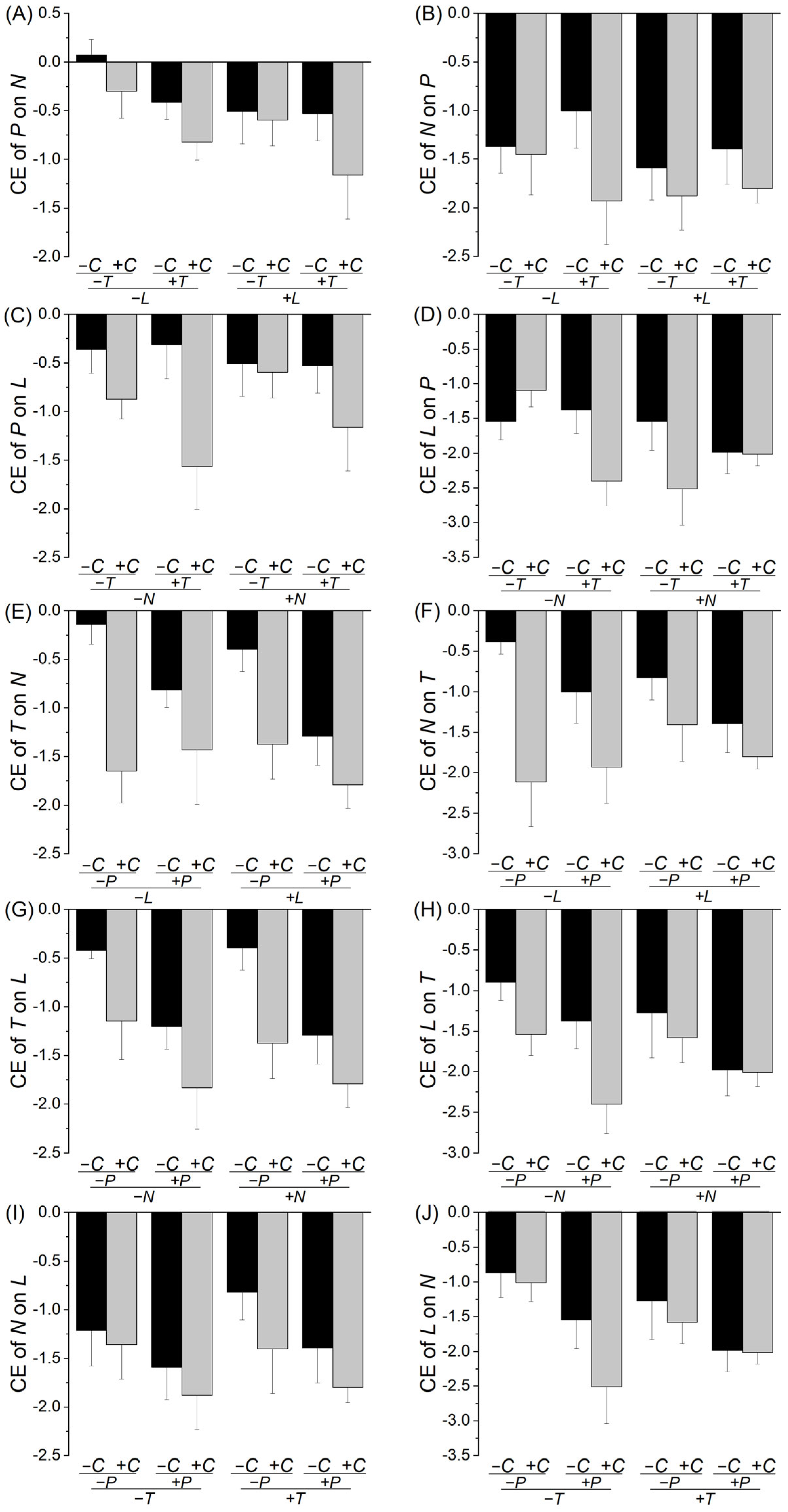
| Effect | F | p | Effect | F | p |
|---|---|---|---|---|---|
| (A) Competitive effect of species C on P | (B) Competitive effect of species P on C | ||||
| T | 1.655 | 0.206 | T | 3.773 | 0.059 |
| N | 3.394 | 0.073 | N | 1.781 | 0.190 |
| L | 0.141 | 0.709 | L | 2.036 | 0.161 |
| T × N | 0.103 | 0.750 | T × N | 0.114 | 0.738 |
| T × L | 3.191 | 0.082 | T × L | 0.489 | 0.488 |
| N × L | 0.437 | 0.512 | N × L | 0.008 | 0.928 |
| T × N × L | 1.075 | 0.306 | T × N × L | 0.370 | 0.547 |
| (C) Competitive effect of species C on T | (D) Competitive effect of species T on C | ||||
| P | 7.243 | 0.010 | P | 3.956 | 0.054 |
| N | 1.130 | 0.294 | N | 0.262 | 0.612 |
| L | 0.554 | 0.461 | L | 4.477 | 0.041 |
| P × N | 0.279 | 0.600 | P × N | 0.085 | 0.772 |
| P × L | 0.536 | 0.468 | P × L | 0.098 | 0.756 |
| P × L | 0.230 | 0.634 | P × L | 0.172 | 0.680 |
| P × N × L | 0.761 | 0.388 | P × N × L | 0.305 | 0.584 |
| (E) Competitive effect of species C on N | (F) Competitive effect of species N on C | ||||
| P | 10.012 | 0.003 | P | 0.287 | 0.595 |
| T | 1.307 | 0.260 | T | 0.730 | 0.398 |
| L | 0.201 | 0.656 | L | 0.280 | 0.599 |
| P × T | 0.048 | 0.827 | P × T | 0.027 | 0.870 |
| P × L | 0.521 | 0.475 | P × L | 1.080 | 0.305 |
| T × L | 2.457 | 0.125 | T × L | 0.837 | 0.366 |
| P × T × L | 0.722 | 0.400 | P × T × L | 0.002 | 0.965 |
| (G) Competitive effect of species C on L | (H) Competitive effect of species L on C | ||||
| P | 3.275 | 0.078 | P | 7.435 | 0.009 |
| T | 0.432 | 0.515 | T | 3.138 | 0.084 |
| N | 0.147 | 0.703 | N | 0.869 | 0.357 |
| P × T | 0.019 | 0.892 | P × T | 0.026 | 0.872 |
| P × N | 0.713 | 0.404 | P × N | 1.229 | 0.274 |
| T × N | 0.038 | 0.845 | T × N | 2.646 | 0.112 |
| P × T × N | 0.300 | 0.587 | P × T × N | 3.867 | 0.056 |
| (I) Competitive effect of species P on T | (J) Competitive effect of species T on P | ||||
| C | 10.705 | 0.002 | C | 1.860 | 0.180 |
| N | <0.001 | 0.988 | N | 2.989 | 0.092 |
| L | 1.892 | 0.177 | L | 2.364 | 0.132 |
| C × N | 1.029 | 0.316 | C × N | 0.956 | 0.334 |
| C × L | 0.672 | 0.417 | C × L | 0.535 | 0.469 |
| N × L | 0.147 | 0.704 | N × L | 1.899 | 0.176 |
| C × N × L | 0.114 | 0.737 | C × N × L | 0.225 | 0.638 |
| (K) Competitive effect of species P on N | (L) Competitive effect of species N on P | ||||
| C | 3.597 | 0.065 | C | 2.963 | 0.093 |
| T | 4.035 | 0.051 | T | 0.026 | 0.873 |
| L | 2.839 | 0.100 | L | 0.830 | 0.368 |
| C × T | 0.538 | 0.468 | C × T | 0.941 | 0.338 |
| C × L | 0.006 | 0.939 | C × L | 0.098 | 0.756 |
| C × L | 0.279 | 0.600 | C × L | 0.151 | 0.699 |
| C × T × L | 0.408 | 0.527 | C × T × L | 0.542 | 0.466 |
| (M) Competitive effect of species P on L | (N) Competitive effect of species L on P | ||||
| C | 7.045 | 0.011 | C | 2.624 | 0.113 |
| T | 1.720 | 0.197 | T | 1.240 | 0.272 |
| N | 0.109 | 0.743 | N | 2.818 | 0.101 |
| C × T | 1.884 | 0.177 | C × T | 0.307 | 0.582 |
| C × N | 1.248 | 0.271 | C × N | 0.190 | 0.665 |
| T × N | 0.004 | 0.951 | T × N | 1.522 | 0.224 |
| C × T × N | 0.046 | 0.832 | C × T × N | 6.193 | 0.017 |
| (O) Competitive effect of species T on N | (P) Competitive effect of species N on T | ||||
| C | 3.834 | 0.057 | C | 11.930 | 0.001 |
| P | 15.772 | <0.001 | P | 1.780 | 0.190 |
| L | 0.805 | 0.375 | L | 0.000 | 0.990 |
| C × P | 2.288 | 0.138 | C × P | 0.856 | 0.360 |
| C × L | 0.876 | 0.355 | C × L | 2.497 | 0.122 |
| P × L | 0.509 | 0.480 | P × L | 0.253 | 0.618 |
| C × P × L | 0.208 | 0.651 | C × P × L | 0.353 | 0.556 |
| (Q) Competitive effect of species T on L | (R) Competitive effect of species L on T | ||||
| C | 10.624 | 0.002 | C | 4.504 | 0.040 |
| P | 11.070 | 0.002 | P | 6.861 | 0.012 |
| N | 0.082 | 0.776 | N | 0.448 | 0.507 |
| C × P | 0.467 | 0.499 | C × P | 0.013 | 0.910 |
| C × N | 0.031 | 0.861 | C × N | 1.985 | 0.167 |
| P × N | 0.021 | 0.886 | P × N | 0.045 | 0.834 |
| C × P × N | 0.196 | 0.660 | C × P × N | 0.494 | 0.486 |
| (S) Competitive effect of species N on L | (T) Competitive effect of species L on N | ||||
| C | 2.181 | 0.148 | C | 1.777 | 0.190 |
| P | 3.715 | 0.061 | P | 9.278 | 0.004 |
| T | 0.411 | 0.525 | T | 0.718 | 0.402 |
| C × P | 0.001 | 0.974 | C × P | 0.253 | 0.618 |
| C × T | 0.323 | 0.573 | C × T | 0.509 | 0.480 |
| P × T | 0.006 | 0.939 | P × T | 0.912 | 0.345 |
| C × P × T | 0.108 | 0.744 | C × P × T | 1.029 | 0.317 |
| Species | Life Form | Morphological Features | Phenology | Typical Habitat |
|---|---|---|---|---|
| Cynodon dactylon (L.) Pers. | Perennial herb | Stems erect or creeping at the base; adventitious roots at nodes; erect parts 10–40 cm tall. | Flowering and fruiting: May–October | Roadsides, field margins, riverbanks, wastelands, hillside grasslands |
| Plantago asiatica L. | Biennial or perennial herb | Leaves basal in a rosette and broadly ovate or oblong; flowers in spikes. | Flowering: April–August; fruiting: June–September | Riverbanks, wetlands, field margins, roadsides, hillside grasslands |
| Taraxacum mongolicum Hand.-Mazz. | Perennial herb | Leaves lanceolate, 4–20 cm long; one to several scapes, 10–25 cm tall; flowers surrounded by a bell-shaped involucre. | Flowering: April–September; fruiting: May–October | Abandoned fields, hillside grasslands, roadsides, and riverbanks. |
| Nepeta cataria L. | Perennial herb | Leaves ovate or triangular-cordate; stems nearly quadrangular at the base, 40–150 cm tall. | Flowering: July–September; fruiting: September–October | Abandoned fields, field margins, roadsides, shrublands, hillside grasslands. |
| Leonurus japonicus Houtt. | Annual or biennial herb | Stems quadrangular with shallow grooves; covered with retrorse hairs, especially dense at nodes and along ridges; usually 30–120 cm tall. | Flowering: June–September; fruiting: September–October | Hillside grasslands, abandoned fields, field margins, roadsides. |
| Trt. No. | C | P | T | N | L | Total Number of Seedlings per Pot |
|---|---|---|---|---|---|---|
| 1 | 1 | - | - | - | - | 1 |
| 2 | 1 | 1 | - | - | - | 2 |
| 3 | 1 | - | 1 | - | - | 2 |
| 4 | 1 | - | - | 1 | - | 2 |
| 5 | 1 | - | - | - | 1 | 2 |
| 6 | 1 | 1 | 1 | - | - | 3 |
| 7 | 1 | 1 | - | 1 | - | 3 |
| 8 | 1 | 1 | - | - | 1 | 3 |
| 9 | 1 | - | 1 | 1 | - | 3 |
| 10 | 1 | - | 1 | - | 1 | 3 |
| 11 | 1 | - | - | 1 | 1 | 3 |
| 12 | 1 | 1 | 1 | 1 | - | 4 |
| 13 | 1 | 1 | 1 | - | 1 | 4 |
| 14 | 1 | 1 | - | 1 | 1 | 4 |
| 15 | 1 | - | 1 | 1 | 1 | 4 |
| 16 | 1 | 1 | 1 | 1 | 1 | 5 |
| 17 | - | 1 | - | - | - | 1 |
| 18 | - | 1 | 1 | - | - | 2 |
| 19 | - | 1 | - | 1 | - | 2 |
| 20 | - | 1 | - | - | 1 | 2 |
| 21 | - | 1 | 1 | 1 | - | 3 |
| 22 | - | 1 | 1 | - | 1 | 3 |
| 23 | - | 1 | - | 1 | 1 | 3 |
| 24 | - | 1 | 1 | 1 | 1 | 4 |
| 25 | - | - | 1 | - | - | 1 |
| 26 | - | - | 1 | 1 | - | 2 |
| 27 | - | - | 1 | - | 1 | 2 |
| 28 | - | - | 1 | 1 | 1 | 3 |
| 29 | - | - | - | 1 | - | 1 |
| 30 | - | - | - | 1 | 1 | 2 |
| 31 | - | - | - | - | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, W.-X.; Xue, W.; Jiao, J.-J.; Yuan, H.-M.; He, L.-X.; Zhang, X.-M.; Xu, T.; Yu, F.-H. Beyond Pairwise Interactions: How Other Species Regulate Competition Between Two Plants? Plants 2025, 14, 2018. https://doi.org/10.3390/plants14132018
Cheng W-X, Xue W, Jiao J-J, Yuan H-M, He L-X, Zhang X-M, Xu T, Yu F-H. Beyond Pairwise Interactions: How Other Species Regulate Competition Between Two Plants? Plants. 2025; 14(13):2018. https://doi.org/10.3390/plants14132018
Chicago/Turabian StyleCheng, Wang-Xin, Wei Xue, Jie-Jie Jiao, Hao-Ming Yuan, Lin-Xuan He, Xiao-Mei Zhang, Tao Xu, and Fei-Hai Yu. 2025. "Beyond Pairwise Interactions: How Other Species Regulate Competition Between Two Plants?" Plants 14, no. 13: 2018. https://doi.org/10.3390/plants14132018
APA StyleCheng, W.-X., Xue, W., Jiao, J.-J., Yuan, H.-M., He, L.-X., Zhang, X.-M., Xu, T., & Yu, F.-H. (2025). Beyond Pairwise Interactions: How Other Species Regulate Competition Between Two Plants? Plants, 14(13), 2018. https://doi.org/10.3390/plants14132018







