Leveraging Biochar Amendments to Enhance Food Security and Plant Resilience Under Climate Change
Abstract
1. Introduction
2. Biochars Mitigate Climate Change for Sustainable Crop Production
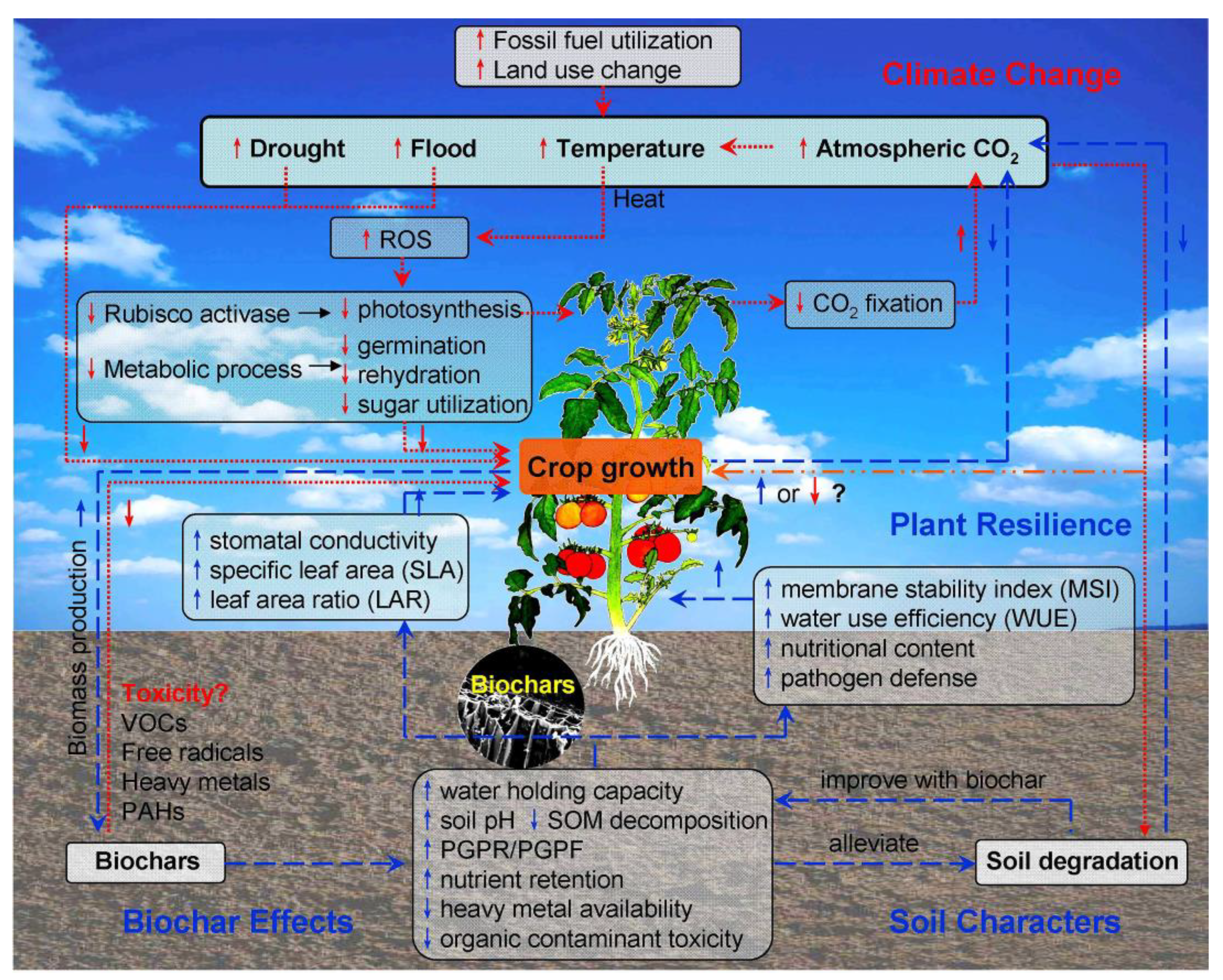
2.1. Effects of Climate Change on Crop Growth and Soil Degradation
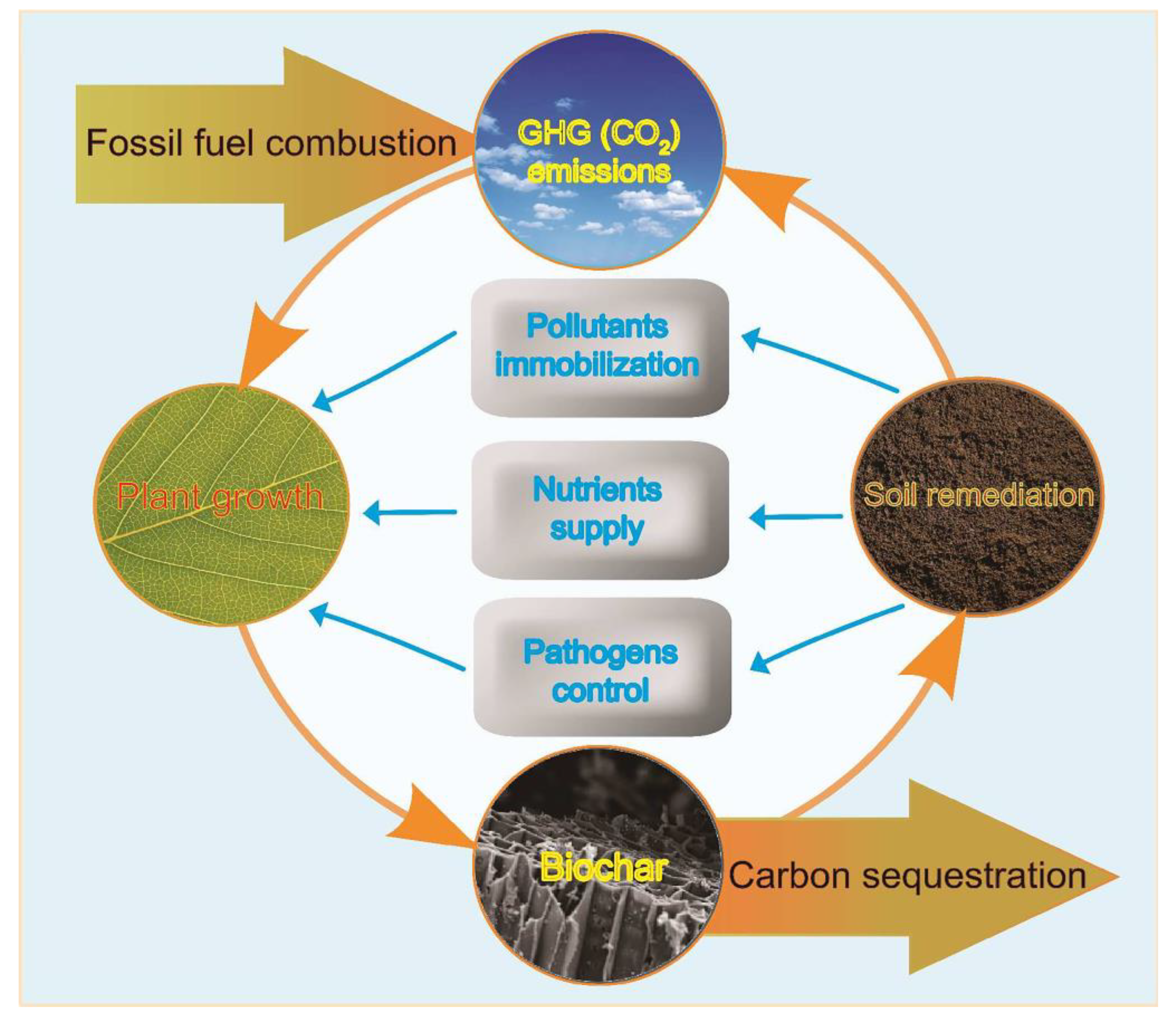
2.2. Biochars Mediate Greenhouse Gas Emissions, Carbon Sequestration, and Plant Responses
2.3. Variability and Standardization Challenges in Biochar Production and Application
3. Biochars Enhance Soil Fertility and Plant Nutrients
4. Biochars Restrain Soil Contaminants to Guarantee Food Safety
4.1. Climate Change Increases Soil Contaminant Availability and Plant Uptake
4.2. Biochars Restrain Crop Uptake and Toxicity of Soil Contaminants
4.2.1. Biochar’s Effects on Bioavailability and Plant Uptake of Heavy Metals
4.2.2. Biochar Effects on Organic Pollutant Toxicity to Plants
4.2.3. Potential Toxicity of Biochar on Plant Growth
5. Biochars Enhance Plant Resistance Against Pathogens
5.1. Climate Change Induces Severer Pest and Pathogen Caused Plant Disease
5.2. Essential Role of Biochars on Plant Resistance Against Pathogens
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Trenberth, K.E. Climate change caused by human activities is happening and it already has major consequences. J. Energy Nat. Resour. Law 2018, 36, 463–481. [Google Scholar] [CrossRef]
- Chiriboga Gavidia, W.G. Greenhouse Gas (CO2, CH4, N2O) Emissions from Ecuadorian Mountainous Streams and High Elevation Lakes. Ph.D. Thesis, University of Liège, Liège, Belgium, 4 May 2023. [Google Scholar]
- Farooqi, Z.U.R.; Sohail, M.; Alserae, H.; Qadir, A.A.; Hussain, T.; Ilic, P.; Riaz, S.; Zafar, Z. Management of Soil Degradation: A Comprehensive Approach for Combating Soil Degradation, Food Insecurity, and Climate Change. In Ecosystem Management: Climate Change and Sustainability; Scrivener Publishing: Austin, TA, USA, 2024; pp. 55–78. [Google Scholar]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Newton, A.C.; Johnson, S.N.; Gregory, P.J. Implications of climate change for diseases, crop yields and food security. Euphytica 2011, 179, 3–18. [Google Scholar] [CrossRef]
- Bastas, K.K. Impact of climate change on food security and plant disease. In Microbial Biocontrol: Food Security and Post Harvest Management; Springer: Berlin/Heidelberg, Germany, 2022; Volume 2, pp. 1–22. [Google Scholar]
- Muluneh, M.G. Impact of climate change on biodiversity and food security: A global perspective—A review article. Agric. Food Secur. 2021, 10, 36. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Jaggard, K.W.; Qi, A.; Ober, E.S. Possible changes to arable crop yields by 2050. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2835–2851. [Google Scholar] [CrossRef]
- Chapman, S.C.; Chakraborty, S.; Dreccer, M.F.; Howden, S.M. Plant adaptation to climate change—Opportunities and priorities in breeding. Crop Pasture Sci. 2012, 63, 251–268. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Kumar, K.; Gambhir, G.; Dass, A.; Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 91. [Google Scholar] [CrossRef]
- Tisserant, A.; Cherubini, F. Potentials, limitations, co-benefits, and trade-offs of biochar applications to soils for climate change mitigation. Land 2019, 8, 179. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Kuryntseva, P.; Karamova, K.; Galitskaya, P.; Selivanovskaya, S.; Evtugyn, G. Biochar functions in soil depending on feedstock and pyrolyzation properties with particular emphasis on biological properties. Agriculture 2023, 13, 2003. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total Environ. 2020, 744, 140714. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Young, P.; Lawrence, J.; Batista, R.; Jensen-Fellows, A.; Richard, B.; Sheridan, T. Biochar Market Profile Report; Worcester Polytechnic Institute: Worcester, MA, USA, 2019; pp. 3–59. [Google Scholar]
- Abhishek, K.; Shrivastava, A.; Vimal, V.; Gupta, A.K.; Bhujbal, S.K.; Biswas, J.K.; Singh, L.; Ghosh, P.; Pandey, A.; Sharma, P. Biochar application for greenhouse gas mitigation, contaminants immobilization and soil fertility enhancement: A state-of-the-art review. Sci. Total Environ. 2022, 853, 158562. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Lee, D.-J.; Siddique, K.H.M. Sustainable agricultural practices for food security and ecosystem services. Environ. Sci. Pollut. Res. 2022, 29, 84076–84095. [Google Scholar] [CrossRef]
- Shah, F.; Wu, W. Soil and crop management strategies to ensure higher crop productivity within sustainable environments. Sustainability 2019, 11, 1485. [Google Scholar] [CrossRef]
- Jakhar, A.M.; Aziz, I.; Kaleri, A.R.; Hasnain, M.; Haider, G.; Ma, J.; Abideen, Z. Nano-fertilizers: A sustainable technology for improving crop nutrition and food security. NanoImpact 2022, 27, 100411. [Google Scholar] [CrossRef]
- Rasool, M.; Akhter, A.; Soja, G.; Haider, M.S. Role of biochar, compost and plant growth promoting rhizobacteria in the management of tomato early blight disease. Sci. Rep. 2021, 11, 6092. [Google Scholar] [CrossRef]
- Fakhar, A.; Galgo, S.J.C.; Canatoy, R.C.; Rafique, M.; Sarfraz, R.; Farooque, A.A.; Khan, M.I. Advancing modified biochar for sustainable agriculture: A comprehensive review on characterization, analysis, and soil performance. Biochar 2025, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Zhang, W.; Xiao, D.; Hu, J.; Li, N.; Yang, J. Biochar-Based Fertilizers: Advancements, Applications, and Future Directions in Sustainable Agriculture—A Review. Agronomy 2025, 15, 1104. [Google Scholar] [CrossRef]
- Iacomino, G.; Idbella, M.; Laudonia, S.; Vinale, F.; Bonanomi, G. The suppressive effects of biochar on above-and belowground plant pathogens and pests: A review. Plants 2022, 11, 3144. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Ren, W.; Tao, H.; Tao, B.; Lindsey, L.E. Impact of biochar amendment on soil microbial biomass carbon enhancement under field experiments: A meta-analysis. Biochar 2025, 7, 2. [Google Scholar] [CrossRef]
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, R.J.; Salvucci, M.E. Rubisco: Structure, regulatory interactions, and possibilities for a better enzyme. Annu. Rev. Plant Biol. 2002, 53, 449–475. [Google Scholar] [CrossRef]
- Fahad, S.; Ihsan, M.Z.; Khaliq, A.; Daur, I.; Saud, S.; Alzamanan, S.; Nasim, W.; Abdullah, M.; Khan, I.A.; Wu, C. Consequences of high temperature under changing climate optima for rice pollen characteristics-concepts and perspectives. Arch. Agron. Soil Sci. 2018, 64, 1473–1488. [Google Scholar] [CrossRef]
- Santiago, J.P.; Sharkey, T.D. Pollen development at high temperature and role of carbon and nitrogen metabolites. Plant. Cell Environ. 2019, 42, 2759–2775. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Hillel, D. Climate Variability and the Global Harvest: Impacts of El Niño and Other Oscillations on Agro-Ecosystems; Oxford University Press: Oxford, UK, 2008; ISBN 0198031475. [Google Scholar]
- Tebaldi, E. The Impacts of El Niño and La Niña on Large Grain Producing Countries in ECA: Yield, Poverty and Policy Response. In Open Knowledge Repository; World Bank Group: Washington, DC, USA, 2018; pp. 1–69. [Google Scholar]
- Jones, M.W.; Peters, G.P.; Gasser, T.; Andrew, R.M.; Schwingshackl, C.; Gütschow, J.; Houghton, R.A.; Friedlingstein, P.; Pongratz, J.; Le Quéré, C. National contributions to climate change due to historical emissions of carbon dioxide, methane, and nitrous oxide since 1850. Sci. Data 2023, 10, 155. [Google Scholar] [CrossRef]
- Janssens-Maenhout, G.; Crippa, M.; Guizzardi, D.; Muntean, M.; Schaaf, E.; Dentener, F.; Bergamaschi, P.; Pagliari, V.; Olivier, J.G.J.; Peters, J.A.H.W. EDGAR v4. 3.2 Global Atlas of the three major Greenhouse Gas Emissions for the period 1970–2012. Earth Syst. Sci. data Discuss. 2017, 2017, 1–55. [Google Scholar]
- Patterson, D.T.; Flint, E.P. Potential effects of global atmospheric CO2 enrichment on the growth and competitiveness of C3 and C4 weed and crop plants. Weed Sci. 1980, 28, 71–75. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Grandis, A.; Arenque, B.C.; Buckeridge, M.S. Impacts of climate changes on crop physiology and food quality. Food Res. Int. 2010, 43, 1814–1823. [Google Scholar] [CrossRef]
- Yaseen, A.A.; Khaleel, N.T.; Al-Azzami, A.A.; Aldossary, A.T.Y.; Ameen, R.A. Climate Change and Its Effect on Nutritional Value: A Review. IOP Conf. Ser. Earth Environ. Sci. 2025, 1449, 12163. [Google Scholar] [CrossRef]
- Woolf, D.; Lehmann, J.; Cowie, A.; Cayuela, M.L.; Whitman, T.; Sohi, S. Biochar for climate change mitigation. In Soil and Climate; CRC Press: Oxfordshire, UK, 2018; pp. 219–248. [Google Scholar]
- Woolf, D.; Lehmann, J.; Ogle, S.; Kishimoto-Mo, A.W.; McConkey, B.; Baldock, J. Greenhouse gas inventory model for biochar additions to soil. Environ. Sci. Technol. 2021, 55, 14795–14805. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Lal, R. Biochar application to soil for climate change mitigation by soil organic carbon sequestration. J. Plant Nutr. Soil Sci. 2014, 177, 651–670. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Zhang, H.; Wang, L.; Zhang, W.; Niu, L.; Wang, P.; Wang, C. The responses of bacterial community and N2O emission to nitrogen input in lake sediment: Estrogen as a co-pollutant. Environ. Res. 2019, 179, 108769. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, H.; Chu, M.; Zhang, C.; Tang, J.; Chang, S.X.; Mašek, O.; Ok, Y.S. Biochar affects greenhouse gas emissions in various environments: A critical review. L. Degrad. Dev. 2022, 33, 3327–3342. [Google Scholar] [CrossRef]
- Shakoor, A.; Arif, M.S.; Shahzad, S.M.; Farooq, T.H.; Ashraf, F.; Altaf, M.M.; Ahmed, W.; Tufail, M.A.; Ashraf, M. Does biochar accelerate the mitigation of greenhouse gaseous emissions from agricultural soil?-A global meta-analysis. Environ. Res. 2021, 202, 111789. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, C.; Li, M.; Zheng, Y.; Ge, C.; Gu, J.; Li, H.; Duan, M.; Wang, X.; Chen, R. Research progress and prospects for using biochar to mitigate greenhouse gas emissions during composting: A review. Sci. Total Environ. 2021, 798, 149294. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, S.; Verheijen, F.G.A.; Kammann, C.; Abalos, D. Biochar effects on methane emissions from soils: A meta-analysis. Soil Biol. Biochem. 2016, 101, 251–258. [Google Scholar] [CrossRef]
- Tarin, M.W.K.; Khaliq, M.A.; Fan, L.; Xie, D.; Tayyab, M.; Chen, L.; He, T.; Rong, J.; Zheng, Y. Divergent consequences of different biochar amendments on carbon dioxide (CO2) and nitrous oxide (N2O) emissions from the red soil. Sci. Total Environ. 2021, 754, 141935. [Google Scholar] [CrossRef] [PubMed]
- Chagas, J.K.M.; de Figueiredo, C.C.; Ramos, M.L.G. Biochar increases soil carbon pools: Evidence from a global meta-analysis. J. Environ. Manag. 2022, 305, 114403. [Google Scholar] [CrossRef]
- Kanan, S.; Samara, F. Dioxins and furans: A review from chemical and environmental perspectives. Trends Environ. Anal. Chem. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Kirkok, S.K.; Kibet, J.K.; Kinyanjui, T.K.; Okanga, F.I. A review of persistent organic pollutants: Dioxins, furans, and their associated nitrogenated analogues. SN Appl. Sci. 2020, 2, 1729. [Google Scholar] [CrossRef]
- Luo, L.; Wang, J.; Lv, J.; Liu, Z.; Sun, T.; Yang, Y.; Zhu, Y.-G. Carbon sequestration strategies in soil using biochar: Advances, challenges, and opportunities. Environ. Sci. Technol. 2023, 57, 11357–11372. [Google Scholar] [CrossRef]
- Sun, J.; Lu, X.; Chen, G.; Luo, N.; Zhang, Q.; Li, X. Biochar promotes soil aggregate stability and associated organic carbon sequestration and regulates microbial community structures in Mollisols from northeast China. Soil 2023, 9, 261–275. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Yadav, G.S. Soil carbon sequestration in crop production. In Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, 2020; pp. 1–39. [Google Scholar]
- Kumar, A.; Bhattacharya, T.; Mukherjee, S.; Sarkar, B. A perspective on biochar for repairing damages in the soil–plant system caused by climate change-driven extreme weather events. Biochar 2022, 4, 22. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Ihab, R.; Rady, M.M.; Belal, H.E.E.; Mostafa, F.A.; Galal, T.M.; Masoudi, L.M.A.; Ali, E.F.; Roulia, M.; Mahmoud, A.E.M. A Novel Nutrient-and Antioxidant-Based Formulation Can Sustain Tomato Production under Full Watering and Drought Stress in Saline Soil. Plants 2023, 12, 3407. [Google Scholar] [CrossRef]
- Zulkarnaini, Z.M.; Sakimin, S.Z.; Mohamed, M.T.M.; Jaafar, H.Z.E. Changes in leaf area index, leaf mass ratio, net assimilation rate, relative growth rate and specific leaf area two cultivars of fig (Ficus carica L.) treated under different concentrations of brassinolide. AGRIVITA J. Agric. Sci. 2019, 41, 158–165. [Google Scholar] [CrossRef]
- Amanullah, M.J.H.; Nawab, K.; Ali, A. Response of specific leaf area (SLA), leaf area index (LAI) and leaf area ratio (LAR) of maize (Zea mays L.) to plant density, rate and timing of nitrogen application. World Appl. Sci. J. 2007, 2, 235–243. [Google Scholar]
- Patrick Jr, W.H.; Mikkelsen, D.S.; Wells, B.R. Plant nutrient behavior in flooded soil. In Fertilizer Technology and Use; Soil Science Society of America, Inc.: Madison, WI, USA, 1985; pp. 197–228. [Google Scholar]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhan, Y.; Zhu, L. Reduced carbon sequestration potential of biochar in acidic soil. Sci. Total Environ. 2016, 572, 129–137. [Google Scholar] [CrossRef]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Arshad, U. Biochar Application: A Sustainable Approach for Mitigating Biotic and Abiotic Stresses in Plants. Integr. Plant Biotechnol. 2024, 2, 77–98. [Google Scholar] [CrossRef]
- Mushtaq, T.; Bano, A.; Ullah, A. Effects of Rhizospheric Microbes, Growth Regulators, and Biochar in Modulating Antioxidant Machinery of Plants Under Stress. J. Plant Growth Regul. 2024, 44, 1846–1867. [Google Scholar] [CrossRef]
- Anbuganesan, V.; Vishnupradeep, R.; Bruno, L.B.; Sharmila, K.; Freitas, H.; Rajkumar, M. Combined application of biochar and plant growth-promoting rhizobacteria improves heavy metal and drought stress tolerance in Zea mays. Plants 2024, 13, 1143. [Google Scholar] [CrossRef]
- Sumedrea, D.I.; Florea, A.; Negru, M.; Oprea, M.; Bdulescu, A. The influence of fertilization and irrigation on the quantitative and qualitative performances of tomatoes grown in greenhouses. Acta Hortic. 2024, 1391, 10. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Nagula, S.; Ijsha, P.B.; Thampatti, M. Tender Coconut Husk Derived Biochar Impact on Soil Properties, Yield and Fruit Quality of Banana. J. Indian Soc. Soil Sci. 2021, 69, 334–338. [Google Scholar] [CrossRef]
- Mankasingh, U.; Choi, P.-C.; Ragnarsdottir, V. Biochar application in a tropical, agricultural region: A plot scale study in Tamil Nadu, India. Appl. Geochem. 2011, 26, S218–S221. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, J.; Yang, W.; He, L.; Lin, A. Evaluation of biochar pyrolyzed from kitchen waste, corn straw, and peanut hulls on immobilization of Pb and Cd in contaminated soil. Environ. Pollut. 2020, 261, 114133. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, J.W.; Speir, R.A.; Harris, K.; Das, K.C.; Lee, R.D.; Morris, L.A.; Fisher, D.S. Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron. J. 2010, 102, 623–633. [Google Scholar] [CrossRef]
- Khan, M.A.; Basir, A.; Fahad, S.; Adnan, M.; Saleem, M.H.; Iqbal, A.; Amanullah; Al-Huqail, A.A.; Alosaimi, A.A.; Saud, S. Biochar optimizes wheat quality, yield, and nitrogen acquisition in low fertile calcareous soil treated with organic and mineral nitrogen fertilizers. Front. Plant Sci. 2022, 13, 879788. [Google Scholar]
- Farrell, M.; Macdonald, L.M.; Butler, G.; Chirino-Valle, I.; Condron, L.M. Biochar and fertiliser applications influence phosphorus fractionation and wheat yield. Biol. Fertil. Soils 2014, 50, 169–178. [Google Scholar] [CrossRef]
- Liu, X.; Li, G.; Chen, C.; Zhang, X.; Zhou, K.; Long, X. Banana stem and leaf biochar as an effective adsorbent for cadmium and lead in aqueous solution. Sci. Rep. 2022, 12, 1584. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Zech, W. The effect of charcoal in banana (Musa Sp.) Planting holes–An on-farm study in central Amazonia, Brazil. In Amazonian Dark Earths: Wim Sombroek’s Vision; Springer: Dordrecht, The Netherlands, 2009; pp. 423–432. [Google Scholar]
- Bass, A.M.; Bird, M.I.; Kay, G.; Muirhead, B. Soil properties, greenhouse gas emissions and crop yield under compost, biochar and co-composted biochar in two tropical agronomic systems. Sci. Total Environ. 2016, 550, 459–470. [Google Scholar] [CrossRef]
- Borchard, N.; Siemens, J.; Ladd, B.; Möller, A.; Amelung, W. Application of biochars to sandy and silty soil failed to increase maize yield under common agricultural practice. Soil Tillage Res. 2014, 144, 184–194. [Google Scholar] [CrossRef]
- Graber, E.R.; Meller Harel, Y.; Kolton, M.; Cytryn, E.; Silber, A.; Rav David, D.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and its broad impacts in soil quality and fertility, nutrient leaching and crop productivity: A review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Bashir, S.; Qayyum, M.A.; Husain, A.; Bakhsh, A.; Ahmed, N.; Hussain, M.B.; Elshikh, M.S.; Alwahibi, M.S.; Almunqedhi, B.M.A.; Hussain, R. Efficiency of different types of biochars to mitigate Cd stress and growth of sunflower (Helianthus L.) in wastewater irrigated agricultural soil. Saudi J. Biol. Sci. 2021, 28, 2453–2459. [Google Scholar] [CrossRef] [PubMed]
- Campion, L.; Bekchanova, M.; Malina, R.; Kuppens, T. The costs and benefits of biochar production and use: A systematic review. J. Clean. Prod. 2023, 408, 137138. [Google Scholar] [CrossRef]
- Pandit, N.R.; Mulder, J.; Hale, S.E.; Zimmerman, A.R.; Pandit, B.H.; Cornelissen, G. Multi-year double cropping biochar field trials in Nepal: Finding the optimal biochar dose through agronomic trials and cost-benefit analysis. Sci. Total Environ. 2018, 637, 1333–1341. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. Gcb Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- He, D.; Luo, Y.; Zhu, B. Feedstock and pyrolysis temperature influence biochar properties and its interactions with soil substances: Insights from a DFT calculation. Sci. Total Environ. 2024, 922, 171259. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.P.; Morais, E.G.; Jindo, K.; Silva, C.A. Biochar N content, pools and aromaticity as affected by feedstock and pyrolysis temperature. Waste Biomass Valoriz 2024, 15, 3599–3619. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Naeem, M.A.; Khalid, M.; Aon, M.; Abbas, G.; Amjad, M.; Murtaza, B.; Khan, W.-D.; Ahmad, N. Combined application of biochar with compost and fertilizer improves soil properties and grain yield of maize. J. Plant Nutr. 2018, 41, 112–122. [Google Scholar] [CrossRef]
- Zhang, M.; Tao, S.; Wang, X. Interactions between organic pollutants and carbon nanomaterials and the associated impact on microbial availability and degradation in soil: A review. Environ. Sci. Nano 2020, 7, 2486–2508. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, B. Insights on the molecular mechanism for the recalcitrance of biochars: Interactive effects of carbon and silicon components. Environ. Sci. Technol. 2014, 48, 9103–9112. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, B.; Zhu, L. Transformation, morphology, and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures. Environ. Sci. Technol. 2014, 48, 3411–3419. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B.; Chen, M. Novel alleviation mechanisms of aluminum phytotoxicity via released biosilicon from rice straw-derived biochars. Sci. Rep. 2016, 6, 29346. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Zimmerman, A.R. Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 2013, 193, 122–130. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, Z.; Chen, B. H/C atomic ratio as a smart linkage between pyrolytic temperatures, aromatic clusters and sorption properties of biochars derived from diverse precursory materials. Sci. Rep. 2016, 6, 22644. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Yanardağ, İ.H.; Zornoza, R.; Cano, A.F.; Yanardağ, A.B.; Mermut, A.R. Evaluation of carbon and nitrogen dynamics in different soil types amended with pig slurry, pig manure and its biochar by chemical and thermogravimetric analysis. Biol. Fertil. Soils 2015, 51, 183–196. [Google Scholar] [CrossRef]
- Smebye, A.; Alling, V.; Vogt, R.D.; Gadmar, T.C.; Mulder, J.; Cornelissen, G.; Hale, S.E. Biochar amendment to soil changes dissolved organic matter content and composition. Chemosphere 2016, 142, 100–105. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xiao, X.; Chen, B.; Zhu, L. Quantification of chemical states, dissociation constants and contents of oxygen-containing groups on the surface of biochars produced at different temperatures. Environ. Sci. Technol. 2015, 49, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity–Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Koide, R.T.; Nguyen, B.T.; Skinner, R.H.; Dell, C.J.; Peoples, M.S.; Adler, P.R.; Drohan, P.J. Biochar amendment of soil improves resilience to climate change. Glob. Change Biol. Bioenerg. 2015, 7, 1084–1091. [Google Scholar] [CrossRef]
- Graber, E.R.; Tsechansky, L.; Gerstl, Z.; Lew, B. High surface area biochar negatively impacts herbicide efficacy. Plant Soil 2012, 353, 95–106. [Google Scholar] [CrossRef]
- Jien, S.-H.; Wang, C.-S. Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 2013, 110, 225–233. [Google Scholar] [CrossRef]
- Omara, P.; Singh, H.; Singh, K.; Sharma, L.; Otim, F.; Obia, A. Short-term effect of field application of biochar on cation exchange capacity, pH, and electrical conductivity of sandy and clay loam temperate soils. Technol. Agron. 2023, 3, 16. [Google Scholar] [CrossRef]
- Šimanský, V.; Horák, J.; Igaz, D.; Balashov, E.; Jonczak, J. Biochar and biochar with N fertilizer as a potential tool for improving soil sorption of nutrients. J. Soils Sediments 2018, 18, 1432–1440. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Ameloot, N.; De Neve, S.; Jegajeevagan, K.; Yildiz, G.; Buchan, D.; Funkuin, Y.N.; Prins, W.; Bouckaert, L.; Sleutel, S. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013, 57, 401–410. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, C.; Ma, E.; Tan, H.; Zhu, T.; Müller, C. Biochar stimulates NH4+ turnover while decreasing NO3− production and N2O emissions in soils under long-term vegetable cultivation. Sci. Total Environ. 2020, 737, 140266. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, M.H.B.; Rafsanjani, M.S.O.; Moussavinik, S.M.; Abdin, M.Z. Effect of salt (NaCl) stress on germination and early seedling growth of Spinach (Spinacia oleracea L.). Ann. Biol. Res. 2011, 2, 490–497. [Google Scholar]
- Meriño-Gergichevich, C.; Alberdi, M.; Ivanov, A.G.; Reyes-Díaz, M. Al3+-Ca2+ interaction in plants growing in acid soils: Al-phytotoxicity response to calcareous amendments. J. Soil Sci. Plant Nutr. 2010, 10, 217–243. [Google Scholar]
- Hou, R.; Ouyang, Z.; Maxim, D.; Wilson, G.; Kuzyakov, Y. Lasting effect of soil warming on organic matter decomposition depends on tillage practices. Soil Biol. Biochem. 2016, 95, 243–249. [Google Scholar] [CrossRef]
- Saha, A.; Ghosh, R.K.; Basak, B.B. Fate and behavior of pesticides and their effect on soil biological properties under climate change scenario. In Sustainable Management of Soil and Environment; Springer: Singapore, 2019; pp. 259–288. [Google Scholar]
- Stokes, J.D.; Paton, G.I.; Semple, K.T. Behaviour and assessment of bioavailability of organic contaminants in soil: Relevance for risk assessment and remediation. Soil Use Manag. 2005, 21, 475–486. [Google Scholar] [CrossRef]
- Li, T.; Tao, Q.; Liang, C.; Yang, X. Elevated CO2 concentration increase the mobility of Cd and Zn in the rhizosphere of hyperaccumulator Sedum alfredii. Environ. Sci. Pollut. Res. 2014, 21, 5899–5908. [Google Scholar] [CrossRef]
- Kumar, R.; Mehrotra, N.K.; Nautiyal, B.D.; Kumar, P.; Singh, P.K. Effect of copper on growth, yield and concentration of Fe, Mn, Zn and Cu in wheat plants (Triticum aestivum L.). J. Environ. Biol. 2009, 30, 485–488. [Google Scholar]
- Derakhshan Nejad, Z.; Jung, M.C.; Kim, K.-H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Health 2018, 40, 927–953. [Google Scholar] [CrossRef]
- Gabriele, I. Application of Phytoremediation Technique for Removal of Polycyclic Aromatic Hydrocarbons and Potential Toxic Elements from Contaminated Soils. Ph.D. Thesis, Università di Cassino e del Lazio Meridionale, Cassino, Italy, 2023. [Google Scholar]
- Wang, Y.; Wang, H.-S.; Tang, C.-S.; Gu, K.; Shi, B. Remediation of heavy-metal-contaminated soils by biochar: A review. Environ. Geotech. 2019, 9, 135–148. [Google Scholar] [CrossRef]
- Jia, M.; Yu, J.; Li, Z.; Wu, L.; Christie, P. Effects of biochar on the migration and transformation of metal species in a highly acid soil contaminated with multiple metals and leached with solutions of different pH. Chemosphere 2021, 278, 130344. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Xia, H.; Liu, R.; Zhang, Y. Enhanced complexation and electrostatic attraction through fabrication of amino-or hydroxyl-functionalized Fe/Ni-biochar composite for the adsorption of Pb (II) and Cd (II). Sep. Purif. Technol. 2024, 328, 125074. [Google Scholar] [CrossRef]
- Qi, X.; Yin, H.; Zhu, M.; Yu, X.; Shao, P.; Dang, Z. MgO-loaded nitrogen and phosphorus self-doped biochar: High-efficient adsorption of aquatic Cu2+, Cd2+, and Pb2+ and its remediation efficiency on heavy metal contaminated soil. Chemosphere 2022, 294, 133733. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Ajibade, F.O.; Atakpa, E.O.; Abdulaha-Al Baquy, M.; Mia, S.; Odii, E.C.; Xu, R. Reduction of heavy metal uptake from polluted soils and associated health risks through biochar amendment: A critical synthesis. J. Hazard. Mater. Adv. 2022, 6, 100086. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; He, L.-Y.; Sheng, X.-F. Increased biomass and reduced heavy metal accumulation of edible tissues of vegetable crops in the presence of plant growth-promoting Neorhizobium huautlense T1-17 and biochar. Agric. Ecosyst. Environ. 2016, 228, 9–18. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.; Tang, L.; Dong, H.; Huang, B. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Mosa, A.; El-Ghamry, A.; Tolba, M. Functionalized biochar derived from heavy metal rich feedstock: Phosphate recovery and reusing the exhausted biochar as an enriched soil amendment. Chemosphere 2018, 198, 351–363. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Xie, S.; Pan, L.; Li, C.; You, F.; Wang, Y. Immobilization of heavy metals in ceramsite produced from sewage sludge biochar. Sci. Total Environ. 2018, 628, 131–140. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, X.; Xiong, T.; Jiang, L.; Wang, H.; Wu, Z. Biochar facilitated hydroxyapatite/calcium silicate hydrate for remediation of heavy metals contaminated soils. Water Air Soil Pollut. 2020, 231, 66. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B.; Hu, D. Effective alleviation of aluminum phytotoxicity by manure-derived biochar. Environ. Sci. Technol. 2013, 47, 2737–2745. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2022, 73, e13203. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef]
- Ashraf, U.; Kanu, A.S.; Mo, Z.; Hussain, S.; Anjum, S.A.; Khan, I.; Abbas, R.N.; Tang, X. Lead toxicity in rice: Effects, mechanisms, and mitigation strategies—A mini review. Environ. Sci. Pollut. Res. 2015, 22, 18318–18332. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Lim, J.E.; Lee, S.E.; Cho, J.S.; Moon, D.H.; Hashimoto, Y.; Ok, Y.S. Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 2014, 95, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Joseph, S.; Cui, L.; Pan, G.; Li, L.; Liu, X.; Zhang, A.; Rutlidge, H.; Wong, S.; Chia, C. A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J. Hazard. Mater. 2014, 272, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Břendová, K.; Zemanová, V.; Pavlíková, D.; Tlustoš, P. Utilization of biochar and activated carbon to reduce Cd, Pb and Zn phytoavailability and phytotoxicity for plants. J. Environ. Manage. 2016, 181, 637–645. [Google Scholar] [CrossRef]
- Zhou, X.-N.; Jiang, Q.-W.; Guo, J.-G.; Lin, D.-D.; Zhu, R.; Yang, G.-J.; Yang, K.; Li, S.-Z.; Xu, J. Road map for transmission interruption of schistosomiasis in China. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi Chin. J. Schistosomiasis Control 2012, 24, 1–4. [Google Scholar]
- Jones, S.; Bardos, R.P.; Kidd, P.S.; Mench, M.; de Leij, F.; Hutchings, T.; Cundy, A.; Joyce, C.; Soja, G.; Friesl-Hanl, W. Biochar and compost amendments enhance copper immobilisation and support plant growth in contaminated soils. J. Environ. Manag. 2016, 171, 101–112. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Liu, A.; Tian, D.; Xiang, Y.; Mo, H. Effects of biochar on growth of Asian lotus (Nelumbo nucifera Gaertn.) and cadmium uptake in artificially cadmium-polluted water. Sci. Hortic. 2016, 198, 311–317. [Google Scholar] [CrossRef]
- Mosa, A.; El-Banna, M.F.; Gao, B. Biochar filters reduced the toxic effects of nickel on tomato (Lycopersicon esculentum L.) grown in nutrient film technique hydroponic system. Chemosphere 2016, 149, 254–262. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Paz-Ferreiro, J.; Beesley, L. Cadmium, lead, and zinc mobility and plant uptake in a mine soil amended with sugarcane straw biochar. Environ. Sci. Pollut. Res. 2015, 22, 17606–17614. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Chen, B. Dual role of biochars as adsorbents for aluminum: The effects of oxygen-containing organic components and the scattering of silicate particles. Environ. Sci. Technol. 2013, 47, 8759–8768. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Waqas, M.; Ding, F.; Shamshad, I.; Arp, H.P.H.; Li, G. The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J. Hazard. Mater. 2015, 300, 243–253. [Google Scholar] [CrossRef]
- Hartley, W.; Dickinson, N.M.; Riby, P.; Lepp, N.W. Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ. Pollut. 2009, 157, 2654–2662. [Google Scholar] [CrossRef]
- Centofanti, T.; McConnell, L.L.; Chaney, R.L.; Beyer, W.N.; Andrade, N.A.; Hapeman, C.J.; Torrents, A.; Nguyen, A.; Anderson, M.O.; Novak, J.M. Organic amendments for risk mitigation of organochlorine pesticide residues in old orchard soils. Environ. Pollut. 2016, 210, 182–191. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Zhang, W.; Wang, F.; Bian, Y.; Boughner, L.A.; Jiang, X. Novel biochar-plant tandem approach for remediating hexachlorobenzene contaminated soils: Proof-of-concept and new insight into the rhizosphere. J. Agric. Food Chem. 2016, 64, 5464–5471. [Google Scholar] [CrossRef]
- Tatarková, V.; Hiller, E.; Vaculík, M. Impact of wheat straw biochar addition to soil on the sorption, leaching, dissipation of the herbicide (4-chloro-2-methylphenoxy) acetic acid and the growth of sunflower (Helianthus annuus L.). Ecotoxicol. Environ. Saf. 2013, 92, 215–221. [Google Scholar] [CrossRef]
- Williams, M.; Martin, S.; Kookana, R.S. Sorption and plant uptake of pharmaceuticals from an artificially contaminated soil amended with biochars. Plant Soil 2015, 395, 75–86. [Google Scholar] [CrossRef]
- Yang, X.-B.; Ying, G.-G.; Peng, P.-A.; Wang, L.I.; Zhao, J.-L.; Zhang, L.-J.; Yuan, P.; He, H.-P. Influence of biochars on plant uptake and dissipation of two pesticides in an agricultural soil. J. Agric. Food Chem. 2010, 58, 7915–7921. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Ying, G.-G.; Kookana, R.S. Reduced plant uptake of pesticides with biochar additions to soil. Chemosphere 2009, 76, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Ogbonnaya, U.; Semple, K.T. Impact of biochar on organic contaminants in soil: A tool for mitigating risk? Agronomy 2013, 3, 349–375. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Johir, M.A.H.; Sun, L.; Asadullah, M.; Belhaj, D. Sorption of hydrophobic organic contaminants on functionalized biochar: Protagonist role of π-π electron-donor-acceptor interactions and hydrogen bonds. J. Hazard. Mater. 2018, 360, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Weldeab, A.O.; Steen, A.; Starkenburg, D.J.; Dal Williams, J.S.; Abboud, K.A.; Xue, J.; Hammer, N.I.; Castellano, R.K.; Watkins, D.L. Tuning the structural and spectroscopic properties of donor–acceptor–donor oligomers via mutual X-bonding, H-bonding, and π–π interactions. J. Mater. Chem. C 2018, 6, 11992–12000. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, R.; Wu, D.; Su, H.; Liu, Z.; Chen, Z. How hydrogen bonding and π–π interactions synergistically facilitate mephedrone adsorption by bio-sorbent: An in-depth microscopic scale interpretation. Environ. Pollut. 2024, 342, 123044. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Ahmad, M.; Seo, D.-C.; Cho, J.-S.; Lee, S.-E.; Lee, S.S.; Ok, Y.S. Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J. Hazard. Mater. 2015, 290, 43–50. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, L.; Moghaddam, T.B.; Chen, M.; Wu, S.; Yuan, X. Adsorption mechanism of polycyclic aromatic hydrocarbons using wood waste-derived biochar. J. Hazard. Mater. 2022, 425, 128003. [Google Scholar] [CrossRef]
- Lima, J.Z.; Ogura, A.P.; da Silva, L.C.M.; Nauerth, I.M.R.; Rodrigues, V.G.S.; Espindola, E.L.G.; Marques, J.P. Biochar-pesticides interactions: An overview and applications of wood feedstock for atrazine contamination. J. Environ. Chem. Eng. 2022, 10, 108192. [Google Scholar] [CrossRef]
- Trigo, C.; Cox, L.; Spokas, K. Influence of pyrolysis temperature and hardwood species on resulting biochar properties and their effect on azimsulfuron sorption as compared to other sorbents. Sci. Total Environ. 2016, 566, 1454–1464. [Google Scholar] [CrossRef]
- Fan, J.; Li, Y.; Yu, H.; Li, Y.; Yuan, Q.; Xiao, H.; Li, F.; Pan, B. Using sewage sludge with high ash content for biochar production and Cu (II) sorption. Sci. Total Environ. 2020, 713, 136663. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, K.; Jin, J.; Han, L.; Sun, H.; Zhao, Y.; Xia, X.; Wu, F.; Xing, B. Metal/metalloid elements and polycyclic aromatic hydrocarbon in various biochars: The effect of feedstock, temperature, minerals, and properties. Environ. Pollut. 2015, 206, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.V.; Sackett, T.E.; Thomas, S.C. Thermal treatment and leaching of biochar alleviates plant growth inhibition from mobile organic compounds. PeerJ 2016, 4, e2385. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.; Moustafa-Farag, M.; Liu, Z.; El-Shazoly, R.M. Combined effect of biochar and salicylic acid in alleviating heavy metal stress, antioxidant enhancement, and Chinese mustard growth in a contaminated soil. J. Soil Sci. Plant Nutr. 2022, 22, 4194–4206. [Google Scholar] [CrossRef]
- Kong, L.; Liu, J.; Zhou, Q.; Sun, Z.; Ma, Z. Sewage sludge derived biochars provoke negative effects on wheat growth related to the PTEs. Biochem. Eng. J. 2019, 152, 107386. [Google Scholar] [CrossRef]
- Buss, W.; Graham, M.C.; Shepherd, J.G.; Mašek, O. Risks and benefits of marginal biomass-derived biochars for plant growth. Sci. Total Environ. 2016, 569, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Ahmed, Z.; Eldin, S.M.; Ali, B.; Bawazeer, S.; Usman, M.; Iqbal, R.; Neupane, D.; Ullah, A.; Khan, A. Biochar-Soil-Plant interactions: A cross talk for sustainable agriculture under changing climate. Front. Environ. Sci. 2023, 11, 1059449. [Google Scholar] [CrossRef]
- Liao, S.; Pan, B.; Li, H.; Zhang, D.; Xing, B. Detecting free radicals in biochars and determining their ability to inhibit the germination and growth of corn, wheat and rice seedlings. Environ. Sci. Technol. 2014, 48, 8581–8587. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I. Climate change impacts on plant pathogens and plant diseases. J. Crop Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Peter Mshelia, L.; Selamat, J.; Iskandar Putra Samsudin, N.; Rafii, M.Y.; Abdul Mutalib, N.-A.; Nordin, N.; Berthiller, F. Effect of temperature, water activity and carbon dioxide on fungal growth and mycotoxin production of acclimatised isolates of Fusarium verticillioides and F. graminearum. Toxins 2020, 12, 478. [Google Scholar] [CrossRef]
- Cotty, P.J.; Jaime-Garcia, R. Influences of Climate on Aflatoxin Producing Fungi and Aflatoxin Contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Achar, P.N.; Sreenivasa, M.Y. Current perspectives of biocontrol agents for management of Fusarium verticillioides and its fumonisin in cereals—A review. J. Fungi 2021, 7, 776. [Google Scholar]
- Kazan, K.; Gardiner, D.M. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: Recent progress and future prospects. Mol. Plant Pathol. 2018, 19, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.M.; Huffaker, A.; Schmelz, E.A.; Dafoe, N.J.; Christensen, S.; Sims, J.; Martins, V.F.; Swerbilow, J.A.Y.; Romero, M.; Alborn, H.T. Effects of elevated [CO2] on maize defence against mycotoxigenic Fusarium verticillioides. Plant. Cell Environ. 2014, 37, 2691–2706. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Delcour, I.; Spanoghe, P.; Uyttendaele, M. Literature review: Impact of climate change on pesticide use. Food Res. Int. 2015, 68, 7–15. [Google Scholar] [CrossRef]
- Casu, A.; Camardo Leggieri, M.; Toscano, P.; Battilani, P. Changing climate, shifting mycotoxins: A comprehensive review of climate change impact on mycotoxin contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13323. [Google Scholar] [CrossRef]
- Meller Harel, Y.; Elad, Y.; Rav-David, D.; Borenstein, M.; Shulchani, R.; Lew, B.; Graber, E.R. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 2012, 357, 245–257. [Google Scholar] [CrossRef]
- He, Z.; Webster, S.; He, S.Y. Growth–defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Trade-offs between plant growth and defense against insect herbivory: An emerging mechanistic synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef]
- Viger, M.; Hancock, R.D.; Miglietta, F.; Taylor, G. More plant growth but less plant defence? First global gene expression data for plants grown in soil amended with biochar. Gcb Bioenergy 2015, 7, 658–672. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Frenkel, O.; Elad, Y.; Lew, B.; Graber, E.R. Non-monotonic influence of biochar dose on bean seedling growth and susceptibility to Rhizoctonia solani: The “Shifted R max-Effect”. Plant Soil 2015, 395, 125–140. [Google Scholar] [CrossRef]
- Elad, Y.; David, D.R.; Harel, Y.M.; Borenshtein, M.; Kalifa, H.B.; Silber, A.; Graber, E.R. Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology 2010, 100, 913–921. [Google Scholar] [CrossRef] [PubMed]

| Biochar Feedstock’s | Crops | Soil Types and Variations | Effect on Crop Yield | Reference |
|---|---|---|---|---|
| Crop residue biochar | ||||
| Rice husk and cotton shell mixture biochar (400 °C). | Tomato | Sandy loamy soil with different irrigation treatments. | Fresh fruit weight ↑ Improved by 20%, 13%, and 6% under high to low water conditions, titratable citric acid concentration. ↑ Enhanced. | [68,69] |
| Rice paddy husk biochar (10 t ha−1). | Banana | ↑ Enhanced soil potassium, magnesium, sodium, and phosphorus. ↑ Enhanced % carbon and % nitrogen. | Crop yield not measured. | [70,71] |
| Peanut hull biochar (400 °C). | Corn | Loamy sandy soil. ↑ Improved soil nitrogen, phosphorus, potassium, sulfur, calcium, magnesium, and soil pH. | Corn grain yield, stover biomass, and corn tissue potassium concentration ↑ Increased. | [72,73] |
| Green waste biochar (500 °C). | Wheat | Haplic calcisol (calcareous soil). No significant influence on soil organic carbon, dissolved organic carbon, microbial biomass carbon, and microbial biomass nitrogen. | Slightly ↓ Reduced wheat grain yield, biochar effect inferior to di-ammonium phosphate fertilizer. | [74,75] |
| Wood biochar | ||||
| Wood biochar. | Banana | ↑ Enhanced soil pH. | ↑ Improved crop nutrient (potassium) uptake, no change in fruit production. | [76,77] |
| Willow wood biochar (500 °C). | Banana and papaya | ↑ Increased soil pH, potassium, calcium, cation exchange capacity, NH4+-N, NO3−-N, % carbon of red clay soil. | Average fruit diameter ↑ Enhanced. | [78] |
| Willow wood biochar (500 °C). | Banana and papaya | Water content, pH, sodium, % carbon, carbon/nitrogen, and carbon stock of red chromosol. | Slightly ↑ Increased average number of fruit per tree. | [78] |
| Pine chip biochar (400 °C). | Corn | Loamy sandy soil, ↓ Reduced soil calcium, and pH. | Corn grain yield and tissue potassium, sulfur, and magnesium concentration ↑ Increased. | [72,73] |
| Hard wood biochar (slow-pyrolysis charcoal at (500 °C) and gasification coke at (1100 °C), soft wood biochar (flash-pyrolysis biochar at (450–550 °C) (15 g kg−1 ≈ 45 t ha−1). | Corn (Zea mays L.) | Sandy Ap horizon of a haplic fluvisol with poor cation exchange capacity and moderate water holding capacity, ailty Ap horizon of a gleyic luvisol with medium potential cation exchange capacity and very high water holding capacity. ↑ Improved soil total carbon and black carbon fraction determined with benzene polycarboxylic acids. Gasification coke ↑ Increased sandy soil pH. Flash-pyrolysis char ↓ Reduced silty soil pH. No influence on soil water holding capacity or aggregates. | Slightly but not significantly ↓ Reduced biomass and corn yield, no influence on nutrient contents or functional traits such as leaf area ratios and specific leaf area. Flash-pyrolysis char ↓ Suppressed the germination of maize kernels. | [79] |
| Hard wood biochar (slow-pyrolysis charcoal at (500 °C) (100 g kg−1 ≈ 300 t ha−1). | Corn (Zea mays L.) | Silty Ap horizon of a gleyic luvisol with medium potential cation exchange capacity and very high water holding capacity. ↑ Enhanced soil water holding capacity. ↑ Improved soil carbon/nitrogen (to a high value of 80) and plant available potassium. | ↓ Reduced corn yield and calcium content. ↑ Increased magnesium and carbon/nitrogen ratio in leaf biomass. | [79] |
| Citrus wood biochar. | Pepper and tomato | Fertigated soil-less media | ↑ Enhanced leaf area, canopy dry weight, number of nodes, and yield of buds, flowers, and fruit of pepper plant. ↑ Increased plant height and leaf size, no effects on flower and fruit yield. | [80] |
| Other biochar types | ||||
| Water-washed gasification coke produced at 1100 °C (15 g kg−1 ≈ 45 t ha−1). | Corn (Zea mays L.) | Silty Ap horizon of a gleyic luvisol with medium potential cation exchange capacity and very high water holding capacity. ↑ Increased soil phosphorus and magnesium, ↓ Reduced potassium. | [79] | |
| Composition | Related Properties | Potential Functions | References |
|---|---|---|---|
| Elemental composition | |||
| C | Carbon stability, sorption capacity | Aromatic carbon increases carbon sequestration and adsorption of pollutants. Amorphous carbon can be utilized by microbes as a carbon source and is responsible for the partition of pollutants. | [90,91] |
| Si | Carbon stability, nutrient concentration | Maintains a stable carbon structure with the formation of a C-Si complex during pyrolysis. Alleviates plant Al uptake with the formation of a Si-Al complex on the plant root cell epidermis. Supplies Si for plants with the release of soluble Si from biochar. | [92,93,94] |
| N | Carbon stability, nutrient concentration, carbon source quality | As a soil fertilizer to release nutrients adsorbed on the biochar surface during aging in a sustained manner for plant nutrient uptake. The C/N ratio of the biochar determines C and N bioavailability to soil microbes and plants. | [95] |
| P | Nutrient concentration | As soil fertilizer to release nutrients adsorbed on the biochar surface during aging in a sustained manner for plant nutrient uptake. | [95] |
| K | Nutrient concentration | As soil fertilizer to increase plant K uptake. | [77] |
| Atomic H/C | Nonpolarity, sorption capacity, carbon stability | Causes a decrease in atomic H/C indicated in the aromatic structure and the recalcitrant nature of biochar. Causes an increase in the adsorption capacity of biochar. | [96] |
| Atomic O/C | Nonpolarity, sorption stability | Causes a decrease in atomic O/C indicated in the aromatic structure and the nonpolarity of biochar. | [91,97] |
| C/N | Carbon stability, carbon source quality | Causes a high C/N decrease and microbial decomposition because of a lack of N supply, thus enhancing carbon stability. High C/N represents poor nutrient quality for plant uptake. | [98] |
| Ash content | pH, nutrient concentration | Has a liming effect to increase the soil pH. Supplies nutrients for plant growth. | [73,99] |
| Structure | |||
| Aromatic carbon | Carbon stability, sorption capacity | Increases carbon sequestration, decreases soil microbial mineralization. Causes the sorption of organic compounds (including pollutants). Decreases GHG emissions. | [90,100,101] |
| Surface functional group | Sorption, pH | Causes the sorption of nutrients and heavy metals and some organic compounds. Reduced acidic functional groups (such as -COOH) with pyrolysis through increased temperature can increase biochar pH. | [102] |
| Porosity | Density, pore volume | High porosity improves soil aeration conditions, which modifies the GHG emission process. Enhances soil water retention, which ensures plant resistance to dry weather. Provides microbial inhibition. | [103,104] |
| SSA | Sorption, CEC | Adsorbs nutrients (as soil fertilizer) and organ pollutants. Forms a soil aggregate to protect SOC from decomposition. | [105,106] |
| Negative surface charge | Sorption, CEC | CEC dependent on environmental pH controls the long-term release of nutrients from biochar or sorbed on biochar from soil. | [107,108,109] |
| VM and VOCs | Carbon stability, potential toxicity | VM represents the labile carbon in biochar. VOCs may inhibit soil-borne pathogens and improve plant growth. | [80,110] |
| Biochars | Pollutants | Plants | Soil Type | Bioavailability | Plant Uptake | Possible Mechanisms | References |
|---|---|---|---|---|---|---|---|
| Heavy metals | |||||||
| Oak wood biochar 400 °C | Pb | Maize | ↓ Decreased Pb bioavailability | ↓ Decreased Pb accumulation in maize shoots | Formation of Pb–phosphate in soil, immobilizing Pb | [135] | |
| Wheat straw biochar 350–550 °C | Pb | Rice | paddy soil | ↓ Decreased extractable Pb | ↓ Decreased Pb only in roots | Precipitation and adsorption, bound to mineral phase of Al, Fe, and P | [136] |
| Wheat straw biochar 350–550 °C | Cd | Rice | paddy soil | ↓ Decreased extractable Cd | ↓ Decreased Cd in rice grain, shoots, and roots, ↑ Increased rice yield | Precipitation and adsorption, bound to mineral phase of Al, Fe, and P | [136] |
| Willow wood biochar 500 °C | Cd, Pb, and Zn | Spinach | modal chernozem | ↓ Decreased Cd and Zn mobility | ↓ Decreased Cd, Pb, and Zn uptake, ↑ Increased biomass | Heavy metal adsorption by biochar | [137] |
| Willow wood biochar 500 °C | Cd, Pb, and Zn | Mustard | modal chernozem | ↓ Decreased Cd and Zn mobility | ↓ Decreased Cd and Zn uptake, ↑ Increased Pb accumulation, ↑ Increase biomass | Heavy metal adsorption by biochar, increased plant tolerance by increasing glutamic acid and glutamine | [137] |
| Rice straw biochar 300 °C | Pb | No plants | utisol and oxisol | ↓ Decreased availability of Pb | No plants | Enhanced negative charge of soil and contributed to the non-electrostatic adsorption of Pb (II); formation of surface complex between oxygen-containing functional groups on biochar with Pb (II) | [138] |
| Poplar wood biochar 525 °C | Cu | Sunflower | Cu-contaminated soil | Significantly reduced leachable Cu | ↑ Increased plant biomass and nutrient (K) concentration, plant height, and root length. ↓ Reduced shoot Cu concentration | Decreased Cu bioavailability, improved soil nutrient and water provision Nutrients (Ca) compete with Cu for plant uptake | [139] |
| Mixed wood biochar | Cu and Pb | Ryegrass | heavily Cu- and Pb-contaminated soil | ↓ Reduced pore water Cu concentration, ↓ reduced shoot | ↓ Reduced Cu and Pb uptake by ryegrass shoots, ↑ Increased biomass | Humified complexes and the formation of minerals such as pyromorphite (with P-rich biochar) reduced Cu in pore water, high pH reduced Pb in pore water | [140] |
| Willow biochar, wheat straw biochar | Cd, Pb, Zn, Cr, Cu, and Ni | Lepidium sativum | heavy-metal-contaminated soil | Biochar ↓ reduced the toxicity of soil leachates | Eliminated root growth inhibition | Adsorption of heavy metals | [80] |
| Pinewood biochar | Cd | Lotus | Cu-polluted water–soil | Not measured | ↑ Increased total and rhizome biomass, ↓ Decreased Cd content in rhizomes, petioles, and leaves, increased Cd transfer efficient from underground to aboveground tissues, ↓ Reduced Cd content in edible part | Biochar formed insoluble chelates or caused precipitation of Cd and alleviate Cd stress, reducing SOD activity of lotus plant | [141] |
| Cotton wood biochar 600 °C | Ni | Tomato | hydroponic system | Not measured | Alleviated fruit yield reduction by 26.6%, minimized the reduction in nutrients concentrations in roots, shoots, and fruits | Precipitation of Ni on biochar in crystal form, ion exchange, and complexation with surface functional groups of biochar; alleviated heavy metal stresses on tomato (distortion of nucleolus, thickening formation in cell wall structure, reduction in chlorophyll content, vacuolization) | [142] |
| Chicken manure biochar and green waste biochar 550 °C | Cd, Cu, and Pb | Indian mustard | metal-spiked soils and naturally metal-contaminated soils | ↓ Reduced NH4NO3 extractable Cd, Cu, and Pb in soils, ↓ Decreased Cd and Cd and Pb but increased Cu concentration in pore water | ↑ Increased shoot and root biomass by 353% and 572% (chicken waste biochar), ↓ Reduced Cd, Cu, and Pb accumulation by Indian mustard, and ↑ Increased nutrient availability of P and K | Metal immobilization by biochar with both specific (coulombic interaction) and non-specific (coordination bonds) adsorption; changed the partitioning of Cd, Cu, and Pb from easily exchangeable phase to organic-bound fraction | [143] |
| Sugarcane straw biochar 700 °C | Cd, Cu, Pb, and Zn | Jack bean and Mucuma aterrima | contaminated Zn mine soil | ↓ Reduced Zn in pore water | ↓ Reduced plant uptake of Cd, Pb, and Zn | Reduced heavy metal toxicity and increased macronutrient (P, K, Ca, and Mg) concentrations in soil | [144] |
| Cattle manure biochar 400 °C | A1 | Wheat | solution | ↓ Reduced Al3+ concentration in solution | ↓ Reduced Al uptake by wheat, enhanced root and shoot elongation, avoided root tip plasma membrane damage | Biochar elevated solution pH, facilitated the transfer of free Al3+ ions to Al(OH)2+ and Al(OH)2+ monomers, which were adsorbed by biochar through surface complexation rather than electrostatic attraction (between Al3+ and biochar negative surface charge) | [145] |
| Sewage sludge, soybean and rice straw, peanut shell biochar 500 °C | Cd, Cu, Pb, and Zn | Turnip | As, heavy metal, and PAHs combined | ↓ Decreased bioaccessibility of Cd, Cu, Pb, and Zn concentration | ↑ Increased root yield by 2% application rate biochar, ↓ Decreased root yield by 5% application rate biochar, ↓ Reduced plant heavy metal accumulation | Precipitation of heavy metal ions (at high pH) with different anions (OH-, SO 2-, HPO-, CO 2-) in soil and O-functional groups in biochar, e.g., forming metal–P precipitates. | [146] |
| Hazardous metalloid | |||||||
| Mixed wood biochar 400 °C | As | Miscanthus | As-contaminated soil | Little effects on As mobility | Little effects on plant yield and As uptake | Increased P availability, P competed with As for the uptake site | [147] |
| Sewage sludge, soybean and rice straw, peanut shell biochar 500 °C | As | Turnip (Brassica rapa L.) | As, heavy metal, and PAHs combined contaminated soil | ↓ Decreased bioaccessibility of As concentration (more with 5% than 2% biochar) | ↑ Increased root yield by 2% application rate biochar, ↓ Decreased root yield by 5% application rate biochar, ↓ Reduced plant As accumulation | P competed with As for plant uptake; Si chelated As; S reduced As availability; 5% biochar application rate increased NH4 +-N availability, causing stress response of plant | [146] |
| Organic contaminants | |||||||
| Willow biochar, wheat straw biochar | PAHs | Lepidium sativum | PAHs-contaminated soil | Biochar ↓ Reduced the toxicity of soil leachates | Eliminated root growth inhibition | Adsorption of PAHs | [80] |
| Pine chip biochar 500 °C | DDT, DDE, and deldrin residue | Orchard grass | old orchard soil with continuous DDT application | Ineffective in lower bioavailability factor of organochlorine pesticide residues | ↑ Increased shoot biomass | Possibly due to low surface areas of biochar in use | [148] |
| Wood charcoal (produced in an earthen pit) and eucalyptus wood biochar 800 °C | S-metolachlor and sulfentrazone | Green Foxtail (Setaria viridis) | Hamra Red Mediterranea n subsoil | ↓ Reduced the bioavailability of herbicides to the weed | Biochar alleviated the inhabitation of weed biomass with the use of herbicides, biochar with high SSA ↓ Reduced herbicide efficacy | High SSA caused high adsorption of herbicides | [105] |
| Sewage sludge, soybean and rice straw, peanut shell biochar 500 °C | PAHs | Turnip (Brassica rapa L.) | As, heavy metal, and PAHs combined contaminated soil | ↓ Decreased the accessible concentrations of ∑16PAHs | ↓ Reduced PAH concentrations in turnip, higher reduction for high-molecular-weight PAHs | High surface area, low polarity, and high C content contributed to PAHs adsorption | [146] |
| Wheat straw biochar 500 °C | Hexachlorobe nzene (HCB) | Ryegrass | ferri-udic Argosols | ↓ Reduced HCB bioavailability to microbes and plants, and ↓ Reduced microbial degradation | ↓ Reduced HCB concentration in shoots and roots | Adsorption of HCB by biochar; ryegrass root exudates (oxalic acid) suppressed HCB sorption to biochar and stimulated HCB microbial rhizodegradation | [149] |
| Wheat straw biochar 300 °C | Ionizable herbicide (4-chloro-2-m ethylphenoxy) acetic acid (MCPA) | Sunflower | soil with 60% silt content | ↓ Increased MCPA sorption and ↓ Decreased desorption, leachability, and dissipation (microbial degradation) | No significant effects in terms of the phytotoxic effects of MCPA on sunflower, biochar ↑ Increased aboveground biomass but ↓ Reduced chlorophyll a,b content | Partition of MCPA to biochar; Mg deficiency may be ascribed to the reduced chlorophyll content | [150] |
| Wheat chaff biochar 450 °C and wood biochar (eucalyptus) 450 °C and 520 °C | Carbamazepin e (CBZ) and propranolol (PRL) | Ryegrass | loamy sand soil | ↓ Reduced active pharmaceutical ingredients (APIs) concentration in pore water | ↓ Reduced plant tissue uptake of APIs | Both partitioning of APIs to biochar amended soil (natural solids in soil) and sorption of APIs to biochar | [151] |
| Cotton straw chips 450 °C and 850 °C | Insecticides (chlorpyrifos and fipronil) | Chinese chives | clay loamy soil (pH = 4.01) | ↑ Increased the half-life of insecticides ↓ Lowered the availability of insecticides to soil microbes | ↓ Reduced plant uptake and microbial degradation ↓ Reduced pesticide residues in both aboveground and underground plant parts ↑ Increased plant biomass | High surface area and microporosity; biochar sequestration and microbial degradation | [152] |
| Eucalyptus spp. wood chips 450 °C and 850 °C | Insecticides (chlorpyrifos and carbofuran) | Spring onion | sand loamy soil | ↓ Decreased the extractable insecticide residues in soil | ↑ Higher biomass under biochar amendment, ↓ Lower insecticide residues in above- and belowground plant parts | Biochar sequestration and microbial degradation | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korai, S.K.; Korai, P.K.; Jaffar, M.A.; Qasim, M.; Younas, M.U.; Shabaan, M.; Zulfiqar, U.; Wang, X.; Artyszak, A. Leveraging Biochar Amendments to Enhance Food Security and Plant Resilience Under Climate Change. Plants 2025, 14, 1984. https://doi.org/10.3390/plants14131984
Korai SK, Korai PK, Jaffar MA, Qasim M, Younas MU, Shabaan M, Zulfiqar U, Wang X, Artyszak A. Leveraging Biochar Amendments to Enhance Food Security and Plant Resilience Under Climate Change. Plants. 2025; 14(13):1984. https://doi.org/10.3390/plants14131984
Chicago/Turabian StyleKorai, Shakal Khan, Punhoon Khan Korai, Muhammad Abuzar Jaffar, Muhammad Qasim, Muhammad Usama Younas, Muhammad Shabaan, Usman Zulfiqar, Xiaoshan Wang, and Arkadiusz Artyszak. 2025. "Leveraging Biochar Amendments to Enhance Food Security and Plant Resilience Under Climate Change" Plants 14, no. 13: 1984. https://doi.org/10.3390/plants14131984
APA StyleKorai, S. K., Korai, P. K., Jaffar, M. A., Qasim, M., Younas, M. U., Shabaan, M., Zulfiqar, U., Wang, X., & Artyszak, A. (2025). Leveraging Biochar Amendments to Enhance Food Security and Plant Resilience Under Climate Change. Plants, 14(13), 1984. https://doi.org/10.3390/plants14131984










